An Exploration of How Solar Radiation Affects the Seasonal Variation of Human Mortality Rates and the Seasonal Variation in Some Other Common Disorders
Abstract
:1. Introduction
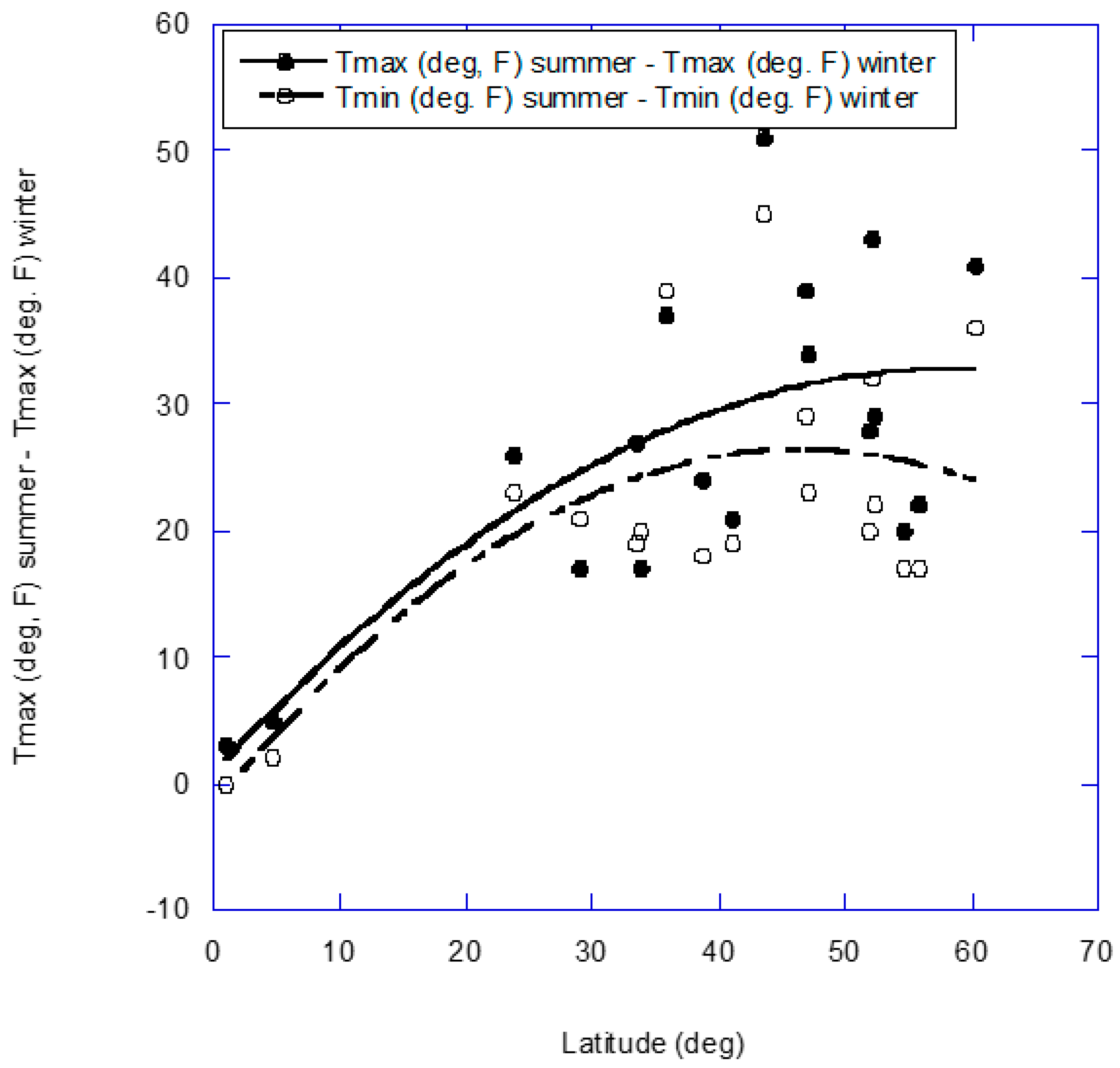
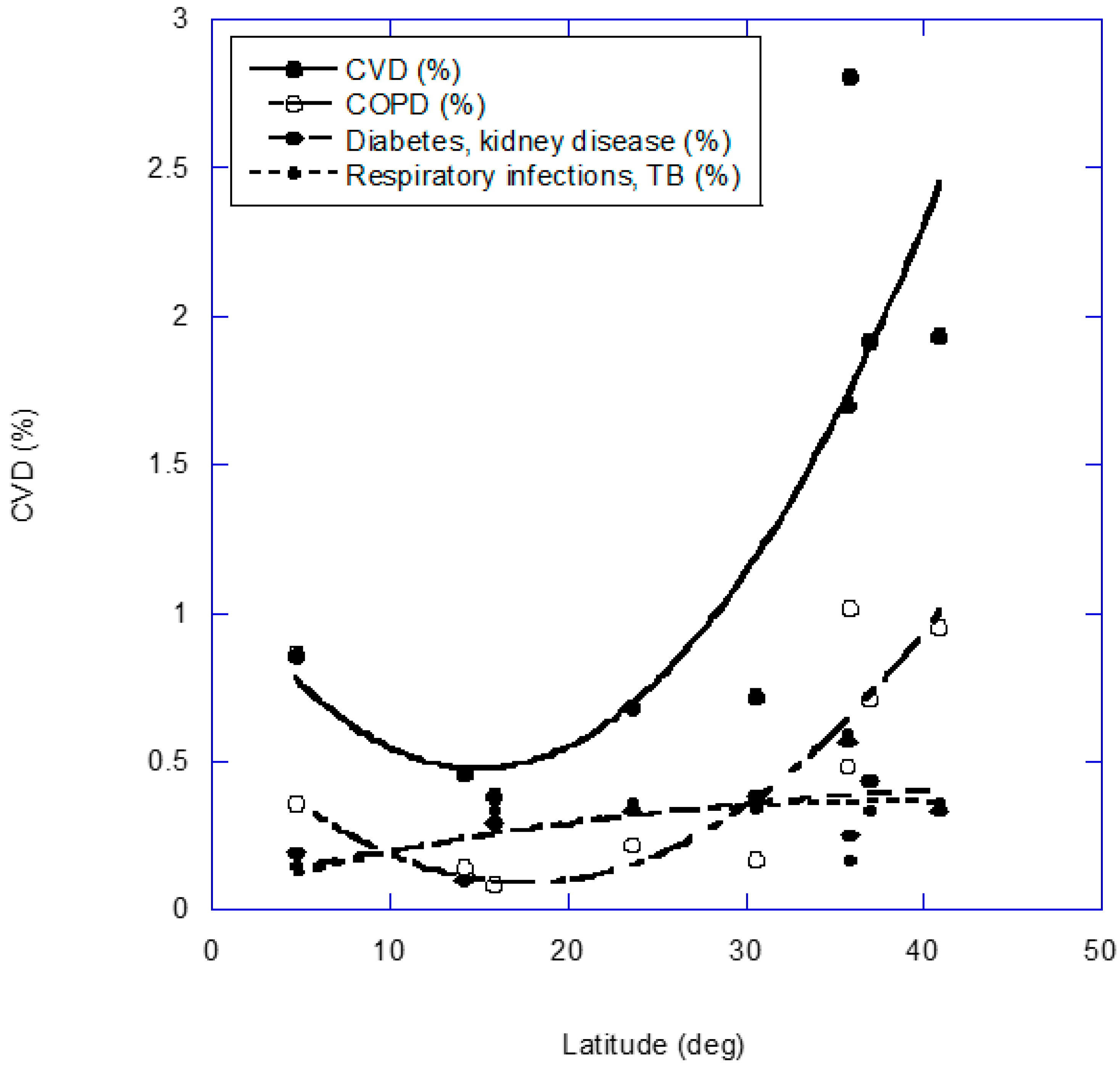
2. Results
2.1. Blood Pressure
2.2. All-Cause Mortality Rate
2.3. Cardiovascular Disease
2.4. Viral Infectious Diseases of the Respiratory Tract
2.5. Cancer
2.6. Other Health Outcomes
| Cause of Death | US 1951–1960 [112] | Australia 2015–2019 [113] | Japan 1970–1999 [114] | Netherlands 1979–1987 [115] | Scotland 1974–1988 [116] |
|---|---|---|---|---|---|
| All causes | 1.17 | 1.10 | 1.04 | 1.33 | |
| Arteriosclerotic heart disease, CHD | 1.28 | 1.15 | 1.14 | 1.34 | 1.28 |
| Cancers | 1.19 | 1.01 | 1.01 | 1.07 | 1.00 |
| Cerebrovascular disease | 1.11 | 1.09 | 1.25 | 1.30 | |
| Chronic respiratory disease | 1.24 | 1.50 | |||
| Cirrhosis of liver | 1.15 | ||||
| Dementia | 1.14 | ||||
| Diabetes mellitus | 1.10 | 1.12 | 1.20 | 1.28 | |
| Digestive diseases | 1.09 | ||||
| Hypertensive heart disease | 1.30 | ||||
| Influenza | 73 | ||||
| Influenza, pneumonia (except newborn) | 2.12 | 1.50 | 1.18 | ||
| Nephritis | 1.22 | 1.30 | |||
| Nonrheumatic chronic endocarditis | 1.24 | ||||
| Pneumonia | 1.33 | 1.88 | |||
| Respiratory | 1.28 | ||||
| Rheumatic fever | 1.21 | ||||
| Septicemia | 1.21 | ||||
| Tuberculosis | 1.17 | 1.59 | |||
| Vascular lesions, CNS | 1.21 |
2.7. Gene Expression
2.8. Air Pollution
3. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marti-Soler, H.; Gonseth, S.; Gubelmann, C.; Stringhini, S.; Bovet, P.; Chen, P.C.; Wojtyniak, B.; Paccaud, F.; Tsai, D.H.; Zdrojewski, T.; et al. Seasonal variation of overall and cardiovascular mortality: A study in 19 countries from different geographic locations. PLoS ONE 2014, 9, e113500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunker, A.; Wildenhain, J.; Vandenbergh, A.; Henschke, N.; Rocklov, J.; Hajat, S.; Sauerborn, R. Effects of Air Temperature on Climate-Sensitive Mortality and Morbidity Outcomes in the Elderly; a Systematic Review and Meta-analysis of Epidemiological Evidence. EBioMedicine 2016, 6, 258–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkart, K.G.; Brauer, M.; Aravkin, A.Y.; Godwin, W.W.; Hay, S.I.; He, J.; Iannucci, V.C.; Larson, S.L.; Lim, S.S.; Liu, J.; et al. Estimating the cause-specific relative risks of non-optimal temperature on daily mortality: A two-part modelling approach applied to the Global Burden of Disease Study. Lancet 2021, 398, 685–697. [Google Scholar] [CrossRef]
- Baggerly, C.A.; Cuomo, R.E.; French, C.B.; Garland, C.F.; Gorham, E.D.; Grant, W.B.; Heaney, R.P.; Holick, M.F.; Hollis, B.W.; McDonnell, S.L.; et al. Sunlight and Vitamin D: Necessary for Public Health. J. Am. Coll. Nutr. 2015, 34, 359–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F. Biological Effects of Sunlight, Ultraviolet Radiation, Visible Light, Infrared Radiation and Vitamin D for Health. Anticancer Res. 2016, 36, 1345–1356. [Google Scholar]
- Grant, W.B.; Bhattoa, H.P.; Boucher, B.J. Seasonal variations of U.S. mortality rates: Roles of solar ultraviolet-B doses, vitamin D, gene expression, and infections. J. Steroid Biochem. Mol. Biol. 2017, 173, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Feelisch, M.; Kolb-Bachofen, V.; Liu, D.; Lundberg, J.O.; Revelo, L.P.; Suschek, C.V.; Weller, R.B. Is sunlight good for our heart? Eur. Heart J. 2010, 31, 1041–1045. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Fernandez, B.O.; Hamilton, A.; Lang, N.N.; Gallagher, J.M.C.; Newby, D.E.; Feelisch, M.; Weller, R.B. UVA irradiation of human skin vasodilates arterial vasculature and lowers blood pressure independently of nitric oxide synthase. J. Investig. Dermatol. 2014, 134, 1839–1846. [Google Scholar] [CrossRef] [Green Version]
- Cherrie, M.; Clemens, T.; Colandrea, C.; Feng, Z.; Webb, D.J.; Weller, R.B.; Dibben, C. Ultraviolet A radiation and COVID-19 deaths in the USA with replication studies in England and Italy. Br. J. Dermatol. 2021, 185, 363–370. [Google Scholar] [CrossRef]
- Grant, W.B.; Bhattoa, H.P.; Pludowski, P. Determinants of Vitamin D Levels from Sun Exposure. In Vitamin D, Ch. 56, 5th ed.; Feldman, D., Pike, J.W., Eds.; Acadenic Press: London, UK, 2022. [Google Scholar]
- Engelsen, O. The relationship between ultraviolet radiation exposure and vitamin D status. Nutrients 2010, 2, 482–495. [Google Scholar] [CrossRef] [Green Version]
- Grant, W.B.; Fakhoury, H.M.A.; Karras, S.N.; Al Anouti, F.; Bhattoa, H.P. Variations in 25-Hydroxyvitamin D in Countries from the Middle East and Europe: The Roles of UVB Exposure and Diet. Nutrients 2019, 11, 2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scragg, R. Seasonality of cardiovascular disease mortality and the possible protective effect of ultra-violet radiation. Int. J. Epidemiol. 1981, 10, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Rostand, S.G. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension 1997, 30, 150–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponsonby, A.L.; McMichael, A.; van der Mei, I. Ultraviolet radiation and autoimmune disease: Insights from epidemiological research. Toxicology 2002, 181–182, 71–78. [Google Scholar] [CrossRef]
- Cannell, J.J.; Vieth, R.; Umhau, J.C.; Holick, M.F.; Grant, W.B.; Madronich, S.; Garland, C.F.; Giovannucci, E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006, 134, 1129–1140. [Google Scholar] [CrossRef]
- Lindqvist, P.G.; Olsson, H.; Landin-Olsson, M. Are active sun exposure habits related to lowering risk of type 2 diabetes mellitus in women, a prospective cohort study? Diabetes Res. Clin. Pract. 2010, 90, 109–114. [Google Scholar] [CrossRef]
- Lindqvist, P.G.; Epstein, E.; Olsson, H. Does an active sun exposure habit lower the risk of venous thrombotic events? A D-lightful hypothesis. J. Thromb. Haemost. 2009, 7, 605–610. [Google Scholar] [CrossRef]
- Lindqvist, P.G.; Epstein, E.; Nielsen, K.; Landin-Olsson, M.; Ingvar, C.; Olsson, H. Avoidance of sun exposure as a risk factor for major causes of death: A competing risk analysis of the Melanoma in Southern Sweden cohort. J. Intern. Med. 2016, 280, 375–387. [Google Scholar] [CrossRef]
- Lindqvist, P.G.; Epstein, E.; Landin-Olsson, M. Sun Exposure—Hazards and Benefits. Anticancer Res. 2022, 42, 1671–1677. [Google Scholar] [CrossRef]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endocrinol. Metab. 1988, 67, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Hypponen, E.; Power, C. Hypovitaminosis D in British adults at age 45 y: Nationwide cohort study of dietary and lifestyle predictors. Am. J. Clin. Nutr. 2007, 85, 860–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroll, M.H.; Bi, C.; Garber, C.C.; Kaufman, H.W.; Liu, D.; Caston-Balderrama, A.; Zhang, K.; Clarke, N.; Xie, M.; Reitz, R.E.; et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS ONE 2015, 10, e0118108. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.S.; Rybchyn, M.S.; Abboud, M.; Brennan-Speranza, T.C.; Fraser, D.R. The Role of Skeletal Muscle in Maintaining Vitamin D Status in Winter. Curr. Dev. Nutr. 2019, 3, nzz087. [Google Scholar] [CrossRef] [PubMed]
- Rybchyn, M.S.; Abboud, M.; Puglisi, D.A.; Gordon-Thomson, C.; Brennan-Speranza, T.C.; Mason, R.S.; Fraser, D.R. Skeletal Muscle and the Maintenance of Vitamin D Status. Nutrients 2020, 12, 3270. [Google Scholar] [CrossRef]
- Katsuki, S.; Arnold, W.; Mittal, C.; Murad, F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J. Cycl. Nucleotide Res. 1977, 3, 23–35. [Google Scholar]
- Gruetter, C.A.; Barry, B.K.; McNamara, D.B.; Gruetter, D.Y.; Kadowitz, P.J.; Ignarro, L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J. Cycl. Nucleotide Res. 1979, 5, 211–224. [Google Scholar]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- SoRelle, R. Nobel prize awarded to scientists for nitric oxide discoveries. Circulation 1998, 98, 2365–2366. [Google Scholar] [CrossRef] [Green Version]
- Ignarro, L.J. Nitric oxide is not just blowing in the wind. Br. J. Pharmacol. 2019, 176, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Weller, R. Nitric oxide—A newly discovered chemical transmitter in human skin. Br. J. Dermatol. 1997, 137, 665–672. [Google Scholar]
- Oplander, C.; Volkmar, C.M.; Paunel-Gorgulu, A.; van Faassen, E.E.; Heiss, C.; Kelm, M.; Halmer, D.; Murtz, M.; Pallua, N.; Suschek, C.V. Whole body UVA irradiation lowers systemic blood pressure by release of nitric oxide from intracutaneous photolabile nitric oxide derivates. Circ. Res. 2009, 105, 1031–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juzeniene, A.; Brekke, P.; Dahlback, A.; Andersson-Engels, S.; Reichrath, J.; Moan, K.; Holick, M.F.; Grant, W.B.; Moan, J. Solar radiation and human health. Rep. Prog. Phys. 2011, 74, 066701. [Google Scholar] [CrossRef] [Green Version]
- Juzeniene, A.; Moan, J. Beneficial effects of UV radiation other than via vitamin D production. Derm. Endocrinol. 2012, 4, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muggeridge, D.J.; Sculthorpe, N.; Grace, F.M.; Willis, G.; Thornhill, L.; Weller, R.B.; James, P.E.; Easton, C. Acute whole body UVA irradiation combined with nitrate ingestion enhances time trial performance in trained cyclists. Nitric Oxide 2015, 48, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorman, S.; Black, L.J.; Feelisch, M.; Hart, P.H.; Weller, R. Can skin exposure to sunlight prevent liver inflammation? Nutrients 2015, 7, 3219–3239. [Google Scholar] [CrossRef] [Green Version]
- Fleury, N.; Feelisch, M.; Hart, P.H.; Weller, R.B.; Smoothy, J.; Matthews, V.B.; Gorman, S. Sub-erythemal ultraviolet radiation reduces metabolic dysfunction in already overweight mice. J. Endocrinol. 2017, 233, 81–92. [Google Scholar] [CrossRef]
- Yu, B.; Jin, S.; Wang, C.; Yan, S.; Zhou, X.; Cui, X.; Tang, Z.; Luan, Q.; Guo, Y.; Bian, Z.; et al. The association of outdoor temperature with blood pressure, and its influence on future cardio-cerebrovascular disease risk in cold areas. J. Hypertens. 2020, 38, 1080–1089. [Google Scholar] [CrossRef]
- Hu, J.; He, G.; Luo, J.; Xu, Y.; Xu, X.; Song, X.; Chen, S.; Ji, G.; Chen, Z.; Jiang, Q.; et al. Temperature-adjusted hypertension prevalence and control rate: A series of cross-sectional studies in Guangdong Province, China. J. Hypertens. 2021, 39, 911–918. [Google Scholar] [CrossRef]
- Group, I.C.R. Intersalt: An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ 1988, 297, 319–328. [Google Scholar]
- Rostand, S.G.; McClure, L.A.; Kent, S.T.; Judd, S.E.; Gutierrez, O.M. Associations of blood pressure, sunlight, and vitamin D in community-dwelling adults. J. Hypertens. 2016, 34, 1704–1710. [Google Scholar] [CrossRef] [Green Version]
- Warren, J.B. Nitric oxide and human skin blood flow responses to acetylcholine and ultraviolet light. FASEB J. 1994, 8, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Andrukhova, O.; Slavic, S.; Zeitz, U.; Riesen, S.C.; Heppelmann, M.S.; Ambrisko, T.D.; Markovic, M.; Kuebler, W.M.; Erben, R.G. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol. Endocrinol. 2014, 28, 53–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, R.B.; Wang, Y.; He, J.; Maddux, F.W.; Usvyat, L.; Zhang, H.; Feelisch, M.; Kotanko, P. Does Incident Solar Ultraviolet Radiation Lower Blood Pressure? J. Am. Heart Assoc. 2020, 9, e013837. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.B.; Feelisch, M.; Kotanko, P. Correspondence on ‘Seasonal variation in blood pressure: Evidence, consensus and recommendations for clinical practice. Consensus statement by the ESH Working Group on Blood Pressure Monitoring and Cardiovascular Variability’. J. Hypertens. 2020, 38, 2077–2079. [Google Scholar] [CrossRef]
- Zhou, A.; Selvanayagam, J.B.; Hypponen, E. Non-linear Mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk. Eur. Heart J. 2021, 43, 1731–1739. [Google Scholar] [CrossRef]
- Mirhosseini, N.; Vatanparast, H.; Kimball, S.M. The Association between Serum 25(OH)D Status and Blood Pressure in Participants of a Community-Based Program Taking Vitamin D Supplements. Nutrients 2017, 9, 1244. [Google Scholar] [CrossRef] [Green Version]
- Kutschenreuter, P.H. A study of the effect of weather on mortality. Trans. N. Y. Acad. Sci. 1959, 126–138. [Google Scholar] [CrossRef]
- Wilkinson, P.; Pattenden, S.; Armstrong, B.; Fletcher, A.; Kovats, R.S.; Mangtani, P.; McMichael, A.J. Vulnerability to winter mortality in elderly people in Britain: Population based study. BMJ 2004, 329, 647. [Google Scholar] [CrossRef] [Green Version]
- Garland, C.F.; Kim, J.J.; Mohr, S.B.; Gorham, E.D.; Grant, W.B.; Giovannucci, E.L.; Baggerly, L.; Hofflich, H.; Ramsdell, J.W.; Zeng, K.; et al. Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. Am. J. Public Health 2014, 104, e43–e50. [Google Scholar] [CrossRef]
- Revich, B.; Shaposhnikov, D. Temperature-induced excess mortality in Moscow, Russia. Int. J. Biometeorol. 2008, 52, 367–374. [Google Scholar] [CrossRef]
- Donaldson, G.C.; Ermakov, S.P.; Komarov, Y.M.; McDonald, C.P.; Keatinge, W.R. Cold related mortalities and protection against cold in Yakutsk, eastern Siberia: Observation and interview study. BMJ 1998, 317, 978–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keatinge, W.R.; Coleshaw, S.R.; Holmes, J. Changes in seasonal mortalities with improvement in home heating in England and Wales from 1964 to 1984. Int. J. Biometeorol. 1989, 33, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Achebak, H.; Devolder, D.; Ballester, J. Trends in temperature-related age-specific and sex-specific mortality from cardiovascular diseases in Spain: A national time-series analysis. Lancet Planet. Health 2019, 3, e297–e306. [Google Scholar] [CrossRef] [Green Version]
- Keatinge, W.R. Winter mortality and its causes. Int. J. Circumpolar Health 2002, 61, 292–299. [Google Scholar] [CrossRef]
- Kenney, W.L.; Munce, T.A. Invited review: Aging and human temperature regulation. J. Appl. Physiol. 2003, 95, 2598–2603. [Google Scholar] [CrossRef]
- Grimes, D.S.; Hindle, E.; Dyer, T. Sunlight, cholesterol and coronary heart disease. QJM 1996, 89, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Zittermann, A.; Schleithoff, S.S.; Koerfer, R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br. J. Nutr. 2005, 94, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Thurston, G.D.; Burnett, R.T.; Turner, M.C.; Shi, Y.; Krewski, D.; Lall, R.; Ito, K.; Jerrett, M.; Gapstur, S.M.; Diver, W.R.; et al. Ischemic Heart Disease Mortality and Long-Term Exposure to Source-Related Components of U.S. Fine Particle Air Pollution. Environ. Health Perspect. 2016, 124, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Acharya, P.; Dalia, T.; Ranka, S.; Sethi, P.; Oni, O.A.; Safarova, M.S.; Parashara, D.; Gupta, K.; Barua, R.S. The Effects of Vitamin D Supplementation and 25-Hydroxyvitamin D Levels on the Risk of Myocardial Infarction and Mortality. J. Endocr. Soc. 2021, 5, bvab124. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Barbarawi, M.; Kheiri, B.; Zayed, Y.; Barbarawi, O.; Dhillon, H.; Swaid, B.; Yelangi, A.; Sundus, S.; Bachuwa, G.; Alkotob, M.L.; et al. Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83000 Individuals in 21 Randomized Clinical Trials: A Meta-analysis. JAMA Cardiol. 2019, 4, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Raed, A.; Bhagatwala, J.; Zhu, H.; Pollock, N.K.; Parikh, S.J.; Huang, Y.; Havens, R.; Kotak, I.; Guo, D.H.; Dong, Y. Dose responses of vitamin D3 supplementation on arterial stiffness in overweight African Americans with vitamin D deficiency: A placebo controlled randomized trial. PLoS ONE 2017, 12, e0188424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerging Risk Factors Collaboration/EPIC-CVD/Vitamin D Studies Collaboration. Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: Observational and Mendelian randomisation analyses. Lancet Diabetes Endocrinol. 2021, 9, 837–846. [Google Scholar] [CrossRef]
- Heaney, R.P. Design and analysis of clinical trials of nutrients: Author reply. Nutr. Rev. 2014, 72, 354. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J.; Bhattoa, H.P.; Lahore, H. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2018, 177, 266–269. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.L.; Vanwoerkom, R.C.; Horne, B.D.; Bair, T.L.; May, H.T.; Lappe, D.L.; Muhlestein, J.B. Parathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: Dependent or independent risk factors? Am. Heart J. 2011, 162, 331–339.e2. [Google Scholar] [CrossRef]
- Valcour, A.; Blocki, F.; Hawkins, D.M.; Rao, S.D. Effects of age and serum 25-OH-vitamin D on serum parathyroid hormone levels. J. Clin. Endocrinol. Metab. 2012, 97, 3989–3995. [Google Scholar] [CrossRef]
- Tomaschitz, A.; Ritz, E.; Pieske, B.; Rus-Machan, J.; Kienreich, K.; Verheyen, N.; Gaksch, M.; Grubler, M.; Fahrleitner-Pammer, A.; Mrak, P.; et al. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease. Metabolism 2014, 63, 20–31. [Google Scholar] [CrossRef]
- Bolland, M.J.; Avenell, A.; Baron, J.A.; Grey, A.; MacLennan, G.S.; Gamble, G.D.; Reid, I.R. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: Meta-analysis. BMJ 2010, 341, c3691. [Google Scholar] [CrossRef] [Green Version]
- Boucher, B.J. Calcium supplements may increase the risk of cardiovascular events in postmenopausal women. Evid. Based Med. 2012, 17, 16–17. [Google Scholar] [CrossRef]
- Lei, J.; Vodovotz, Y.; Tzeng, E.; Billiar, T.R. Nitric oxide, a protective molecule in the cardiovascular system. Nitric Oxide 2013, 35, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.B. Sunlight Has Cardiovascular Benefits Independently of Vitamin D. Blood Purif. 2016, 41, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.F.; Clemens, T.L.; Hastie, C.E.; Cherrie, M.P.C.; Dibben, C.; Pell, J.P. UVA and Seasonal Patterning of 56 370 Myocardial Infarctions Across Scotland, 2000–2011. J. Am. Heart Assoc. 2019, 8, e012551. [Google Scholar] [CrossRef]
- Bhatnagar, A. Environmental Determinants of Cardiovascular Disease. Circ. Res. 2017, 121, 162–180. [Google Scholar] [CrossRef]
- Hope-Simpson, R.E. The role of season in the epidemiology of influenza. J. Hyg. 1981, 86, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Aloia, J.F.; Li-Ng, M. Re: Epidemic influenza and vitamin D. Epidemiol. Infect. 2007, 135, 1095–1096. [Google Scholar] [CrossRef] [Green Version]
- Li-Ng, M.; Aloia, J.F.; Pollack, S.; Cunha, B.A.; Mikhail, M.; Yeh, J.; Berbari, N. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol. Infect. 2009, 137, 1396–1404. [Google Scholar] [CrossRef]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef] [Green Version]
- Shaman, J.; Jeon, C.Y.; Giovannucci, E.; Lipsitch, M. Shortcomings of vitamin D-based model simulations of seasonal influenza. PLoS ONE 2011, 6, e20743. [Google Scholar] [CrossRef]
- Tamerius, J.D.; Shaman, J.; Alonso, W.J.; Bloom-Feshbach, K.; Uejio, C.K.; Comrie, A.; Viboud, C. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLoS Pathog. 2013, 9, e1003194. [Google Scholar] [CrossRef]
- Ianevski, A.; Zusinaite, E.; Shtaida, N.; Kallio-Kokko, H.; Valkonen, M.; Kantele, A.; Telling, K.; Lutsar, I.; Letjuka, P.; Metelitsa, N.; et al. Low Temperature and Low UV Indexes Correlated with Peaks of Influenza Virus Activity in Northern Europe during 2010–2018. Viruses 2019, 11, 207. [Google Scholar] [CrossRef] [Green Version]
- Harper, G.J. Airborne micro-organisms: Survival tests with four viruses. J. Hyg. 1961, 59, 479–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Audi, A.; AlIbrahim, M.; Kaddoura, M.; Hijazi, G.; Yassine, H.M.; Zaraket, H. Seasonality of Respiratory Viral Infections: Will COVID-19 Follow Suit? Front. Public Health 2020, 8, 567184. [Google Scholar] [CrossRef] [PubMed]
- Eccles, R. An explanation for the seasonality of acute upper respiratory tract viral infections. Acta Otolaryngol. 2002, 122, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Khalis, M.; Toure, A.B.; El Badisy, I.; Khomsi, K.; Najmi, H.; Bouaddi, O.; Marfak, A.; Al-Delaimy, W.K.; Berraho, M.; Nejjari, C. Relationship between Meteorological and Air Quality Parameters and COVID-19 in Casablanca Region, Morocco. Int. J. Environ. Res. Public Health 2022, 19, 4989. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [Green Version]
- Oristrell, J.; Oliva, J.C.; Casado, E.; Subirana, I.; Dominguez, D.; Toloba, A.; Balado, A.; Grau, M. Vitamin D supplementation and COVID-19 risk: A population-based, cohort study. J. Endocrinol. Investig. 2022, 45, 167–179. [Google Scholar] [CrossRef]
- Seal, K.H.; Bertenthal, D.; Carey, E.; Grunfeld, C.; Bikle, D.D.; Lu, C.M. Association of Vitamin D Status and COVID-19-Related Hospitalization and Mortality. J. Gen. Intern. Med. 2022, 37, 853–861. [Google Scholar] [CrossRef]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcala Diaz, J.F.; Lopez Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Villasis-Keever, M.A.; Lopez-Alarcon, M.G.; Miranda-Vovales, G.; Zurita-Cruz, J.N.; Barrada-Vazquez, A.Z. Efficacy and Safety of Vitamin D Supplementation to Prevent COVID-19 in Frontline Healthcare Workers. A Randomized Clinical Trial. Arch. Med. Res. 2022, 53, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.R.; Celli, B.; Anderson, J.A.; Ferguson, G.T.; Jones, P.W.; Vestbo, J.; Yates, J.C.; Calverley, P.M. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur. Respir. J. 2012, 39, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.C.; Wedzicha, J.A. The causes and consequences of seasonal variation in COPD exacerbations. Int. J. Chron. Obstruct. Pulmon. Dis. 2014, 9, 1101–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’h, N.; Falgarone, G.; Boissier, M.C. Vitamin D and inflammation. Jt. Bone Spine 2010, 77, 552–557. [Google Scholar] [CrossRef]
- Ma, W.; Nguyen, L.H.; Yue, Y.; Ding, M.; Drew, D.A.; Wang, K.; Merino, J.; Rich-Edwards, J.W.; Sun, Q.; Camargo, C.A.; et al. Associations between predicted vitamin D status, vitamin D intake, and risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and coronavirus disease 2019 (COVID-19) severity. Am. J. Clin. Nutr. 2022, 115, 1123–1133. [Google Scholar] [CrossRef]
- Lau, F.H.; Powell, C.E.; Adonecchi, G.; Danos, D.M.; DiNardo, A.R.; Chugden, R.J.; Wolr, P.; Castilla, C.F. Pilot Phase Results of a Prospective, Randomized Controlled Trial of Narrowband Ultraviolet B Phototherapy in Hospitalized COVID-19 Patients. Exp. Dermatol. 2022. [Google Scholar] [CrossRef]
- Walrand, S. Autumn COVID-19 surge dates in Europe correlated to latitudes, not to temperature-humidity, pointing to vitamin D as contributing factor. Sci. Rep. 2021, 11, 1981. [Google Scholar] [CrossRef]
- Gorman, S.; Weller, R.B. Investigating the Potential for Ultraviolet Light to Modulate Morbidity and Mortality From COVID-19: A Narrative Review and Update. Front. Cardiovasc. Med. 2020, 7, 616527. [Google Scholar] [CrossRef]
- Muñoz, A.; Grant, W.B. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, T.; Panwar, M.S.; Sharma, D.; Bundel, R.; Hamilton, R.T.; Radosevich, J.A.; Mandal, C.C. Colder environments are associated with a greater cancer incidence in the female population of the United States. Tumour Biol. 2017, 39, 1010428317724784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandyopadhayaya, S.; Ford, B.; Mandal, C.C. Cold-hearted: A case for cold stress in cancer risk. J. Therm. Biol. 2020, 91, 102608. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wei, Q.; Bondy, M.L.; Yu, T.K.; Li, D.; Brewster, A.; Shete, S.; Sahin, A.; Meric-Bernstam, F.; Wang, L.E. Promoter polymorphism (-786t>C) in the endothelial nitric oxide synthase gene is associated with risk of sporadic breast cancer in non-Hispanic white women age younger than 55 years. Cancer 2006, 107, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Kang, D.; Park, S.K.; Berndt, S.I.; Reding, D.; Chatterjee, N.; Chanock, S.; Huang, W.Y.; Hayes, R.B. Nitric oxide synthase gene polymorphisms and prostate cancer risk. Carcinogenesis 2009, 30, 621–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, T.; Haruma, K.; Kitadai, Y.; Ito, M.; Yoshihara, M.; Sumii, K.; Hayakawa, N.; Kajiyama, G. Enhanced expression of inducible nitric oxide synthase and nitrotyrosine in gastric mucosa of gastric cancer patients. Clin. Cancer Res. 1999, 5, 1411–1415. [Google Scholar]
- Mason, B.H.; Holdaway, I.M.; Stewart, A.W.; Neave, L.M.; Kay, R.G. Season of tumour detection influences factors predicting survival of patients with breast cancer. Breast Cancer Res. Treat. 1990, 15, 27–37. [Google Scholar] [CrossRef]
- Porojnicu, A.C.; Dahlback, A.; Moan, J. Sun exposure and cancer survival in Norway: Changes in the risk of death with season of diagnosis and latitude. Adv. Exp. Med. Biol. 2008, 624, 43–54. [Google Scholar]
- Chandler, P.D.; Chen, W.Y.; Ajala, O.N.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.M.; Giovannucci, E.L.; Willett, W.; Buring, J.E.; et al. Effect of Vitamin D3 Supplements on Development of Advanced Cancer: A Secondary Analysis of the VITAL Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2025850. [Google Scholar] [CrossRef]
- Madden, J.M.; Murphy, L.; Zgaga, L.; Bennett, K. De novo vitamin D supplement use post-diagnosis is associated with breast cancer survival. Breast Cancer Res. Treat. 2018, 172, 179–190. [Google Scholar] [CrossRef]
- Mohr, S.B.; Gorham, E.D.; Alcaraz, J.E.; Kane, C.I.; Macera, C.A.; Parsons, J.K.; Wingard, D.L.; Horst, R.; Garland, C.F. Serum 25-hydroxyvitamin D and breast cancer in the military: A case-control study utilizing pre-diagnostic serum. Cancer Causes Control. 2013, 24, 495–504. [Google Scholar] [CrossRef]
- Rosenwaike, I. Seasonal variation of deaths in the United States, 1951–1960. J. Am. Stat. Assoc. 1966, 61, 706–719. [Google Scholar]
- Gregory, G.; Zhu, L.; Hayen, A.; Bell, K.J.L. Learning from the pandemic: Mortality trends and seasonality of deaths in Australia in 2020. Int. J. Epidemiol. 2022, 51, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Nakaji, S.; Parodi, S.; Fontana, V.; Umeda, T.; Suzuki, K.; Sakamoto, J.; Fukuda, S.; Wada, S.; Sugawara, K. Seasonal changes in mortality rates from main causes of death in Japan (1970–1999). Eur. J. Epidemiol. 2004, 19, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Mackenbach, J.P.; Kunst, A.E.; Looman, C.W. Seasonal variation in mortality in The Netherlands. J. Epidemiol. Community Health 1992, 46, 261–265. [Google Scholar] [CrossRef]
- Douglas, A.S.; Allan, T.M.; Rawles, J.M. Composition of seasonality of disease. Scott. Med. J. 1991, 36, 76–82. [Google Scholar] [CrossRef]
- Ebi, K.L.; Capon, A.; Berry, P.; Broderick, C.; de Dear, R.; Havenith, G.; Honda, Y.; Kovats, R.S.; Ma, W.; Malik, A.; et al. Hot weather and heat extremes: Health risks. Lancet 2021, 398, 698–708. [Google Scholar] [CrossRef]
- Vandebergh, M.; Degryse, N.; Dubois, B.; Goris, A. Environmental risk factors in multiple sclerosis: Bridging Mendelian randomization and observational studies. J. Neurol. 2022. [Google Scholar] [CrossRef]
- Wang, R. Mendelian randomization study updates the effect of 25-hydroxyvitamin D levels on the risk of multiple sclerosis. J. Transl. Med. 2022, 20, 3. [Google Scholar] [CrossRef]
- Sloka, S.; Grant, M.; Newhook, L.A. The geospatial relation between UV solar radiation and type 1 diabetes in Newfoundland. Acta Diabetol. 2010, 47, 73–78. [Google Scholar] [CrossRef]
- Disanto, G.; Chaplin, G.; Morahan, J.M.; Giovannoni, G.; Hypponen, E.; Ebers, G.C.; Ramagopalan, S.V. Month of birth, vitamin D and risk of immune-mediated disease: A case control study. BMC Med. 2012, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Boucher, B.J.; Mannan, N.; Noonan, K.; Hales, C.N.; Evans, S.J. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. Diabetologia 1995, 38, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Niroomand, M.; Fotouhi, A.; Irannejad, N.; Hosseinpanah, F. Does high-dose vitamin D supplementation impact insulin resistance and risk of development of diabetes in patients with pre-diabetes? A double-blind randomized controlled trial. Diabetes Res. Clin. Pract. 2019, 148, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bejar, C.A.; Goyal, S.; Afzal, S.; Mangino, M.; Zhou, A.; van der Most, P.J.; Bao, Y.; Gupta, V.; Smart, M.C.; Walia, G.K.; et al. A Bidirectional Mendelian Randomization Study to evaluate the causal role of reduced blood vitamin D levels with type 2 diabetes risk in South Asians and Europeans. Nutr. J. 2021, 20, 71. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Staten, M.A.; Knowler, W.C.; Nelson, J.; Vickery, E.M.; LeBlanc, E.S.; Neff, L.M.; Park, J.; Pittas, A.G.; Group, D.d.R. Intratrial Exposure to Vitamin D and New-Onset Diabetes Among Adults With Prediabetes: A Secondary Analysis From the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care 2020, 43, 2916–2922. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, C.; Wang, L. Temperature exposure during pregnancy and birth outcomes: An updated systematic review of epidemiological evidence. Environ. Pollut. 2017, 225, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Samuels, L.; Nakstad, B.; Roos, N.; Bonell, A.; Chersich, M.; Havenith, G.; Luchters, S.; Day, L.T.; Hirst, J.E.; Singh, T.; et al. Physiological mechanisms of the impact of heat during pregnancy and the clinical implications: Review of the evidence from an expert group meeting. Int. J. Biometeorol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhou, L.; Wang, S.; Yin, H.; Yang, X.; Hao, L. Effect of maternal vitamin D status on risk of adverse birth outcomes: A systematic review and dose-response meta-analysis of observational studies. Eur. J. Nutr. 2022. [Google Scholar] [CrossRef]
- Nausheen, S.; Habib, A.; Bhura, M.; Rizvi, A.; Shaheen, F.; Begum, K.; Iqbal, J.; Ariff, S.; Shaikh, L.; Raza, S.S.; et al. Impact evaluation of the efficacy of different doses of vitamin D supplementation during pregnancy on pregnancy and birth outcomes: A randomised, controlled, dose comparison trial in Pakistan. BMJ Nutr. Prev. Health 2021, 4, 425–434. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hollis, B.W. The Implications of Vitamin D Status During Pregnancy on Mother and her Developing Child. Front. Endocrinol. 2018, 9, 500. [Google Scholar] [CrossRef] [Green Version]
- Dopico, X.C.; Evangelou, M.; Ferreira, R.C.; Guo, H.; Pekalski, M.L.; Smyth, D.J.; Cooper, N.; Burren, O.S.; Fulford, A.J.; Hennig, B.J.; et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat. Commun. 2015, 6, 7000. [Google Scholar] [CrossRef]
- Goldinger, A.; Shakhbazov, K.; Henders, A.K.; McRae, A.F.; Montgomery, G.W.; Powell, J.E. Seasonal effects on gene expression. PLoS ONE 2015, 10, e0126995. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Spira, A.; Holick, M.F. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: A randomized double-blind clinical trial. PLoS ONE 2013, 8, e58725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. Disassociation of Vitamin D’s Calcemic Activity and Non-calcemic Genomic Activity and Individual Responsiveness: A Randomized Controlled Double-Blind Clinical Trial. Sci. Rep. 2019, 9, 17685. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, A.; Kalajian, T.A.; Song, A.; Allen, R.; Charoenngam, N.; Lewanczuk, R.; Holick, M.F. Variable Genomic and Metabolomic Responses to Varying Doses of Vitamin D Supplementation. Anticancer Res. 2020, 40, 535–543. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Bogdan, C. Nitric oxide and the regulation of gene expression. Trends Cell Biol. 2001, 11, 66–75. [Google Scholar] [CrossRef]
- Hemish, J.; Nakaya, N.; Mittal, V.; Enikolopov, G. Nitric oxide activates diverse signaling pathways to regulate gene expression. J. Biol. Chem. 2003, 278, 42321–42329. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z. Spatial analysis of MODIS aerosol optical depth, PM2.5, and chronic coronary heart disease. Int. J. Health Geogr. 2009, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Feigin, V.L.; Roth, G.A.; Naghavi, M.; Parmar, P.; Krishnamurthi, R.; Chugh, S.; Mensah, G.A.; Norrving, B.; Shiue, I.; Ng, M.; et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016, 15, 913–924. [Google Scholar] [CrossRef] [Green Version]
- Lelieveld, J.; Klingmuller, K.; Pozzer, A.; Poschl, U.; Fnais, M.; Daiber, A.; Munzel, T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 2019, 40, 1590–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stieb, D.M.; Judek, S.; Burnett, R.T. Meta-analysis of time-series studies of air pollution and mortality: Effects of gases and particles and the influence of cause of death, age, and season. J. Air Waste Manag. Assoc. 2002, 52, 470–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corless, D.; Gupta, S.P.; Switala, S.; Barragry, J.M.; Boucher, B.J.; Cohen, R.D.; Diffey, B.L. Response of plasma-25-hydroxyvitamin D to ultraviolet irradiation in long-stay geriatric patients. Lancet 1978, 2, 649–651. [Google Scholar] [CrossRef]
- Chuck, A.; Todd, J.; Diffey, B. Subliminal ultraviolet-B irradiation for the prevention of vitamin D deficiency in the elderly: A feasibility study. Photodermatol. Photoimmunol. Photomed. 2001, 17, 168–171. [Google Scholar] [CrossRef]
- Chandra, P.; Wolfenden, L.L.; Ziegler, T.R.; Tian, J.; Luo, M.; Stecenko, A.A.; Chen, T.C.; Holick, M.F.; Tangpricha, V. Treatment of vitamin D deficiency with UV light in patients with malabsorption syndromes: A case series. Photodermatol. Photoimmunol. Photomed. 2007, 23, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Dabai, N.S.; Pramyothin, P.; Holick, M.F. The effect of ultraviolet radiation from a novel portable fluorescent lamp on serum 25-hydroxyvitamin D3 levels in healthy adults with Fitzpatrick skin types II and III. Photodermatol. Photoimmunol. Photomed. 2012, 28, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Park, D.-H.; Oh, S.-T.; Lim, J.-H. Development of UVB LED Lighting System Based on UV Dose Calculation Algorithm to Meet Individual Daily UV Dose. Appl. Sci. 2019, 9, 2479. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.Y.; Lim, J.-H. Development and Effect Analysis of UVB-LED General Lighting to Support Vitamin D Synthesis. Appl. Sci. 2020, 10, 889. [Google Scholar] [CrossRef] [Green Version]
- Ames, B.N.; Grant, W.B.; Willett, W.C. Does the High Prevalence of Vitamin D Deficiency in African Americans Contribute to Health Disparities? Nutrients 2021, 13, 499. [Google Scholar] [CrossRef]
- Jablonski, N.G.; Chaplin, G. Colloquium paper: Human skin pigmentation as an adaptation to UV radiation. Proc. Natl. Acad. Sci. USA 2010, 107 (Suppl. 2), 8962–8968. [Google Scholar] [CrossRef] [Green Version]
- Mohania, D.; Chandel, S.; Kumar, P.; Verma, V.; Digvijay, K.; Tripathi, D.; Choudhury, K.; Mitten, S.K.; Shah, D. Ultraviolet Radiations: Skin Defense-Damage Mechanism. Adv. Exp. Med. Biol. 2017, 996, 71–87. [Google Scholar] [PubMed]
- Scott, J.F.; Das, L.M.; Ahsanuddin, S.; Qiu, Y.; Binko, A.M.; Traylor, Z.P.; Debanne, S.M.; Cooper, K.D.; Boxer, R.; Lu, K.Q. Oral Vitamin D Rapidly Attenuates Inflammation from Sunburn: An Interventional Study. J. Investig. Dermatol. 2017, 137, 2078–2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.F.; Lu, K.Q. Vitamin D as a Therapeutic Option for Sunburn: Clinical and Biologic Implications. DNA Cell Biol. 2017, 36, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.; Correa, M.P.; Holick, M.F.; Melo, E.V.; Lazaretti-Castro, M. Sun-induced production of vitamin D3 throughout 1 year in tropical and subtropical regions: Relationship with latitude, cloudiness, UV-B exposure and solar zenith angle. Photochem. Photobiol. Sci. 2021, 20, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J.; Vogt, T.; Holick, M.F.; Friedrich, M. Abstracts of the Joint International Symposia “Vitamin D in Prevention and Therapy” and “Biologic Effects of Light”. Anticancer Res. 2022, 42, 2193–2222. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grant, W.B.; Boucher, B.J. An Exploration of How Solar Radiation Affects the Seasonal Variation of Human Mortality Rates and the Seasonal Variation in Some Other Common Disorders. Nutrients 2022, 14, 2519. https://doi.org/10.3390/nu14122519
Grant WB, Boucher BJ. An Exploration of How Solar Radiation Affects the Seasonal Variation of Human Mortality Rates and the Seasonal Variation in Some Other Common Disorders. Nutrients. 2022; 14(12):2519. https://doi.org/10.3390/nu14122519
Chicago/Turabian StyleGrant, William B., and Barbara J. Boucher. 2022. "An Exploration of How Solar Radiation Affects the Seasonal Variation of Human Mortality Rates and the Seasonal Variation in Some Other Common Disorders" Nutrients 14, no. 12: 2519. https://doi.org/10.3390/nu14122519
APA StyleGrant, W. B., & Boucher, B. J. (2022). An Exploration of How Solar Radiation Affects the Seasonal Variation of Human Mortality Rates and the Seasonal Variation in Some Other Common Disorders. Nutrients, 14(12), 2519. https://doi.org/10.3390/nu14122519







