Wheat Biscuits Enriched with Plant-Based Protein Contribute to Weight Loss and Beneficial Metabolic Effects in Subjects with Overweight/Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
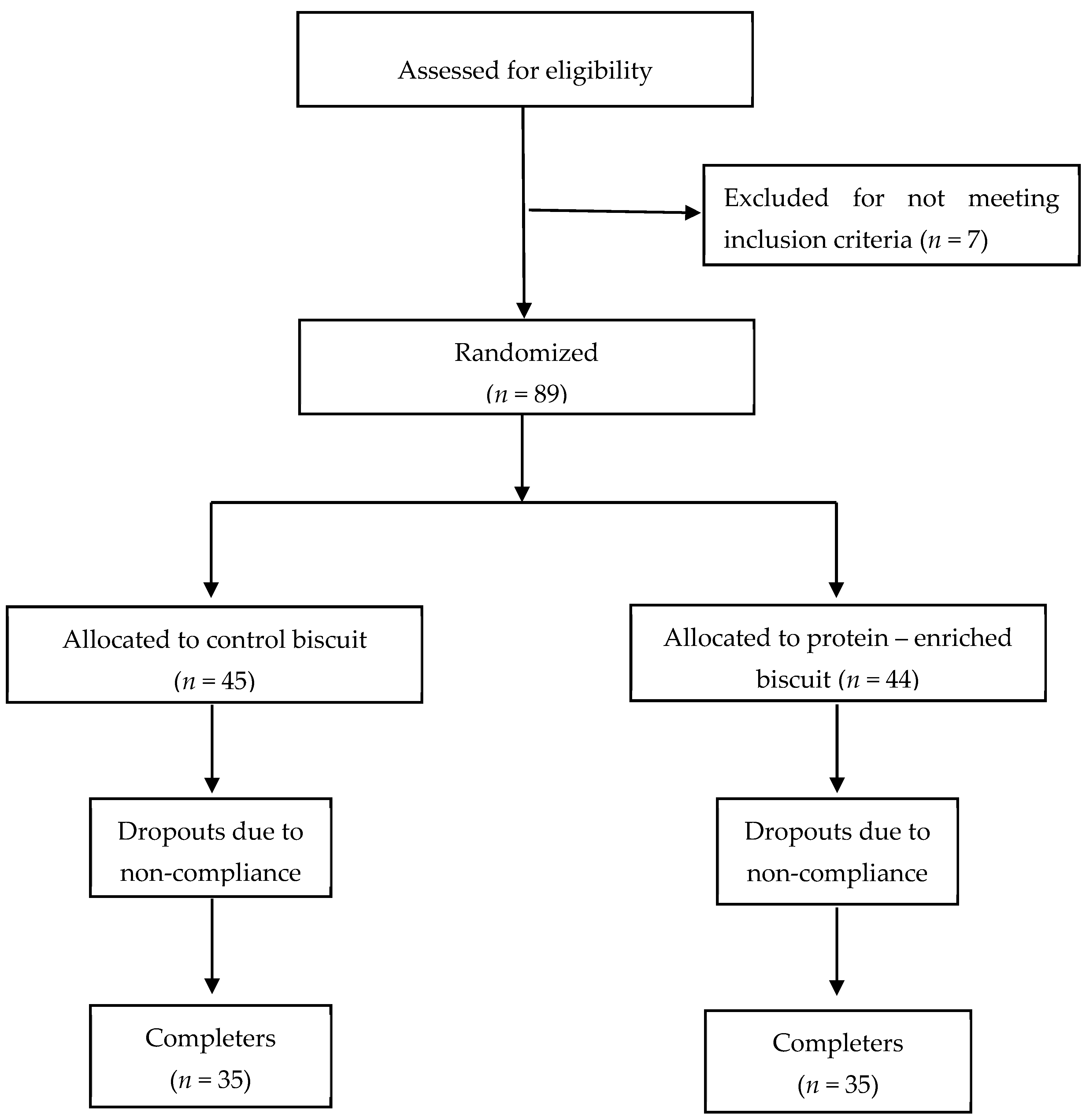
2.2. Study Design
2.3. Blood Analyses
2.4. Test Biscuits
2.5. Statistical Analysis
3. Results
Study Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 27 March 2021).
- Blundell, J.E.; Dulloo, A.G.; Salvador, J.; Frühbeck, G.; On Behalf of the EASO SAB Working Group on BMI. Beyond BMI—Phenotyping the obesities. Obes. Facts 2014, 7, 322–328. [Google Scholar] [CrossRef] [PubMed]
- McManus, K. A Practical Guide to the Mediterranean Diet; Harvard Health Publishing: Boston, MA, USA, 2019. [Google Scholar]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, E.Z.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food and Agriculture Organization of the United Nations Pulses: Nutritious Seeds for a Sustainable Future. International Year of Pulses. 2016. Available online: http://www.fao.org/3/a-i5528e.pdf (accessed on 1 December 2021).
- Qamar, S.; Manrique, Y.J.; Parekh, H.; Falconer, J.R. Nuts, cereas, seeds and legume proteins derived emulsifiers saasource of plant protein beverages: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2742–2762. [Google Scholar] [CrossRef] [PubMed]
- Binou, P.; Yanni, A.E.; Karathanos, V.T. Physical properties, sensory acceptance, postprandial glycemic response, and satiety of cereal based foods enriched with legume flours: A review. Crit. Rev. Food Sci. Nutr. 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Halton, T.L.; Hu, F.B. The effects of high protein diets on thermogenesis, satiety and weight loss: A critical review. J. Am. Coll. Nutr. 2004, 23, 373–385. [Google Scholar] [CrossRef]
- Tremblay, A.; Bellisle, F. Nutrients, satiety, and control of energy intake. Appl. Physiol. Nutr. Metab. 2015, 40, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, M.; Stenberg, M.; Frid, A.H.; Holst, J.J.; Björck, I.M.E. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: The role of plasma amino acids and incretins. Am. J. Clin. Nutr. 2004, 80, 1246–1253. [Google Scholar] [CrossRef]
- Batterham, R.L.; Heffron, H.; Kapoor, S.; Chivers, J.E.; Chandarana, K.; Herzog, H.; Le Roux, C.W.; Thomas, E.L.; Bell, J.D.; Withers, D.J. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006, 4, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Simonson, M.; Boirie, Y.; Guillet, C. Protein, amino acids and obesity treatment. Rev. Endocr. Metab. Disord. 2020, 21, 341–353. [Google Scholar] [CrossRef]
- Psichas, A.; Reimann, F.; Gribble, F.M. Gut chemosensing mechanisms. J. Clin. Investig. 2015, 125, 908–917. [Google Scholar] [CrossRef] [Green Version]
- McGavigan, A.K.; O’Hara, H.C.; Amin, A.; Kinsey-Jones, J.; Spreckley, E.; Alamshah, A.; Agahi, A.; Banks, K.; France, R.; Hyberg, G. L-Cysteine suppresses ghrelin and reduces appetite in rodents and humans. Int. J. Obes. 2015, 39, 447–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, A.; Neophytou, C.; Thein, S.; Martin, N.M.; Alamshah, A.; Spreckley, E.; Bloom, S.R.; Murphy, K.G. L-Arginine Increases Postprandial Circulating GLP-1 and PYY Levels in Humans. Obesity 2018, 261, 721–1726. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.E.; Tamini, S.; Cicolini, S.; De Col, A.; Caroli, D.; Mai, S.; Rondinelli, E.; Saezza, A.; Cella, S.G.; Sartorio, A. Evaluation of an Amino Acid Mix on the Secretion of Gastrointestinal Peptides, Glucometabolic Homeostasis, and Appetite in Obese Adolescents Administered with a Fixed-Dose or ad Libitum Meal. J. Clin. Med. 2020, 9, 3054. [Google Scholar] [CrossRef] [PubMed]
- Dashtabi, A.; Mazloom, Z.; Fararouei, M.; Hejazi, N. Oral L-Arginine Administration Improves Anthropometric and Biochemical Indices Associated with Cardiovascular Diseases in Obese Patients: A Randomized, Single Blind Placebo Controlled Clinical Trial. Res. Cardiovasc. Med. 2015, 5, e29419. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.M.; Milajerdi, A.; Fatahi, S.; Rahmani, J.; Zarezadeh, M.; Ghaedi, E.; Varkaneh, H.K. The effect of L-arginine supplementation on obesity-related indices: A systematic review and meta-analysis of randomized clinical trials. Int. J. Vitam. Nutr. Res. 2021, 91, 164–174. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Leoncini, R.; De Col, A.; Tamini, S.; Cicolini, S.; Abbruzzese, L.; Cella, S.G.; Sartorio, A. The Appetite-Suppressant and GLP-1-Stimulating Effects of Whey Proteins in Obese Subjects are Associated with Increased Circulating Levels of Specific Amino Acids. Nutrients 2020, 12, 775. [Google Scholar] [CrossRef] [Green Version]
- Bolster, D.R.; Rahn, M.; Kamil, A.G.; Bristol, L.T.; Goltz, S.R.; Leidy, H.J.; Blaze, M.; Nunez, M.A.; Guo, E.; Wang, J.; et al. Consuming Lower-Protein Nutrition Bars with Added Leucine Elicits Postprandial Changes in Appetite Sensations in Healthy Women. J. Nutr. 2018, 148, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Traylor, D.A.; Kamal, M.; Nunes, E.A.; Prior, T.; Gorissen, S.; Lees, M.; Gesel, F.; Lim, C.; Phillips, S.M. Consumption of High-Leucine-Containing Protein Bar Following Breakfast Impacts Aminoacidemia and Subjective Appetite in Older Persons. Curr. Dev. Nutr. 2021, 5, nzab080. [Google Scholar] [CrossRef]
- Blouet, C.; Jo, Y.H.; Li, X.; Schwartz, G.J. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J. Neurosci. 2009, 29, 8302–8311. [Google Scholar] [CrossRef]
- Purpera, M.N.; Shen, L.; Taghavi, M.; Munzberg, H.; Martin, R.J.; Hutson, S.M.; Morrison, C.D. Impaired branched chain amino acid metabolism alters feeding behavior and increases orexigenic neuropeptide expression in the hypothalamus. J. Endocrinol. 2012, 212, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Leidy, H.J.; Todd, C.B.; Zino, A.Z.; Immel, J.E.; Mukherjea, R.; Shafer, R.S.; Ortinau, L.C.; Braun, M. Consuming High-Protein Soy Snacks Affects Appetite Control, Satiety, and Diet Quality in Young People and Influences Select Aspects of Mood and Cognition. J. Nutr. 2015, 145, 1614–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, G.; Noakes, M.; Keogh, J.; Foster, P.; Clifton, P. High protein high fibre snack bars reduce food intake and improve short term glucose and insulin profiles compared with high fat snack bars. Asia Pac. J. Clin. Nutr. 2006, 15, 443–450. [Google Scholar] [PubMed]
- Douglas, S.M.; Ortinau, L.C.; Hoertel, H.A.; Leidy, H.J. Low, moderate, or high protein yogurt snacks on appetite control and subsequent eating in healthy women. Appetite 2013, 60, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Leidy, H.J.; Tang, M.; Armstrong, C.L.; Martin, C.B.; Campbell, W.W. The effects of consuming frequent, higher protein meals on appetite and satiety during weight loss in overweight/obese men. Obesity 2011, 19, 818–824. [Google Scholar] [CrossRef] [Green Version]
- Haghighat, N.; Ashtary-Larky, D.; Bagheri, R.; Wong, A.; Cheraghloo, N.; Moradpour, G.; Nordvall, M.; Asbaghi, O.; Moeinvaziri, N.; Amini, M.; et al. Effects of 6 Months of Soy-Enriched High Protein Compared to Eucaloric Low Protein Snack Replacement on Appetite, Dietary Intake, and Body Composition in Normal-Weight Obese Women: A Randomized Controlled Trial. Nutrients 2021, 13, 2266. [Google Scholar] [CrossRef]
- Ropelle, E.R.; Pauli, J.R.; Fernandes, M.F.; Rocco, S.A.; Marin, R.M.; Morari, J.; Souza, K.K.; Dias, M.M.; Gomes-Marcondes, M.C.; Gontijo, J.A.R.; et al. A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in high-protein diet-induced weight loss. Diabetes 2008, 57, 594–605. [Google Scholar] [CrossRef] [Green Version]
- Mace, O.J.; Schindler, M.; Patel, S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J. Physiol. 2012, 590, 2917–2936. [Google Scholar] [CrossRef]
- Alamshah, A.; McGavigan, A.K.; Spreckley, E.; Kinsey-Jones, J.S.; Amin, A.; Tough, I.R.; O’Hara, H.C.; Moolla, A.; Banks, K.; France, R.; et al. L-Arginine promotes gut hormone release and reduces food intake in rodents. Diabetes Obes. Metab. 2016, 18, 508–518. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Kang, C.; Xian, Y.; Zhang, M.; Chen, X.; Pei, M.; Zhu, W.; Hang, S. Sensing of L-Arginine by Gut-Expressed Calcium Sensing Receptor Stimulates Gut Satiety Hormones Cholecystokinin and Glucose-Dependent Insulinotropic Peptide Secretion in Pig Model. J. Food Sci. 2018, 83, 2394–2401. [Google Scholar] [CrossRef]
- Oya, M.; Kitaguchi, T.; Pais, R.; Reimann, F.; Gribble, F.; Tsuboi, T. The G protein-coupled receptor family C group 6 subtype A (GPRC6A) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells. J. Biol. Chem. 2013, 288, 4513–4521. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Murthy, K.S.; Grider, J.R. Expression of putative umami/L-amino acid receptors in mouse intestine and colon, and a murine enteroendocrine cell line. FASEB J. 2017, 3, 1090.2. [Google Scholar]
- Abete, I.; Parra, D.; Crujeiras, A.B.; Goyenechea, E.; Martinez, J.A. Specific insulin sensitivity and leptin responses to a nutritional treatment of obesity via a combination of energy restriction and fatty fish intake. J. Hum. Nutr. Diet 2008, 21, 591–600. [Google Scholar] [CrossRef]
- De Luis, D.A.; Izaola, O.; Primo, D.; Ovalle, H.F.; Lopez, J.J.; Gomez, E.; Ortola, A.; Aller, R. Biochemical, Anthropometric and Lifestyle Factors Related with Weight Maintenance after Weight Loss Secondary to a Hypocaloric Mediterranean Diet. Ann. Nutr. Metab. 2017, 71, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Pelissari Kravchychyn, A.C.; Munhoz da Silveira Campos, R.; Oliveira, E.; Silva, L.; Alaby Martins Ferreira, Y.; Campos Corgosinho, F.; Landi Masquio, D.C.; Emanuelle de Castro Ferreira Vicente, S.; Missae Oyama, L.; Tock, L.; et al. Adipocytokine and appetite-regulating hormone response to weight loss in adolescents with obesity: Impact of weight loss magnitude. Nutrition 2021, 87–88, 111188. [Google Scholar] [CrossRef] [PubMed]
- Farr, O.M.; Gavrieli, A.; Mantzoros, C.S. Leptin applications in 2015: What have we learned about leptin and obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Morioka, T.; Mori, K.; Motoyama, K.; Emoto, M. Ectopic fat accumulation and glucose homeostasis: Role of leptin in glucose and lipid metabolism and mass maintenance in skeletal muscle. In Musculoskeletal Disease Associated with Diabetes Mellitus; Springer: Japan, Tokyo, 2016; pp. 201–213. [Google Scholar]
- Banks, W.A.; William, A. Role of the blood-brain barrier in the evolution of feeding and cognition. Ann. N. Y. Acad. Sci. 2012, 1264, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Lien, L.F.; Haqq, A.M.; Arlotto, M.; Slentz, C.A.; Muehlbauer, M.J.; McMahon, R.L.; Rochon, J.; Gallup, D.; Bain, J.R.; Ilkayeva, O.; et al. The STEDMAN project: Biophysical, biochemical and metabolic effects of a behavioral weight loss intervention during weight loss, maintenance, and regain. OMICS 2009, 13, 21–35. [Google Scholar] [CrossRef]
- Hernández Morante, J.J.; Díaz Soler, I.; Muñoz, J.S.G.; Sánchez, H.P.; Barberá Ortega, M.D.C.; Martínez, C.M.; Morillas Ruiz, J.M. Moderate Weight Loss Modifies Leptin and Ghrelin Synthesis Rhythms but Not the Subjective Sensations of Appetite in Obesity Patients. Nutrients 2020, 12, 916. [Google Scholar] [CrossRef] [Green Version]
- Forsythe, L.K.; Wallace, J.M.; Livingstone, M.B. Obesity and inflammation: The effects of weight loss. Nutr. Res. Rev. 2008, 21, 117–133. [Google Scholar] [CrossRef]
- Bianchi, V.E. Weight loss is a critical factor to reduce inflammation. Clin. Nutr. ESPEN 2018, 28, 21–35. [Google Scholar] [CrossRef]
- De Mello, V.D.; Kolehmainen, M.; Schwab, U.; Mager, U.; Laaksonen, D.E.; Pulkkinen, L.; Niskanen, L.; Gylling, H.; Atalay, M.; Rauramaa, R.; et al. Effect of weight loss on cytokine messenger RNA expression in peripheral blood mononuclear cells of obese subjects with the metabolic syndrome. Metabolism 2008, 57, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Vink, R.; Roumans, N.; Fazelzadeh, P.; Tareen, S.H.K.; Boekschoten, M.V.; van Baak, M.A.; Mariman, E.C. Adipose tissue gene expression is differentially regulated with different rates of weight loss in overweight and obese humans. Int. J. Obes. 2017, 41, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, D.L.; Mamula, K.A.; Blackburn, H.L.; McDyer, F.A.; Jellema, G.L.; van Laar, R.; Costantino, N.S.; Engler, R.J.; Vernalis, M.N. Importance of substantial weight loss for altering gene expression during cardiovascular lifestyle modification. Obesity 2015, 23, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B.; Bruckbauer, A. Effects of a leucine and pyridoxine-containing nutraceutical on fat oxidation, and oxidative and inflammatory stress in overweight and obese subjects. Nutrients 2012, 4, 529–541. [Google Scholar] [CrossRef] [Green Version]
- Ohno, T.; Tanaka, Y.; Sugauchi, F.; Orito, E.; Hasegawa, I.; Nukaya, H.; Kato, A.; Matunaga, S.; Endo, M.; Tanaka, Y.; et al. Suppressive effect of oral administration of branched-chain amino acid granules on oxidative stress and inflammation in HCV-positive patients with liver cirrhosis. Hepatol. Res. 2008, 38, 683–688. [Google Scholar] [CrossRef]
- Korish, A.A. Multiple antioxidants and L-arginine modulate inflammation and dyslipidemia in chronic renal failure rats. Ren. Fail. 2010, 32, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Quirino, I.E.; Carneiro, M.B.; Cardoso, V.N.; das Graças Carvalho Dos Santos, R.; Vieira, L.Q.; Fiuza, J.A.; Alvarez-Leite, J.I.; de Vasconcelos Generoso, S.; Correia, M.I. Arginine Supplementation Induces Arginase Activity and Inhibits TNF-α Synthesis in Mice Spleen Macrophages after Intestinal Obstruction. JPEN J. Parenter. Enteral Nutr. 2016, 40, 417–422. [Google Scholar] [CrossRef]
- Wells, B.J.; Mainous, A.G.; Everett, C.J. Association between dietary arginine and C-reactive protein. Nutrition 2005, 21, 125–130. [Google Scholar] [CrossRef]
- Hu, S.; Han, M.; Rezaei, A.; Li, D.; Wu, G.; Ma, X. L-Arginine Modulates Glucose and Lipid Metabolism in Obesity and Diabetes. Curr. Protein Pept. Sci. 2017, 18, 599–608. [Google Scholar] [CrossRef]
- Han, T.S.; Richmond, P.; Avenell, A.; Lean, M.E. Waist circumference reduction and cardiovascular benefits during weight loss in women. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1997, 21, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Konieczna, J.; Romaguera, D.; Pereira, V.; Fiol, M.; Razquin, C.; Estruch, R.; Asensio, E.M.; Babio, N.; Fitó, M.; Gómez-Gracia, E.; et al. Longitudinal association of changes in diet with changes in body weight and waist circumference in subjects at high cardiovascular risk: The PREDIMED trial. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 139. [Google Scholar] [CrossRef]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef] [Green Version]
- Piers, L.S.; Walker, K.Z.; Stoney, R.M.; Soares, M.J.; O’Dea, K. Substitution of saturated with monounsaturated fat in a 4-week diet affects body weight and composition of overweight and obese men. Br. J. Nutr. 2003, 90, 717–727. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, H.; Ito, H.; Egami, Y.; Kaji, Y.; Maruyama, T.; Koike, G.; Jingu, S.; Harada, M. Waist circumference is the main determinant of elevated C-reactive protein in metabolic syndrome. Diabetes Res. Clin. Pract. 2008, 79, 330–336. [Google Scholar] [CrossRef]
- Huffman, F.G.; Whisner, S.; Zarini, G.G.; Nath, S. Waist circumference and BMI in relation to serum high sensitivity C-reactive protein (hs-CRP) in Cuban Americans with and without type 2 diabetes. Int. J. Environ. Res. Public Health 2010, 7, 842–852. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.C.; Lopes, C.; Guimarães, J.T.; Barros, H. Central obesity as a major determinant of increased high-sensitivity C-reactive protein in metabolic syndrome. Int. J. Obes. 2005, 29, 1452–1456. [Google Scholar] [CrossRef] [Green Version]
- Morales, F.E.; Tinsley, G.M.; Gordon, P.M. Acute and Long-Term Impact of High-Protein Diets on Endocrine and Metabolic Function, Body Composition, and Exercise-Induced Adaptations. J. Am. Coll. Nutr. 2017, 36, 295–305. [Google Scholar] [CrossRef]
- Brehm, B.J.; D’Alessio, D.A. Benefits of high-protein weight loss diets: Enough evidence for practice? Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 416–421. [Google Scholar] [CrossRef]
- Moon, J.; Koh, G. Clinical Evidence and Mechanisms of High-Protein Diet-Induced Weight Loss. J. Obes. Metab. Syndr. 2020, 29, 166–173. [Google Scholar] [CrossRef]
| Biscuit | Energy Content (Kcal) | Carbohydrates (g) | Total Dietary Fiber (g) | Protein (g) | BCAAs (g) | Arg (g) | Fat (g) | Saturated Fatty Acids (g) | Monounsaturated Fatty Acids (g) | Polyunsaturated Fatty Acids (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| per 100 g | ||||||||||
| CB | 445 | 71.0 | 1.5 | 7.3 | 0.98 | 0.24 | 14.0 | 6.70 | 5.19 | 2.11 |
| PB | 451 | 56.8 | 5.6 | 14.5 | 2.05 | 1.05 | 17.2 | 7.70 | 6.70 | 2.80 |
| Control Biscuit | Enriched Biscuit | |||||
|---|---|---|---|---|---|---|
| Characteristic | Baseline | Endpoint | p-Value * | Baseline | Endpoint | p-Value * |
| Sex (male/female) | 14/21 | 14/21 | 12/23 | 12/23 | ||
| Age (years) | 46.0 ± 9.1 | 46.0 ± 9.1 | 42.9 ± 14.7 | 42.9 ± 14.7 | ||
| Weight (kg) | 85.1 ± 13.3 | 79.9 ± 12.5 | <0.001 | 85.3 ± 16.7 | 78.8 ± 15.4 | <0.001 |
| BMI (kg/m2) | 30.9 ± 3.7 | 29.0 ± 3.7 | <0.001 | 30.6 ± 4.2 | 28.2 ± 3.7 | <0.001 |
| WC (cm) | 99.6 ± 13.6 | 93.7 ± 13.0 | <0.001 | 97.0 ± 13.2 | 90.2 ± 12.4 | <0.001 |
| Body fat (%) | 35.1 ± 8.1 | 32.5 ± 9.4 | <0.001 | 36.1 ± 6.4 | 32.7 ± 6.5 | <0.001 |
| Body fat mass (kg) | 29.9 ± 8.5 | 26.0 ± 9.2 | <0.001 | 30.9 ± 8.1 | 25.8 ± 7.3 | <0.001 |
| Lean mass (kg) | 55.3 ± 11.4 | 53.8 ± 11.2 | <0.001 | 54.8 ± 12.8 | 53.3 ± 12.2 | <0.001 |
| Physical activity (min/week) | 137.3 ± 104.7 | 125.1 ± 105.3 | 0.156 | 145.6 ± 88.0 | 146.9 ± 84.9 | 0.895 |
| SBP (mmHg) | 120.3 ± 12.2 | 113.3 ± 11.5 | 0.001 | 118.8 ± 18.1 | 108.8 ± 22.0 | 0.027 |
| DBP (mmHg) | 81.0 ± 9.9 | 77.8 ± 9.0 | 0.029 | 79.9 ± 10.6 | 74.7 ± 8.2 | <0.001 |
| Glucose (mg/dL) | 90.4 ± 12.6 | 90.3 ± 6.3 | 0.989 | 90.6 ± 11.9 | 90.1 ± 10.9 | 0.677 |
| Cholesterol (mg/dL) | 185.0 ± 35.6 | 189.6 ± 38.5 | 0.364 | 179.8 ± 32.9 | 181.1 ± 32.4 | 0.732 |
| HDL-C (mg/dL) | 59.7 ± 9.6 | 59.5 ± 9.2 | 0.174 | 59.8 ± 10.9 | 60.0 ± 10.4 | 0.386 |
| LDL-C (mg/dL) | 104.6 ± 34.7 | 108.9 ± 38.2 | 0.354 | 102.3 ± 30.5 | 103.6 ± 28.9 | 0.707 |
| Triacylglycerols (mg/dL) | 103.6 ± 63.6 | 99.3 ± 41.3 | 0.595 | 87.4 ± 43.2 | 89.1 ± 44.9 | 0.746 |
| AST (U/L) | 17.0 ± 5.6 | 16.9 ± 4.2 | 0.888 | 19.7 ± 11.1 | 15.8 ± 4.8 | 0.018 |
| ALT (U/L) | 16.7 ± 8.9 | 17.7 ± 5.5 | 0.417 | 18.9 ± 14.1 | 15.0 ± 6.2 | 0.044 |
| γ-GT (U/L) | 23.8 ± 13.8 | 24.5 ± 12.8 | 0.611 | 21.7 ± 9.9 | 20.1 ± 9.5 | 0.135 |
| Urea (mg/dL) | 30.2 ± 7.5 | 29.1 ± 5.9 | 0.382 | 29.7 ± 5.0 | 30.5 ± 5.8 | 0.444 |
| Creatinine (mg/dL) | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.697 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.400 |
| Uric acid (mg/dL) | 4.3 ± 1.1 | 4.5 ± 1.1 | 0.378 | 4.5 ± 1.5 | 4.5 ± 1.4 | 0.800 |
| Total proteins (mg/dL) | 7.0 ± 0.3 | 7.0 ± 0.2 | 0.821 | 6.9 ± 0.4 | 6.9 ± 0.3 | 0.903 |
| Characteristic | Control Biscuit | Enriched Biscuit | p-Value * |
|---|---|---|---|
| Weight loss (%) | 6.2 ± 2.7 | 7.6 ± 2.7 | 0.025 |
| BMI decrease (kg/m2) | 1.9 ± 0.9 | 2.4 ± 0.9 | 0.038 |
| WC decrease (cm) | 5.9 ± 3.4 | 6.8 ± 3.1 | 0.262 |
| Body fat percentage decrease (%) | 2.6 ± 2.3 | 3.3 ± 2.1 | 0.181 |
| Body fat mass decrease (kg) | 3.9 ± 2.4 | 4.9 ± 2.2 | 0.059 |
| Baseline | Endpoint | |||||
|---|---|---|---|---|---|---|
| Characteristic | Control Biscuit | Enriched Biscuit | p-Value * | Control Biscuit | Enriched Biscuit | p-Value * |
| Calorie intake (kcal) | 2529.2 ± 471.1 | 2463.3 ± 441.1 | 0.554 | 1917.3 ± 262.3 | 1736.2 ± 291.6 | 0.009 |
| Protein intake (g) | 91.6 ± 22.5 | 87.2 ± 19.2 | 0.390 | 80.1 ± 14.7 | 76.8 ± 14.0 | 0.347 |
| Carbohydrate intake (g) | 239.8 ± 56.8 | 226.4 ± 57.6 | 0.336 | 210.8 ± 41.8 | 186.4 ± 41.2 | 0.018 |
| Fat intake (g) | 134.0 ± 22.9 | 134.4 ± 22.9 | 0.940 | 82.5 ± 10.1 | 77.9 ± 11.0 | 0.078 |
| Physical activity (min/week) | 137.3 ± 104.7 | 145.6 ± 88.0 | 0.721 | 125.1 ± 105.3 | 146.9 ± 84.9 | 0.345 |
| Control Biscuit | Enriched Biscuit | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Baseline | Endpoint | p-Value * | Baseline | Endpoint | p-Value * | p-Value * (Endpoint between Groups) |
| Adiponectin (mg/L) | 10.8 ± 0.8 | 9.9 ± 0.8 | 0.013 | 10.8 ± 1.0 | 10.7 ± 1.3 | 0.938 | 0.598 |
| Leptin (ng/mL) | 43.9 ± 5.0 | 31.1 ± 27.6 | <0.001 | 45.9 ± 4.8 | 26.0 ± 2.7 | <0.001 | 0.347 |
| hs-CRP (mg/L) | 2.2 ± 0.5 | 2.5 ± 0.6 | 0.366 | 2.3 ± 0.5 | 2.0 ± 0.3 | 0.332 | 0.405 |
| IL-6 (pg/mL) | 2.4 ± 0.2 | 2.8 ± 0.4 | 0.166 | 2.4 ± 0.2 | 2.2 ± 0.1 | 0.443 | 0.139 |
| IL-1β (pg/mL) | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.276 | 0.7 ± 0.2 | 0.3 ± 0.1 | 0.081 | 0.470 |
| TNF-a (pg/mL) | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.953 | 0.5 ± 0.0 | 0.3 ± 0.0 | <0.001 | 0.394 |
| Adiponectin decrease (mg/L) | −0.9 ± 0.4 | −0.1 ± 0.6 | 0.233 | ||||
| Leptin decrease (ng/mL) | −12.8 ± 2.2 | −19.9 ± 3.1 | 0.066 | ||||
| hs-CRP decrease (mg/L) | +0.4 ± 0.4 | −0.3 ± 0.3 | 0.189 | ||||
| IL-6 decrease (pg/mL) | +0.4 ± 0.3 | −0.2 ± 0.2 | 0.107 | ||||
| IL-1β decrease (pg/mL) | −0.1 ± 0.1 | −0.4 ± 0.2 | 0.170 | ||||
| TNF-a decrease (pg/mL) | 0.0 ± 0.0 | −0.2 ± 0.1 | <0.001 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binou, P.; Yanni, A.E.; Kartsioti, K.; Barmpagianni, A.; Konstantopoulos, P.; Karathanos, V.T.; Kokkinos, A. Wheat Biscuits Enriched with Plant-Based Protein Contribute to Weight Loss and Beneficial Metabolic Effects in Subjects with Overweight/Obesity. Nutrients 2022, 14, 2516. https://doi.org/10.3390/nu14122516
Binou P, Yanni AE, Kartsioti K, Barmpagianni A, Konstantopoulos P, Karathanos VT, Kokkinos A. Wheat Biscuits Enriched with Plant-Based Protein Contribute to Weight Loss and Beneficial Metabolic Effects in Subjects with Overweight/Obesity. Nutrients. 2022; 14(12):2516. https://doi.org/10.3390/nu14122516
Chicago/Turabian StyleBinou, Panagiota, Amalia E. Yanni, Klio Kartsioti, Aikaterini Barmpagianni, Panagiotis Konstantopoulos, Vaios T. Karathanos, and Alexander Kokkinos. 2022. "Wheat Biscuits Enriched with Plant-Based Protein Contribute to Weight Loss and Beneficial Metabolic Effects in Subjects with Overweight/Obesity" Nutrients 14, no. 12: 2516. https://doi.org/10.3390/nu14122516
APA StyleBinou, P., Yanni, A. E., Kartsioti, K., Barmpagianni, A., Konstantopoulos, P., Karathanos, V. T., & Kokkinos, A. (2022). Wheat Biscuits Enriched with Plant-Based Protein Contribute to Weight Loss and Beneficial Metabolic Effects in Subjects with Overweight/Obesity. Nutrients, 14(12), 2516. https://doi.org/10.3390/nu14122516








