Hypercalcemia in Pregnancy Due to CYP24A1 Mutations: Case Report and Review of the Literature
Abstract
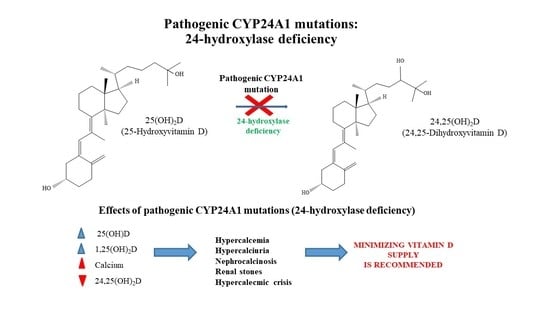
:1. Introduction
2. Case Report
3. Literature Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bollerslev, J.; Rejnmark, L.; Zahn, A.; Heck, A.; Appelman-Dijkstra, N.M.; Cardoso, L.; Hannan, F.M.; Cetani, F.; Sikjaer, T.; Formenti, A.M.; et al. European Expert Consensus on Practical Management of Specific Aspects of Parathyroid Disorders in Adults and in Pregnancy: Recommendations of the ESE Educational Program of Parathyroid Disorders. Eur. J. Endocrinol. 2021, 186, R33–R63. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S. Maternal Mineral and Bone Metabolism during Pregnancy, Lactation, and Post-Weaning Recovery. Physiol. Rev. 2016, 96, 449–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dandurand, K.; Ali, D.S.; Khan, A.A. Hypercalcemia in Pregnancy. Endocrinol. Metab. Clin. N. Am. 2021, 50, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Cappellani, D.; Brancatella, A.; Morganti, R.; Borsari, S.; Baldinotti, F.; Caligo, M.A.; Kaufmann, M.; Jones, G.; Marcocci, C.; Cetani, F. Hypercalcemia due to CYP24A1 mutations: A systematic descriptive review. Eur. J. Endocrinol. 2021, 186, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Molin, A.; Lemoine, S.; Kaufmann, M.; Breton, P.; Nowoczyn, M.; Ballandonne, C.; Coudray, N.; Mittre, H.; Richard, N.; Ryckwaert, A.; et al. Overlapping Phenotypes Associated with CYP24A1, SLC34A1, and SLC34A3 Mutations: A Cohort Study of Patients with Hypersensitivity to Vitamin D. Front. Endocrinol. 2021, 12, 736240. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Anastasilakis, A.D. Parathyroid Disease in Pregnancy and Lactation: A Narrative Review of the Literature. Biomedicines 2021, 9, 475. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Kaufmann, M.; Weber, S.; Irwin, A.; Goos, C.; John, U.; Misselwitz, J.; Klaus, G.; Kuwertz-Broking, E.; Fehrenbach, H.; et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N. Engl. J. Med. 2011, 365, 410–421. [Google Scholar] [CrossRef]
- Carpenter, T.O. CYP24A1 loss of function: Clinical phenotype of monoallelic and biallelic mutations. J. Steroid Biochem. Mol. Biol. 2017, 173, 337–340. [Google Scholar] [CrossRef]
- De Paolis, E.; Scaglione, G.L.; De Bonis, M.; Minucci, A.; Capoluongo, E. CYP24A1 and SLC34A1 genetic defects associated with idiopathic infantile hypercalcemia: From genotype to phenotype. Clin. Chem. Lab. Med. 2019, 57, 1650–1667. [Google Scholar] [CrossRef]
- Pronicka, E.; Ciara, E.; Halat, P.; Janiec, A.; Wojcik, M.; Rowinska, E.; Rokicki, D.; Pludowski, P.; Wojciechowska, E.; Wierzbicka, A.; et al. Biallelic mutations in CYP24A1 or SLC34A1 as a cause of infantile idiopathic hypercalcemia (IIH) with vitamin D hypersensitivity: Molecular study of 11 historical IIH cases. J. Appl. Genet. 2017, 58, 349–353. [Google Scholar] [CrossRef] [Green Version]
- Zelzer, S.; Meinitzer, A.; Enko, D.; Simstich, S.; Le Goff, C.; Cavalier, E.; Herrmann, M.; Goessler, W. Simultaneous determination of 24,25- and 25,26-dihydroxyvitamin D3 in serum samples with liquid-chromatography mass spectrometry—A useful tool for the assessment of vitamin D metabolism. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1158, 122394. [Google Scholar] [CrossRef] [PubMed]
- Zelzer, S.; Pruller, F.; Curcic, P.; Sloup, Z.; Holter, M.; Herrmann, M.; Mangge, H. Vitamin D Metabolites and Clinical Outcome in Hospitalized COVID-19 Patients. Nutrients 2021, 13, 2129. [Google Scholar] [CrossRef] [PubMed]
- Trummer, C.; Schwetz, V.; Pandis, M.; Grubler, M.R.; Verheyen, N.; Gaksch, M.; Zittermann, A.; Marz, W.; Aberer, F.; Lang, A.; et al. Effects of Vitamin D Supplementation on IGF-1 and Calcitriol: A Randomized-Controlled Trial. Nutrients 2017, 9, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilz, S.; Gaksch, M.; Kienreich, K.; Grubler, M.; Verheyen, N.; Fahrleitner-Pammer, A.; Treiber, G.; Drechsler, C.; ó Hartaigh, B.; Obermayer-Pietsch, B.; et al. Effects of vitamin D on blood pressure and cardiovascular risk factors: A randomized controlled trial. Hypertension 2015, 65, 1195–1201. [Google Scholar] [CrossRef]
- McBride, L.; Crosthwaite, A.; Houlihan, C.; Stark, Z.; Rodda, C. Rare cause of maternal and neonatal hypercalcaemia. J. Paediatr. Child Health 2019, 55, 232–235. [Google Scholar] [CrossRef]
- McBride, L.; Houlihan, C.; Quinlan, C.; Messazos, B.; Stark, Z.; Crosthwaite, A. Outcomes Following Treatment of Maternal Hypercalcemia Due to CYP24A1 Pathogenic Variants. Kidney Int. Rep. 2019, 4, 888–892. [Google Scholar] [CrossRef] [Green Version]
- Hedberg, F.; Pilo, C.; Wikner, J.; Torring, O.; Calissendorff, J. Three Sisters with Heterozygous Gene Variants of CYP24A1: Maternal Hypercalcemia, New-Onset Hypertension, and Neonatal Hypoglycemia. J. Endocr. Soc. 2019, 3, 387–396. [Google Scholar] [CrossRef]
- Arnold, N.; O’Toole, V.; Huynh, T.; Smith, H.C.; Luxford, C.; Clifton-Bligh, R.; Eastman, C.J. Intractable hypercalcaemia during pregnancy and the postpartum secondary to pathogenic variants in CYP24A1. Endocrinol. Diabetes Metab. Case Rep. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Griffin, T.P.; Joyce, C.M.; Alkanderi, S.; Blake, L.M.; O’Keeffe, D.T.; Bogdanet, D.; Islam, M.N.; Dennedy, M.C.; Gillan, J.E.; Morrison, J.J.; et al. Biallelic CYP24A1 variants presenting during pregnancy: Clinical and biochemical phenotypes. Endocr. Connect. 2020, 9, 530–541. [Google Scholar] [CrossRef]
- Woods, G.N.; Saitman, A.; Gao, H.; Clarke, N.J.; Fitzgerald, R.L.; Chi, N.W. A Young Woman with Recurrent Gestational Hypercalcemia and Acute Pancreatitis Caused by CYP24A1 Deficiency. J. Bone Miner. Res. 2016, 31, 1841–1844. [Google Scholar] [CrossRef] [Green Version]
- Romasovs, A.; Jaunozola, L.; Berga-Svitina, E.; Daneberga, Z.; Miklasevics, E.; Pirags, V. Hypercalcemia and CYP24A1 Gene Mutation Diagnosed in the 2nd Trimester of a Twin Pregnancy: A Case Report. Am. J. Case Rep. 2021, 22, e931116. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.D.; Hsiao, E.C.; O’Donnell, B.; Salmeen, K.; Nussbaum, R.; Krebs, M.; Baumgartner-Parzer, S.; Kaufmann, M.; Jones, G.; Bikle, D.D.; et al. Maternal Hypercalcemia Due to Failure of 1,25-Dihydroxyvitamin-D3 Catabolism in a Patient with CYP24A1 Mutations. J. Clin. Endocrinol. Metab. 2015, 100, 2832–2836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinour, D.; Davidovits, M.; Aviner, S.; Ganon, L.; Michael, L.; Modan-Moses, D.; Vered, I.; Bibi, H.; Frishberg, Y.; Holtzman, E.J. Maternal and infantile hypercalcemia caused by vitamin-D-hydroxylase mutations and vitamin D intake. Pediatr. Nephrol. 2015, 30, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, C.; Upton, T.; Hunt, P.; Phillips, I.; Kaufmann, M.; Florkowski, C.; Soule, S.; Jones, G. Vitamin D supplementation in pregnancy: A word of caution. Familial hypercalcaemia due to disordered vitamin D metabolism. Ann. Clin. Biochem. 2020, 57, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Kwong, W.T.; Fehmi, S.M. Hypercalcemic Pancreatitis Triggered by Pregnancy with a CYP24A1 Mutation. Pancreas 2016, 45, e31–e32. [Google Scholar] [CrossRef]
- Colussi, G.; Ganon, L.; Penco, S.; De Ferrari, M.E.; Ravera, F.; Querques, M.; Primignani, P.; Holtzman, E.J.; Dinour, D. Chronic hypercalcaemia from inactivating mutations of vitamin D 24-hydroxylase (CYP24A1): Implications for mineral metabolism changes in chronic renal failure. Nephrol. Dial. Transplant. 2014, 29, 636–643. [Google Scholar] [CrossRef] [Green Version]
- Stathopoulos, I.P.; Liakou, C.G.; Katsalira, A.; Trovas, G.; Lyritis, G.G.; Papaioannou, N.A.; Tournis, S. The use of bisphosphonates in women prior to or during pregnancy and lactation. Hormones 2011, 10, 280–291. [Google Scholar] [CrossRef]
- Sokal, A.; Elefant, E.; Leturcq, T.; Beghin, D.; Mariette, X.; Seror, R. Pregnancy and newborn outcomes after exposure to bisphosphonates: A case-control study. Osteoporos. Int. 2019, 30, 221–229. [Google Scholar] [CrossRef]
- Machairiotis, N.; Ntali, G.; Kouroutou, P.; Michala, L. Clinical evidence of the effect of bisphosphonates on pregnancy and the infant. Horm. Mol. Biol. Clin. Investig. 2019, 40, 20190021. [Google Scholar] [CrossRef]
- Green, S.B.; Pappas, A.L. Effects of maternal bisphosphonate use on fetal and neonatal outcomes. Am. J. Health Syst. Pharm. 2014, 71, 2029–2036. [Google Scholar] [CrossRef]
- Pilz, S.; Zittermann, A.; Obeid, R.; Hahn, A.; Pludowski, P.; Trummer, C.; Lerchbaum, E.; Perez-Lopez, F.R.; Karras, S.N.; Marz, W. The Role of Vitamin D in Fertility and during Pregnancy and Lactation: A Review of Clinical Data. Int. J. Environ. Res. Public Health 2018, 15, 2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appelman-Dijkstra, N.M.; Ertl, D.A.; Zillikens, M.C.; Rjenmark, L.; Winter, E.M. Hypercalcemia during pregnancy: Management and outcomes for mother and child. Endocrine 2021, 71, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Janiec, A.; Halat-Wolska, P.; Obrycki, L.; Ciara, E.; Wojcik, M.; Pludowski, P.; Wierzbicka, A.; Kowalska, E.; Ksiazyk, J.B.; Kulaga, Z.; et al. Long-term outcome of the survivors of infantile hypercalcaemia with CYP24A1 and SLC34A1 mutations. Nephrol. Dial. Transplant. 2021, 36, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Sayers, J.; Hynes, A.M.; Srivastava, S.; Dowen, F.; Quinton, R.; Datta, H.K.; Sayer, J.A. Successful treatment of hypercalcaemia associated with a CYP24A1 mutation with fluconazole. Clin. Kidney J. 2015, 8, 453–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkes, C.P.; Li, D.; Hakonarson, H.; Meyers, K.E.; Thummel, K.E.; Levine, M.A. CYP3A4 Induction by Rifampin: An Alternative Pathway for Vitamin D Inactivation in Patients with CYP24A1 Mutations. J. Clin. Endocrinol. Metab. 2017, 102, 1440–1446. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Wu, L.; Zhang, L.; Zhang, L. Fetal outcomes after maternal exposure to oral antifungal agents during pregnancy: A systematic review and meta-analysis. Int. J. Gynaecol. Obstet. 2020, 148, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Orazulike, N.; Sharma, J.B.; Sharma, S.; Umeora, O.U.J. Tuberculosis (TB) in pregnancy—A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 259, 167–177. [Google Scholar] [CrossRef]
- Maretzke, F.; Bechthold, A.; Egert, S.; Ernst, J.B.; Melo van Lent, D.; Pilz, S.; Reichrath, J.; Stangl, G.I.; Stehle, P.; Volkert, D.; et al. Role of Vitamin D in Preventing and Treating Selected Extraskeletal Diseases-An Umbrella Review. Nutrients 2020, 12, 969. [Google Scholar] [CrossRef] [Green Version]
- Pludowski, P.; Takacs, I.; Boyanov, M.; Belaya, Z.; Diaconu, C.C.; Mokhort, T.; Zherdova, N.; Rasa, I.; Payer, J.; Pilz, S. Clinical Practice in the Prevention, Diagnosis and Treatment of Vitamin D Deficiency: A Central and Eastern European Expert Consensus Statement. Nutrients 2022, 14, 1483. [Google Scholar] [CrossRef]
- Pilz, S.; Trummer, C.; Theiler-Schwetz, V.; Grubler, M.R.; Verheyen, N.D.; Odler, B.; Karras, S.N.; Zittermann, A.; Marz, W. Critical Appraisal of Large Vitamin D Randomized Controlled Trials. Nutrients 2022, 14, 303. [Google Scholar] [CrossRef]
- Buttriss, J.L.; Lanham-New, S.A.; Steenson, S.; Levy, L.; Swan, G.E.; Darling, A.L.; Cashman, K.D.; Allen, R.E.; Durrant, L.R.; Smith, C.P.; et al. Implementation strategies for improving vitamin D status and increasing vitamin D intake in the UK: Current controversies and future perspectives: Proceedings of the 2nd Rank Prize Funds Forum on vitamin D. Br. J. Nutr. 2021, 127, 1567–1587. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, F.R.; Pilz, S.; Chedraui, P. Vitamin D supplementation during pregnancy: An overview. Curr. Opin. Obstet. Gynecol. 2020, 32, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Molin, A.; Baudoin, R.; Kaufmann, M.; Souberbielle, J.C.; Ryckewaert, A.; Vantyghem, M.C.; Eckart, P.; Bacchetta, J.; Deschenes, G.; Kesler-Roussey, G.; et al. CYP24A1 Mutations in a Cohort of Hypercalcemic Patients: Evidence for a Recessive Trait. J. Clin. Endocrinol. Metab. 2015, 100, E1343–E1352. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, M.; Gallagher, J.C.; Peacock, M.; Schlingmann, K.P.; Konrad, M.; DeLuca, H.F.; Sigueiro, R.; Lopez, B.; Mourino, A.; Maestro, M.; et al. Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J. Clin. Endocrinol. Metab. 2014, 99, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Fabregat-Cabello, N.; Farre-Segura, J.; Huyghebaert, L.; Peeters, S.; Le Goff, C.; Souberbielle, J.C.; Cavalier, E. A fast and simple method for simultaneous measurements of 25(OH)D, 24,25(OH)2D and the Vitamin D Metabolite Ratio (VMR) in serum samples by LC-MS/MS. Clin. Chim. Acta 2017, 473, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Francic, V.; Ursem, S.R.; Dirks, N.F.; Keppel, M.H.; Theiler-Schwetz, V.; Trummer, C.; Pandis, M.; Borzan, V.; Grubler, M.R.; Verheyen, N.D.; et al. The Effect of Vitamin D Supplementation on its Metabolism and the Vitamin D Metabolite Ratio. Nutrients 2019, 11, 2539. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.C.Y.; Nicholls, H.; Piec, I.; Washbourne, C.J.; Dutton, J.J.; Jackson, S.; Greeves, J.; Fraser, W.D. Reference intervals for serum 24,25-dihydroxyvitamin D and the ratio with 25-hydroxyvitamin D established using a newly developed LC-MS/MS method. J. Nutr. Biochem. 2017, 46, 21–29. [Google Scholar] [CrossRef] [Green Version]
| Parameter (Unit) | Reference Range | 1st Visit | 2nd Visit | 3rd Visit | 4th Visit | 5th Visit |
|---|---|---|---|---|---|---|
| Gestational week | 24 | 27 | 31 | 33 | Two months after giving birth | |
| Albumin adjusted serum calcium (mmol/L) | 2.20 to 2.65 | 3.08 | 3.00 | 3.07 | 2.95 | 2.35 |
| Ionized serum calcium (mmol/L) | 1.15 to 1.35 | 1.57 | 1.51 | 1.57 | 1.50 | 1.29 |
| Total serum calcium (mmol/L) | 2.20 to 2.65 | 2.97 | 2.93 | 2.99 | 2.84 | 2.58 |
| Serum phosphate (mmol/L) | 0.84 to 1.45 | 0.74 | 0.72 | 0.89 | 0.95 | 1.19 |
| Serum magnesium (mmol/L) | 0.70 to 1.10 | 0.55 | 0.55 | 0.55 | 0.66 | 0.74 |
| Serum creatinine (mg/dL) | up to 1.00 | 0.78 | 0.82 | 0.88 | 0.75 | 1.02 |
| eGFR (CKD-EPI) (ml/min/1.73 m2) | 90 to 120 | 106 | 100 | 92 | 111 | 76 |
| Spot urine calcium/creatinine ratio (mmol/mmol) | up to 0.60 | 1.02 | 0.82 | 0.25 | 0.54 | 0.29 |
| Parathyroid hormone (pg/mL) | 15.0 to 65.0 | 8.0 | 7.1 | 7.6 | 8.4 | |
| 25-hydroxyvitamin D (nmol/L) * | 75 to 150 | 87 | 75 | 75 | 45 | |
| 1.25-dihydroxyvitamin D (pmol/L) * | 52 to 267 | 279 | 325 | 295 | 73 | |
| Bone-specific alkaline phosphatase (µg/L) | 4.7 to 27.0 | 6.9 | 19.0 | |||
| Osteocalcin (ng/mL) | 1.0 to 35.0 | 19.3 | 24.3 | 29.6 | 42.6 | |
| Procollagen type 1 N-terminal propetide (ng/mL) | 15 to 49 | 50.0 | 81.3 | 87.9 | 94.4 | |
| C-terminal telopeptide of type 1 collagen (ng/mL) | 0.03 to 0.37 | 0.29 | 0.35 | 0.67 | 0.55 | |
| Fibroblast-growth-factor-23 (pg/mL) | 14.0 to 48.0 | 176.1 | 156.6 | 56.0 | ||
| Parathyroid hormone-related peptide (pmol/L) | 0.0 to 1.3 | 1.2 | 1.2 | 0.5 | ||
| 25-hydroxyvitamin D3 (nmol/L) ** | NA | 90.2 | 71.7 | |||
| 25-hydroxyvitamin D2 (nmol/L) ** | NA | 1.8 | 2.7 | |||
| 25-hydroxyvitamin D2 + D3 (nmol/L) ** | 75 to 150 | 92.0 | 74.4 | |||
| 24,25-dihydroxyvitamin D3 (nmol/L) ** | NA | 0.66 | 0.13 | |||
| 24,25-hydroxyvitamin D3 to 25-hydoxyvitamin D3 ratio (%) ** | >3 | 0.73 | 0.18 |
| Case Number of the Mother | Reference Number | Age (Years) | Number of Fetuses | Peak Serum Calcium in Pregnancy (mmol/L) * | Major Maternal Pregnancy Complications | Type of Delivery ** | Major Maternal Postpartum Complications | Live Birth | Breast-Feeding | Major Newborn Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | [15] | 27 | 1 | Not reported | Pre-eclampsia, polyhydramnios | Caesarian section | Acute kidney injury | Yes | Yes | Symptomatic hypercalcemia at 5 days |
| [16] | 32 | 1 | Ionized serum calcium > 1.5 | Hypertension, worsening renal function | Caesarian section | Worsening renal function | Yes | No | Symptomatic hypocalcemia at 3 months | |
| 2 | [17] | 23 | 1 | NA | None | NA | Pre-eclampsia, hypercalcemic crisis | Yes | NA | Convulsions, hypoglycemia, necrotizing enterocolitis |
| NA | 1 | 2.89 | Hypertension | Vaginal | Not reported | Yes | NA | Hypoglycemia, hypercalcemia | ||
| NA | 1 | 3.44 | Symptomatic hypercalcemia | Vaginal | None | Yes | NA | Hypercalcemia | ||
| NA | 1 | 2.88 | None | Vaginal | Hypertension, hypercalcemic crisis | Yes | NA | Hypoglycemia | ||
| NA | 1 | 2.92 | Hypertension | Vaginal | Hypercalcemic crisis | Yes | NA | None | ||
| 3 | [17] | 21 | 1 | 2.87 | None | Vaginal | Hypercalcemia | Yes | NA | Hypercalcemia |
| NA | 1 | 2.83 | Hypertension | NA | Hypercalcemia | Yes | NA | Hypercalcemia | ||
| 4 | [18] | 47 | 2 | 3.11 | Hypertension, diabetes | Caesarian section | Hypercalcemia | Yes for both | No | None |
| 5 | [18] | 36 | 2 | NA | NA | NA | Hypercalcemic crisis | Yes for both | NA | None |
| NA | 1 | NA | None | NA | None | Yes | NA | None | ||
| NA | 1 | NA | None | NA | None | Yes | NA | None | ||
| 6 | [19] | 32 | 1 | 3.27 | Pre-eclampsia | Caesarian section | Hypertension, acute kidney injury | Yes | NA | Mild hypercalcemia |
| 7 | [19] | 32 | NA | NA | Nephrolithiasis | NA | NA | NA | NA | NA |
| 8 | [20] | 20 | 2 | 3.07 | Hypertension | Vaginal | Hypercalcemic crisis, acute pancreatitis | No | No | Not alive (intrauterine demise at 26 weeks) |
| 20 | 1 | 2.87 | Acute pancreatitis | Vaginal | None | Yes | No | None | ||
| 9 | [21] | 33 | 2 | 3.4 | Hypertension | Caesarian section | NA | Yes for one | NA | Development disorder, anorectal malformation, asymptomatic hypercalcemia |
| 10 | [25] | 20 | 2 | 3.82 | Acute pancreatitis | NA | Acute pancreatitis | No | No | Not alive (intrauterine demise at 26 weeks) |
| 20 | 1 | 2.99 | Pre-eclampsia, acute pancreatitis | Vaginal | None | Yes | No | None | ||
| 11 | [22] | 24 | NA | 3.07 | NA | NA | NA | NA | NA | NA |
| 26 | NA | 3.07 | NA | NA | NA | NA | NA | NA | ||
| 27 | NA | 2.92 | NA | NA | NA | NA | NA | NA | ||
| 28 | 1 | 3.04 | Pre-eclampsia | NA | NA | Yes | Yes | Slight hypocalcemia | ||
| 12 | [23] | NA | 1 | 2.92 | Intrauterine growth retardation | NA | NA | Yes | NA | None |
| NA | 1 | NA | NA | Yes | NA | None | ||||
| 35 | 2 | 2.99 | Rupture of membranes | Vaginal and caesarian section | Hypercalcemic crisis | Yes | NA | None | ||
| 13 | [24] | Mid 20 | 1 | 3.3 | Idiopathic cholestasis | NA | NA | Yes | NA | None |
| End 20 | 1 | 2.6 | Idiopathic cholestasis | NA | NA | Yes | NA | None |
| Case Number of the Mother | Reference Number | Intravenous Hydration | Loop Diuretics | Glucocorticoids | Phosphate Supplements | Calcitonin |
|---|---|---|---|---|---|---|
| 1 | [15] | No * | No | No | No | No |
| [16] | Yes | No | Yes | Yes | Yes | |
| 2 | [17] | No | No | No | No | No |
| No | No | No | No | No | ||
| No | Yes | No | No | No | ||
| No | No | No | No | No | ||
| No | No | No | No | No | ||
| 3 | [17] | No | No | No | No | No |
| No | No | No | No | No | ||
| 4 | [18] | Yes | Yes | Yes | No | No |
| 5 | [18] | No | No | No | No | No |
| No | No | No | No | No | ||
| No | No | No | No | No | ||
| 6 | [19] | Yes | No | Yes | No | No |
| 7 | [19] | No | No | No | No | No |
| 8 | [20] | No | No | No | No | No |
| Yes | No | No | Yes ** | Yes | ||
| 9 | [21] | No | Yes | No | No | No |
| 10 | [25] | No | No | No | No | No |
| No | No | No | No | No | ||
| 11 | [22] | No | No | No | No | No |
| No | No | No | No | No | ||
| No | No | No | No | No | ||
| No | No | No | No | No | ||
| 12 | [23] | No | No | No | No | No |
| No | No | No | No | No | ||
| No | No | No | No | No | ||
| 13 | [24] | No | No | Yes | No | No |
| No | No | No | No | No |
| Case Number of the Mother | Reference Number | Intravenous Hydration | Loop Diuretics | Glucocorticoids | Potassium Supplements | Calcitonin | Denosumab | Bisphosphonates |
|---|---|---|---|---|---|---|---|---|
| 1 | [15] | No * | No | No | No | No | No | No |
| [16] | No | No | No | No | Yes | No | No | |
| 2 | [17] | Yes | Yes | No | No | Yes | No | Yes |
| No | No | No | No | No | No | No | ||
| No | Yes | No | No | No | No | Yes | ||
| Yes | No | No | No | No | No | No | ||
| Yes | No | No | No | No | No | No | ||
| 3 | [17] | Yes | No | No | No | No | No | No |
| Yes | No | No | No | No | No | No | ||
| 4 | [18] | Yes | Yes | Yes | No | No | Yes | No |
| 5 | [18] | Yes | No | Yes | No | No | Yes | Yes |
| No | No | No | No | No | No | No | ||
| No | No | No | No | No | No | No | ||
| 6 | [19] | Yes | No | Yes | No | No | No | No |
| 7 | [19] | No | No | No | No | No | No | No |
| 8 | [20] | Yes | No | Yes | No | Yes | No | No |
| No | No | No | No | Yes | No | No | ||
| 9 | [21] | No | No | No | No | No | No | No |
| 10 | [25] | Yes | No | No | No | Yes | No | No |
| No | No | No | No | No | No | No | ||
| 11 | [22] | No | No | No | No | No | No | No |
| No | No | No | No | No | No | No | ||
| No | No | No | No | No | No | No | ||
| No | No | No | No | No | No | No | ||
| 12 | [23] | No | No | No | No | No | No | No |
| No | No | No | No | No | No | No | ||
| Yes | No | Yes | No | No | No | Yes | ||
| 13 | [24] | No | No | No | No | No | No | No |
| No | No | No | No | No | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilz, S.; Theiler-Schwetz, V.; Pludowski, P.; Zelzer, S.; Meinitzer, A.; Karras, S.N.; Misiorowski, W.; Zittermann, A.; März, W.; Trummer, C. Hypercalcemia in Pregnancy Due to CYP24A1 Mutations: Case Report and Review of the Literature. Nutrients 2022, 14, 2518. https://doi.org/10.3390/nu14122518
Pilz S, Theiler-Schwetz V, Pludowski P, Zelzer S, Meinitzer A, Karras SN, Misiorowski W, Zittermann A, März W, Trummer C. Hypercalcemia in Pregnancy Due to CYP24A1 Mutations: Case Report and Review of the Literature. Nutrients. 2022; 14(12):2518. https://doi.org/10.3390/nu14122518
Chicago/Turabian StylePilz, Stefan, Verena Theiler-Schwetz, Pawel Pludowski, Sieglinde Zelzer, Andreas Meinitzer, Spyridon N. Karras, Waldemar Misiorowski, Armin Zittermann, Winfried März, and Christian Trummer. 2022. "Hypercalcemia in Pregnancy Due to CYP24A1 Mutations: Case Report and Review of the Literature" Nutrients 14, no. 12: 2518. https://doi.org/10.3390/nu14122518
APA StylePilz, S., Theiler-Schwetz, V., Pludowski, P., Zelzer, S., Meinitzer, A., Karras, S. N., Misiorowski, W., Zittermann, A., März, W., & Trummer, C. (2022). Hypercalcemia in Pregnancy Due to CYP24A1 Mutations: Case Report and Review of the Literature. Nutrients, 14(12), 2518. https://doi.org/10.3390/nu14122518