A Systematic Review of the Influence of Bovine Colostrum Supplementation on Leaky Gut Syndrome in Athletes: Diagnostic Biomarkers and Future Directions
Abstract
:1. Introduction
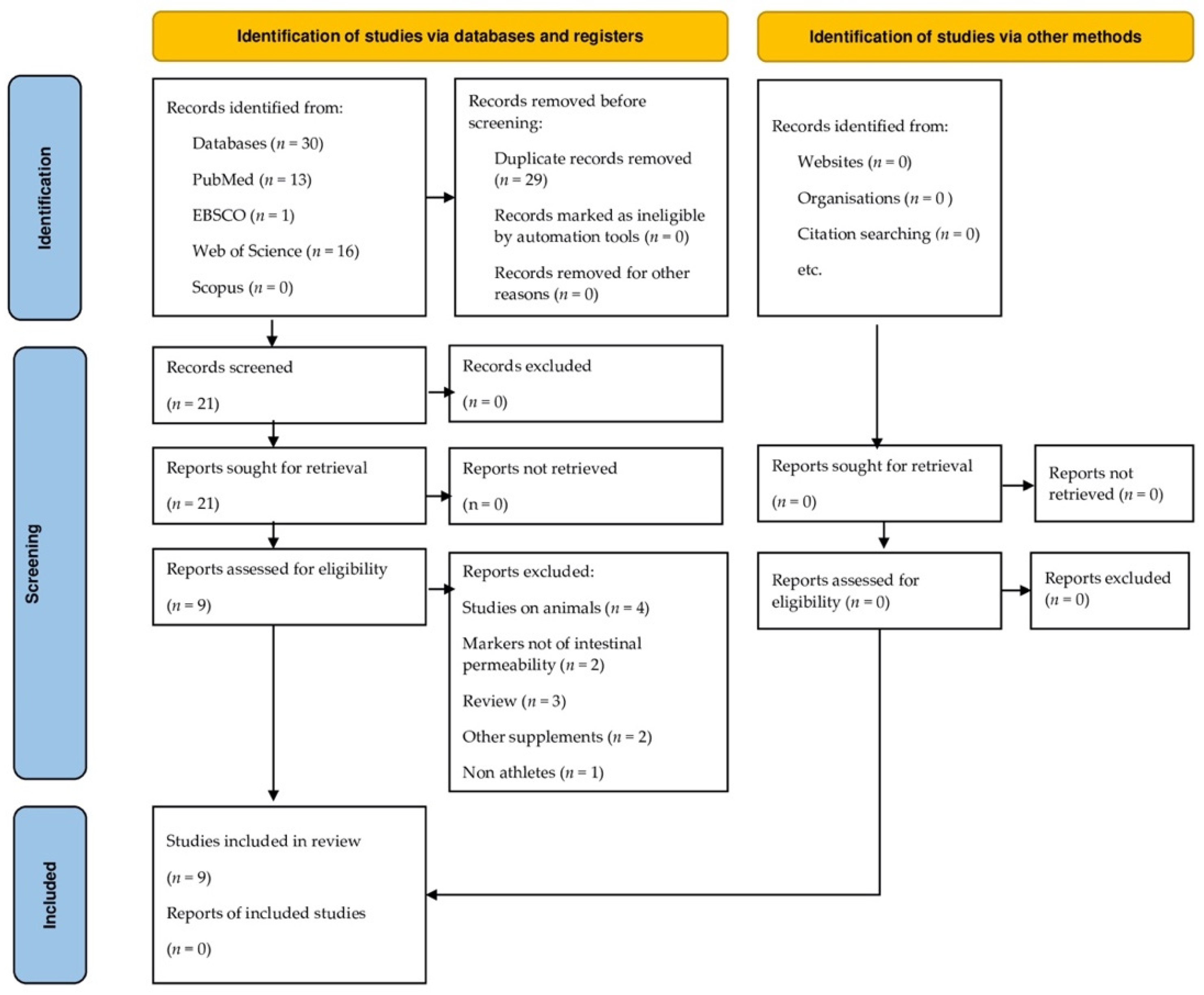
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
- •
- Since bovine colostrum is a food rather than a drug, its adverse effects may be minimal, provided the product is obtained from a reliable source and is prepared using good manufacturing practices (GLP).
- •
- Being a dietary supplement and without any narcotic, steroidal, or euphorigenic properties, it is suggested that BC should be removed from the WADA anti-doping list. While BC products are well tolerated, some patients allergic to dairy products may experience undesirable side effects such as gastrointestinal problems like flatulence and nausea.
- •
- Well-designed, randomized, and placebo-controlled clinical trials are needed to assess the therapeutic potential to alleviate gastrointestinal injury, long-term safety, and efficacy as well as optimal dose levels of colostrum products in athletes and physically active men and women.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and Exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, E.P.; Burini, R.C.; Jeukendrup, A. Gastrointestinal Complaints during Exercise: Prevalence, Etiology, and Nutritional Recommendations. Sports Med. 2014, 44 (Suppl. 1), S79–S85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, F.M.; Petriz, B.; Marques, G.; Kamilla, L.H.; Franco, O.L. Is There an Exercise-Intensity Threshold Capable of Avoiding the Leaky Gut? Front. Nutr. 2021, 8, 627289. [Google Scholar] [CrossRef]
- Gleeson, M.; Pyne, D.B. Respiratory Inflammation and Infections in High-Performance Athletes. Immunol. Cell Biol. 2016, 94, 124–131. [Google Scholar] [CrossRef]
- Edwards, K.H.; Ahuja, K.D.; Watson, G.; Dowling, C.; Musgrave, H.; Reyes, J.; Cherry, J.; Kitic, C.M. The Influence of Exercise Intensity and Exercise Mode on Gastrointestinal Damage. Appl. Physiol. Nutr. Metab. 2021, 46, 1105–1110. [Google Scholar] [CrossRef]
- Aune, S.K.; Cwikiel, J.; Flaa, A.; Arnesen, H.; Solheim, S.; Awoyemi, A.; Trøseid, M.; Seljeflot, I.; Helseth, R. Gut Leakage Markers in Response to Strenuous Exercise in Patients with Suspected Coronary Artery Disease. Cells 2021, 10, 2193. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic Review: Exercise-Induced Gastrointestinal Syndrome-Implications for Health and Intestinal Disease. Aliment. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef] [Green Version]
- Rathe, M.; Müller, K.; Sangild, P.T.; Husby, S. Clinical Applications of Bovine Colostrum Therapy: A Systematic Review. Nutr. Rev. 2014, 72, 237–254. [Google Scholar] [CrossRef]
- Arslan, A.; Kaplan, M.; Duman, H.; Bayraktar, A.; Ertürk, M.; Henrick, B.M.; Frese, S.A.; Karav, S. Bovine Colostrum and Its Potential for Human Health and Nutrition. Front. Nutr. 2021, 8, 651721. [Google Scholar] [CrossRef]
- Hałasa, M.; Maciejewska-Markiewicz, D.; Baśkiewicz-Hałasa, M.; Safranow, K.; Stachowska, E. Post-Delivery Milking Delay Influence on the Effect of Oral Supplementation with Bovine Colostrum as Measured with Intestinal Permeability Test. Medicina 2020, 56, 495. [Google Scholar] [CrossRef] [PubMed]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory Effects of Lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, F.; Panella, G.; Leboffe, L.; Antonini, G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals 2016, 9, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, M.; Wakabayashi, H.; Yamauchi, K.; Teraguchi, S.; Hayasawa, H. Bovine Lactoferrin and Lactoferricin Derived from Milk: Production and Applications. Biochem. Cell Biol. 2002, 80, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Suresh, G. Enteral Lactoferrin Supplementation for Prevention of Sepsis and Necrotizing Enterocolitis in Preterm Infants. Cochrane Database Syst. Rev. 2017, 6, CD007137. [Google Scholar] [CrossRef]
- Główka, N.; Durkalec-Michalski, K.; Woźniewicz, M. Immunological Outcomes of Bovine Colostrum Supplementation in Trained and Physically Active People: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1023. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Iacucci, M. Diverse Immune Effects of Bovine Colostrum and Benefits in Human Health and Disease. Nutrients 2021, 13, 3798. [Google Scholar] [CrossRef]
- Mehra, R.; Singh, R.; Nayan, V.; Buttar, H.S.; Kumar, N.; Kumar, S.; Bhardwaj, A.; Kaushik, R.; Kumar, H. Nutritional Attributes of Bovine Colostrum Components in Human Health and Disease: A Comprehensive Review. Food Biosci. 2021, 40, 100907. [Google Scholar] [CrossRef]
- Lal, S.O.; Wolf, S.E.; Herndon, D.N. Growth Hormone, Burns and Tissue Healing. Growth Horm. IGF Res. 2000, 10 (Suppl. B), S39–S43. [Google Scholar] [CrossRef]
- Corpeleijn, W.E.; van Vliet, I.; de Gast-Bakker, D.-A.H.; van der Schoor, S.R.D.; Alles, M.S.; Hoijer, M.; Tibboel, D.; van Goudoever, J.B. Effect of Enteral IGF-1 Supplementation on Feeding Tolerance, Growth, and Gut Permeability in Enterally Fed Premature Neonates. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 184–190. [Google Scholar] [CrossRef]
- Mero, A.; Miikkulainen, H.; Riski, J.; Pakkanen, R.; Aalto, J.; Takala, T. Effects of Bovine Colostrum Supplementation on Serum IGF-I, IgG, Hormone, and Saliva IgA during Training. J. Appl. Physiol. 1997, 83, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Mero, A.; Nykänen, T.; Keinänen, O.; Knuutinen, J.; Lahti, K.; Alen, M.; Rasi, S.; Leppäluoto, J. Protein Metabolism and Strength Performance after Bovine Colostrum Supplementation. Amino Acids 2005, 28, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, H.; van Breda, E.; Verlaan, G.; Smeets, R. Effects of Oral Bovine Colostrum Supplementation on Serum Insulin-like Growth Factor-I Levels. Nutrition 2002, 18, 566–567. [Google Scholar] [CrossRef]
- Buckley, J.D.; Brinkworth, G.D.; Abbott, M.J. Effect of Bovine Colostrum on Anaerobic Exercise Performance and Plasma Insulin-like Growth Factor I. J. Sports Sci. 2003, 21, 577–588. [Google Scholar] [CrossRef]
- Coombes, J.S.; Conacher, M.; Austen, S.K.; Marshall, P.A. Dose Effects of Oral Bovine Colostrum on Physical Work Capacity in Cyclists. Med. Sci. Sports Exerc. 2002, 34, 1184–1188. [Google Scholar] [CrossRef] [Green Version]
- Duff, W.R.D.; Chilibeck, P.D.; Rooke, J.J.; Kaviani, M.; Krentz, J.R.; Haines, D.M. The Effect of Bovine Colostrum Supplementation in Older Adults during Resistance Training. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 276–285. [Google Scholar] [CrossRef]
- Davison, G.; Jones, A.W.; Marchbank, T.; Playford, R.J. Oral Bovine Colostrum Supplementation Does Not Increase Circulating Insulin-like Growth Factor-1 Concentration in Healthy Adults: Results from Short- and Long-Term Administration Studies. Eur. J. Nutr. 2020, 59, 1473–1479. [Google Scholar] [CrossRef] [Green Version]
- O’Callaghan, T.F.; O’Donovan, M.; Murphy, J.P.; Sugrue, K.; Mannion, D.; McCarthy, W.P.; Timlin, M.; Kilcawley, K.N.; Hickey, R.M.; Tobin, J.T. Evolution of the Bovine Milk Fatty Acid Profile—From Colostrum to Milk Five Days Post Parturition. Int. Dairy J. 2020, 104, 104655. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial Dysbiosis-Induced Obesity: Role of Gut Microbiota in Homoeostasis of Energy Metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Davison, G. Bovine Colostrum and Immune Function after Exercise. Med. Sport Sci. 2012, 59, 62–69. [Google Scholar] [CrossRef]
- Shing, C.M.; Hunter, D.C.; Stevenson, L.M. Bovine Colostrum Supplementation and Exercise Performance: Potential Mechanisms. Sports Med. 2009, 39, 1033–1054. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W.; March, D.S.; Curtis, F.; Bridle, C. Bovine Colostrum Supplementation and Upper Respiratory Symptoms during Exercise Training: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. BMC Sports Sci. Med. Rehabil. 2016, 8, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Główka, N.; Woźniewicz, M. Potential Use of Colostrum Bovinum Supplementation in Athletes—A Review. Acta Sci. Pol. Technol. Aliment. 2019, 18, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Chantler, S.; Griffiths, A.; Matu, J.; Davison, G.; Holliday, A.; Jones, B. A Systematic Review: Role of Dietary Supplements on Markers of Exercise-Associated Gut Damage and Permeability. PLoS ONE 2022, 17, e0266379. [Google Scholar] [CrossRef]
- Group OLoEW. The Oxford 2011 Levels of Evidence; Oxford Centre for Evidence-Based Medicine Oxford: Oxford, UK, 2011; Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 26 May 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Buckley, J.D.; Butler, R.N.; Southcott, E.; Brinkworth, G.D. Bovine Colostrum Supplementation During Running Training Increases Intestinal Permeability. Nutrients 2009, 1, 224. [Google Scholar] [CrossRef] [Green Version]
- March, D.S.; Marchbank, T.; Playford, R.J.; Jones, A.W.; Thatcher, R.; Davison, G. Intestinal Fatty Acid-Binding Protein and Gut Permeability Responses to Exercise. Eur. J. Appl. Physiol. 2017, 117, 931–941. [Google Scholar] [CrossRef] [Green Version]
- McKenna, Z.; Berkemeier, Q.; Naylor, A.; Kleint, A.; Gorini, F.; Ng, J.; Kim, J.-K.; Sullivan, S.; Gillum, T. Bovine Colostrum Supplementation Does Not Affect Plasma I-FABP Concentrations Following Exercise in a Hot and Humid Environment. Eur. J. Appl. Physiol. 2017, 117, 2561–2567. [Google Scholar] [CrossRef]
- Davison, G.; Marchbank, T.; March, D.S.; Thatcher, R.; Playford, R.J. Zinc Carnosine Works with Bovine Colostrum in Truncating Heavy Exercise-Induced Increase in Gut Permeability in Healthy Volunteers. Am. J. Clin. Nutr. 2016, 104, 526–536. [Google Scholar] [CrossRef] [Green Version]
- March, D.S.; Jones, A.W.; Thatcher, R.; Davison, G. The Effect of Bovine Colostrum Supplementation on Intestinal Injury and Circulating Intestinal Bacterial DNA Following Exercise in the Heat. Eur. J. Nutr. 2019, 58, 1441–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, S.A.; Cheung, S.S.; Cotter, J.D. Bovine Colostrum, Training Status, and Gastrointestinal Permeability during Exercise in the Heat: A Placebo-Controlled Double-Blind Study. Appl. Physiol. Nutr. Metab. 2014, 39, 1070–1082. [Google Scholar] [CrossRef] [PubMed]
- Marchbank, T.; Davison, G.; Oakes, J.R.; Ghatei, M.A.; Patterson, M.; Moyer, M.P.; Playford, R.J. The Nutriceutical Bovine Colostrum Truncates the Increase in Gut Permeability Caused by Heavy Exercise in Athletes. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G477–G484. [Google Scholar] [CrossRef] [Green Version]
- Hałasa, M.; Maciejewska, D.; Baśkiewicz-Hałasa, M.; Machaliński, B.; Safranow, K.; Stachowska, E. Oral Supplementation with Bovine Colostrum Decreases Intestinal Permeability and Stool Concentrations of Zonulin in Athletes. Nutrients 2017, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Harper, K.M.; Mutasa, M.; Prendergast, A.J.; Humphrey, J.; Manges, A.R. Environmental Enteric Dysfunction Pathways and Child Stunting: A Systematic Review. PLoS Negl. Trop. Dis. 2018, 12, e0006205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denno, D.M.; VanBuskirk, K.; Nelson, Z.C.; Musser, C.A.; Burgess, D.C.H.; Tarr, P.I. Use of the Lactulose to Mannitol Ratio to Evaluate Childhood Environmental Enteric Dysfunction: A Systematic Review. Clin. Infect. Dis. 2014, 59 (Suppl. 4), S213–S219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funaoka, H.; Kanda, T.; Fujii, H. Intestinal fatty acid-binding protein (I-FABP) as a new biomarker for intestinal diseases. Rinsho Byori 2010, 58, 162–168. [Google Scholar]
- Lamprecht, M.; Bogner, S.; Schippinger, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J.F. Probiotic Supplementation Affects Markers of Intestinal Barrier, Oxidation, and Inflammation in Trained Men; a Randomized, Double-Blinded, Placebo-Controlled Trial. J. Int. Soc. Sports Nutr. 2012, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Bölke, E.; Jehle, P.M.; Hausmann, F.; Däubler, A.; Wiedeck, H.; Steinbach, G.; Storck, M.; Orth, K. Preoperative Oral Application of Immunoglobulin-Enriched Colostrum Milk and Mediator Response during Abdominal Surgery. Shock 2002, 17, 9–12. [Google Scholar] [CrossRef]
- Hałasa, M.; Maciejewska, D.; Ryterska, K.; Baśkiewicz-Hałasa, M.; Safranow, K.; Stachowska, E. Assessing the Association of Elevated Zonulin Concentration in Stool with Increased Intestinal Permeability in Active Professional Athletes. Medicina 2019, 55, 710. [Google Scholar] [CrossRef] [Green Version]
- Seethaler, B.; Basrai, M.; Neyrinck, A.M.; Nazare, J.-A.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Biomarkers for Assessment of Intestinal Permeability in Clinical Practice. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G11–G17. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, G.; Ardehali, S.H.; Baghestani, A.-R.; Vahdat Shariatpanahi, Z. Effects of Early Enteral Bovine Colostrum Supplementation on Intestinal Permeability in Critically Ill Patients: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrition 2019, 60, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Leaky Gut: Mechanisms, Measurement and Clinical Implications in Humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Duranti, S.; Mancabelli, L.; Mancino, W.; Anzalone, R.; Longhi, G.; Statello, R.; Carnevali, L.; Sgoifo, A.; Bernasconi, S.; Turroni, F.; et al. Exploring the Effects of COLOSTRONONI on the Mammalian Gut Microbiota Composition. PLoS ONE 2019, 14, e0217609. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bhardwaj, P.; Singh, R. Administration of Lactobacillus Casei and Bifidobacterium Bifidum Ameliorated Hyperglycemia, Dyslipidemia, and Oxidative Stress in Diabetic Rats. Int. J. Prev. Med. 2016, 7, 102. [Google Scholar] [CrossRef]
- Grigas, J.; Ruzauskas, M.; Pautienius, A.; Bartkiene, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Bernatoniene, J.; Ivanauskas, L.; et al. Investigation of Immunomodulatory and Gut Microbiota-Altering Properties of Multicomponent Nutraceutical Prepared from Lactic Acid Bacteria, Bovine Colostrum, Apple Production By-Products, and Essential Oils. Foods 2021, 10, 1313. [Google Scholar] [CrossRef]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia Muciniphila and Its Role in Regulating Host Functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.; Macdonald, C.; Wicks, A.C.; Holt, M.P.; Floyd, D.; Ghosh, S.; Wright, N.A.; Playford, R.J. Use of the “Nutriceutical”, Bovine Colostrum, for the Treatment of Distal Colitis: Results from an Initial Study. Aliment. Pharmacol. Ther. 2002, 16, 1917–1922. [Google Scholar] [CrossRef]
- Galdino, A.B.D.S.; Rangel, A.H.D.N.; Buttar, H.S.; Nascimento, M.S.L.; Gavioli, E.C.; Oliveira, R.D.P.; Sales, D.C.; Urbano, S.A.; Anaya, K. Bovine Colostrum: Benefits for the Human Respiratory System and Potential Contributions for Clinical Management of COVID-19. Food Agric. Immunol. 2021, 32, 143–162. [Google Scholar] [CrossRef]
| Evidence Level (Treatment Benefits) |
| Level 1—Systematic review of randomized trials or n-of-1 trials |
| Level 2—Randomized trial or observational study with dramatic effect |
| Level 3—Non-randomized controlled cohort/follow-up study |
| Level 4—Case series, case–control study, or historically controlled study |
| Level 5—Mechanism-based reasoning |
| Bucley (2009) [38] | ? | + | + | + | + | ? |
| March (2017) [39] | + | + | + | + | + | |
| McKenne (2017) [40] | ? | ? | + | + | + | ? |
| Davison (2016) [41] | + | + | + | + | + | |
| March (2018) [42] | + | + | + | + | + | |
| Morrison (2014) [43] | ? | ? | ? | + | + | ? |
| Marchbank (2021) [44] | ? | + | + | + | + | ? |
| Hałasa (2020) [11] | + | + | + | + | + | |
| Hałasa (2017) [45] | + | ? | + | + | + | ? |
| Randomisation proecess | Devation from intended intervetion | Missing outcome data | Measurment of the outcome | Selection of the reported results | Overall |
| Autor/Year/OCEMB | Age (y) | N/Sex | Dose | Duration | Sugar Test/Duration of the Urine Collection | I-FABP | Participants | Exercise Test |
|---|---|---|---|---|---|---|---|---|
| Bucley et al./2009/1 st level [38] | 25+/−4.7 | BC group = 9, Whey group = 8, Control group = 13, M | 60 g/day | 8 weeks | ↑, 5 h | - | Regular exercise training for at least three months before the study. | Treadmill running test until the participant reached volitional exhaustion. |
| March et al./2017/1 st level [39] | 26+/−5 | 18, M | 20 g/day | 14 weeks | ↓, 5 h | ↓ | All regular exercisers. VO2 peak 56.3 +/−6.5 mL·kg/min | 20 min run at a constant speed equivalent to 80% VO2 peak on treadmill, 1 % grade, 22.1+/−1.7 C, 37+/−8% humidity. |
| McKenne et al./2017/1 st level [40] | 20+/−2 | 10, M | 20 g/day, two doses daily 10 g each | 14 weeks | - | ← | VO2 max 55.8 +/−3.79 mL·kg/min, variable running time in two conditions CON to BC | Participants ran for 46 ± 7.75 min at 40 °C and 50% RH using a temperature- and humidity-controlled environmental chamber. Exercise was terminated after 60 min, if Tcore exceeded 40 °C, heart rate rose above 195 bpm, or if participants asked to terminate the trial. |
| Davison et al./2016/1 st level [41] | Mean 25 | 8, M | 20 g bovine colostrum, 10 g BC capsule, each were taken 2 times | 14 days | - | ↓ | Active individuals who regularly exercised 4 times/week VO2 max 59.6 +/−1.8 mL·kg/min | Treadmill running for 20 min at 80% of the VO2 max. |
| March et al./2018/1 st level [42] | 26+/−6 | 12, M | 20 g/day, 10 g in the morning, and the same with the evening meal | 14 days | - | ↓ | VO2 peak 55.8 +/−4.8 mL·kg/min | Constant speed equivalent to 70% VO2 peak treadmill with a 1% grade for 60 min or until core temperature reached 40 °C. Climatic chamber was maintained at 30.0 ± 0.1 °C, and 60 ± 0% relative humidity. |
| Morrison et al./2014/1 st level [43] | 23+/−4, 21+/−2 | n = 7 trained group (TG), n = 8 untrained group (UG), M | 1.7 g/kgmc/day | 7 days | ←, 5 h | ← | TG 60 mL·kg−1·min−1 and trained at least 6 times per week for at least 60 min per training session, UG less than 50 mL·kg−1·min−1 and participated in physical activity less than 3 times per week | Exercise consisted of 15 min cycling at a fixed load, initially eliciting 50% HRR (cycle 1), running for 30 min at a fixed speed initially eliciting 80% HRR (run 1), 30 min running maximal-distance trial (run 2), 15 min cycle. Environmental chamber (30 °C, 50% RH) with graded airflow based on the participant’s running speed (wind speed: 3.5 m·s−1 to 4.5 m·s−1). |
| Marchbank et al./2021/1 st level [44] | Mean 26 | 12, M | 20 g/day | 14 days | ↓, 5 h | - | All subjects were regular exercisers and took part in running as part of their training; seven were runners, two participated in boxing, and three participated in rugby. VO2 max 53.3 +/−6.8 mL·kg/min | 20 min run at a constant speed equivalent to 80% VO2 peak on treadmill, 1 % grade, 22.1+/−1.7 C, 37+/−8% humidity |
| Hałasa et al./2020/1 st level [11] | Mean 34.5 | n = 7, n = 8, n = 9, n = 9, M, F | 500 mg 2/day harvested in 2 h | 20 days | ↓, 6 h | - | A group of 36 healthy volunteers, 30 males and 6 females, active athletes from various sport disciplines, including mixed martial arts (10), triathlon (11), cycling (9), water polo (6). | No exercise test. |
| Hałasa et al./2017/1 st level [45] | Mean 27.5 | n = 7, n = 7, M | 500 mg 2/day in the morning and in the evening, m 30 min before meal | 20 days | ↓, 6 h | - | Martial arts fitters in active training at the middle of the competition season. | No exercise test. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziewiecka, H.; Buttar, H.S.; Kasperska, A.; Ostapiuk-Karolczuk, J.; Domagalska, M.; Cichoń, J.; Skarpańska-Stejnborn, A. A Systematic Review of the Influence of Bovine Colostrum Supplementation on Leaky Gut Syndrome in Athletes: Diagnostic Biomarkers and Future Directions. Nutrients 2022, 14, 2512. https://doi.org/10.3390/nu14122512
Dziewiecka H, Buttar HS, Kasperska A, Ostapiuk-Karolczuk J, Domagalska M, Cichoń J, Skarpańska-Stejnborn A. A Systematic Review of the Influence of Bovine Colostrum Supplementation on Leaky Gut Syndrome in Athletes: Diagnostic Biomarkers and Future Directions. Nutrients. 2022; 14(12):2512. https://doi.org/10.3390/nu14122512
Chicago/Turabian StyleDziewiecka, Hanna, Harpal S. Buttar, Anna Kasperska, Joanna Ostapiuk-Karolczuk, Małgorzata Domagalska, Justyna Cichoń, and Anna Skarpańska-Stejnborn. 2022. "A Systematic Review of the Influence of Bovine Colostrum Supplementation on Leaky Gut Syndrome in Athletes: Diagnostic Biomarkers and Future Directions" Nutrients 14, no. 12: 2512. https://doi.org/10.3390/nu14122512
APA StyleDziewiecka, H., Buttar, H. S., Kasperska, A., Ostapiuk-Karolczuk, J., Domagalska, M., Cichoń, J., & Skarpańska-Stejnborn, A. (2022). A Systematic Review of the Influence of Bovine Colostrum Supplementation on Leaky Gut Syndrome in Athletes: Diagnostic Biomarkers and Future Directions. Nutrients, 14(12), 2512. https://doi.org/10.3390/nu14122512






