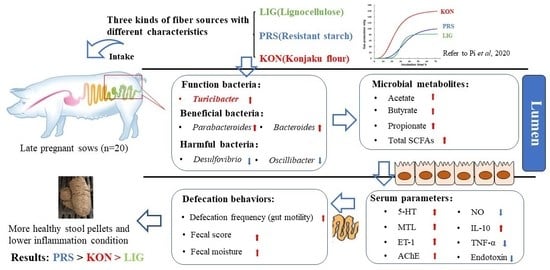
Consumption of Dietary Fiber with Different Physicochemical Properties during Late Pregnancy Alters the Gut Microbiota and Relieves Constipation in Sow Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Designs
2.2. Monitoring of the Defection Frequency, Fecal Consistency Score, and Fecal Moisture during Late Pregnancy
2.3. Blood and Fecal Sample Collection
2.4. Reproductive Performance and Parturition Duration Recording
2.5. Fecal DNA Extraction, 16S rRNA Amplification, and Sequencing
2.6. Analysis of 16S rRNA Sequencing Data
2.7. Determination of the SCFAs Profile in Feces
2.8. Determination of Gut Motility Regulatory Factors, Endotoxin, and Inflammatory Factors in Serum
2.9. Statistical Analysis
3. Results
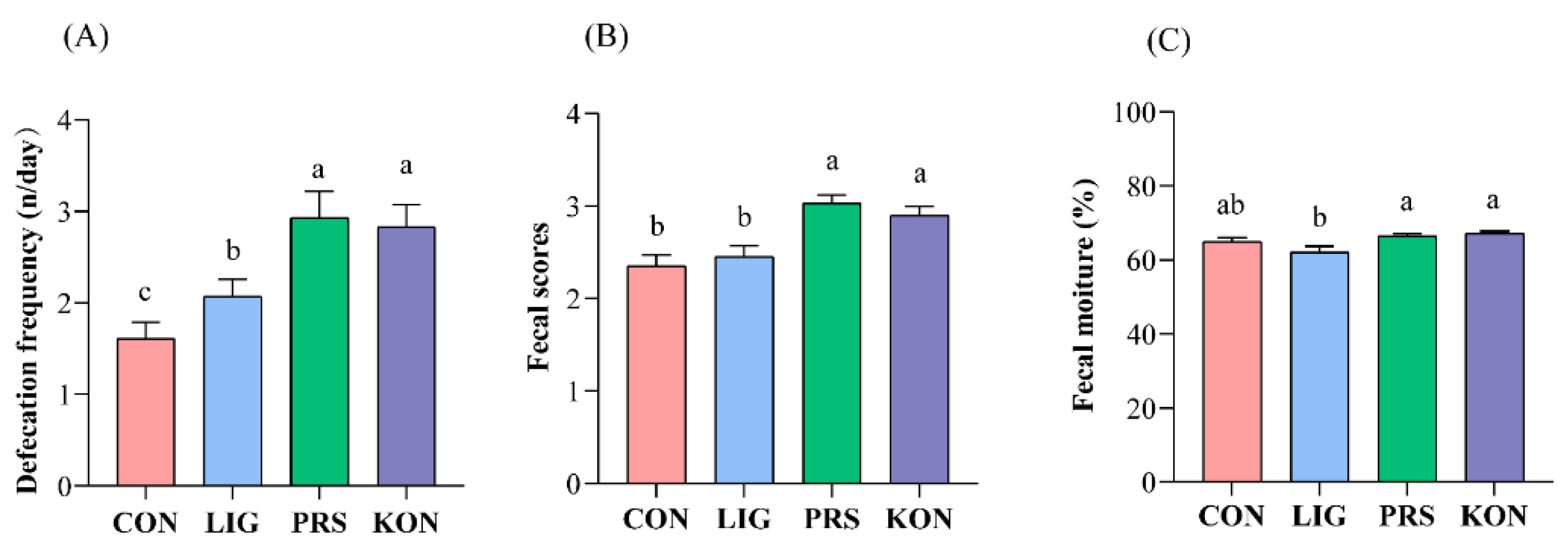
3.1. Effects of Different Fiber Sources on the Defecation Frequency and Fecal Parameters of Sows
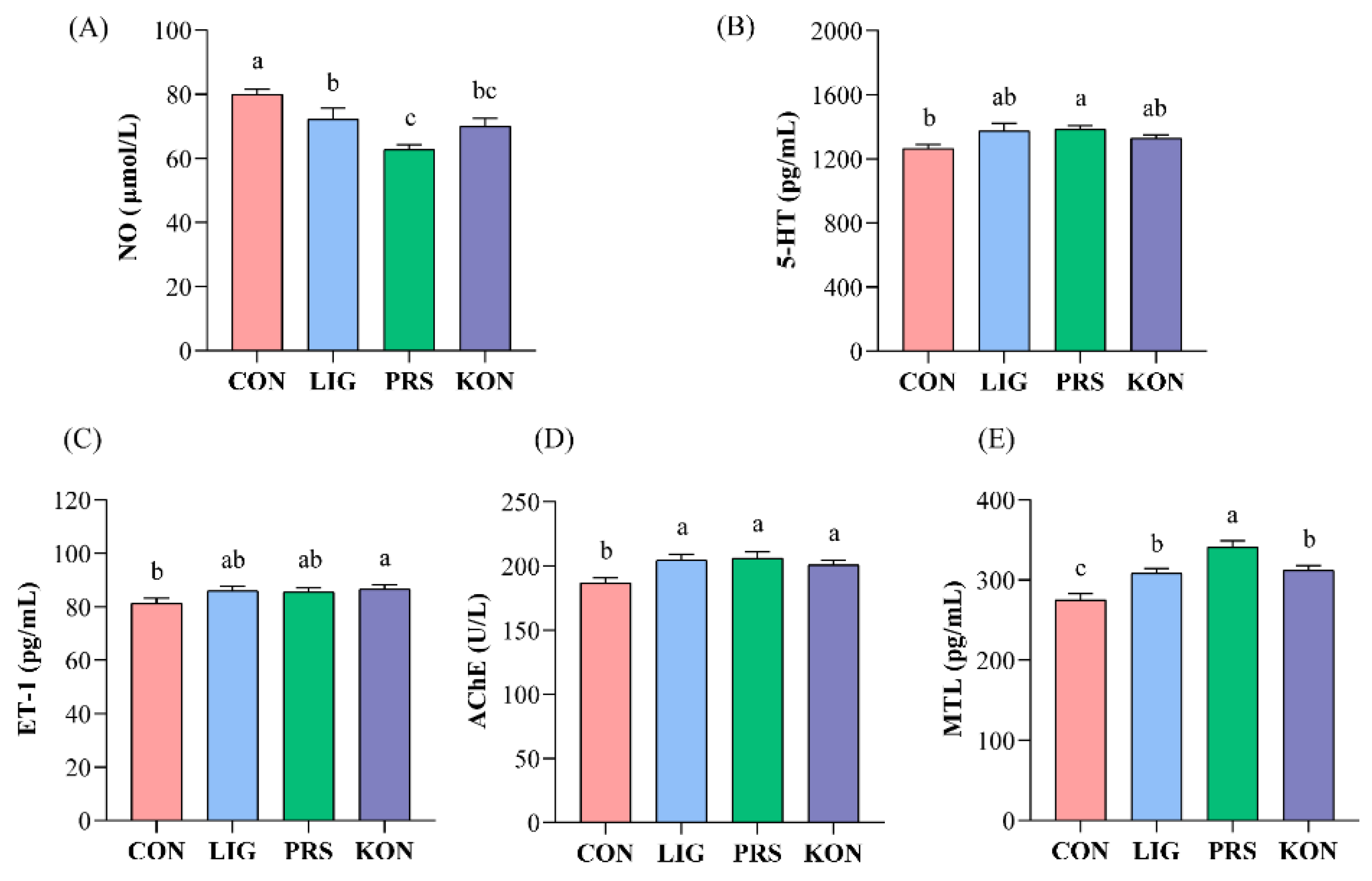
3.2. Effects of Different Fiber Sources on the Serum Gut Motility Regulatory Factors of Sows
3.3. Effects of Different Fiber Sources on the Reproductive Parameters and Parturition Duration of Sows
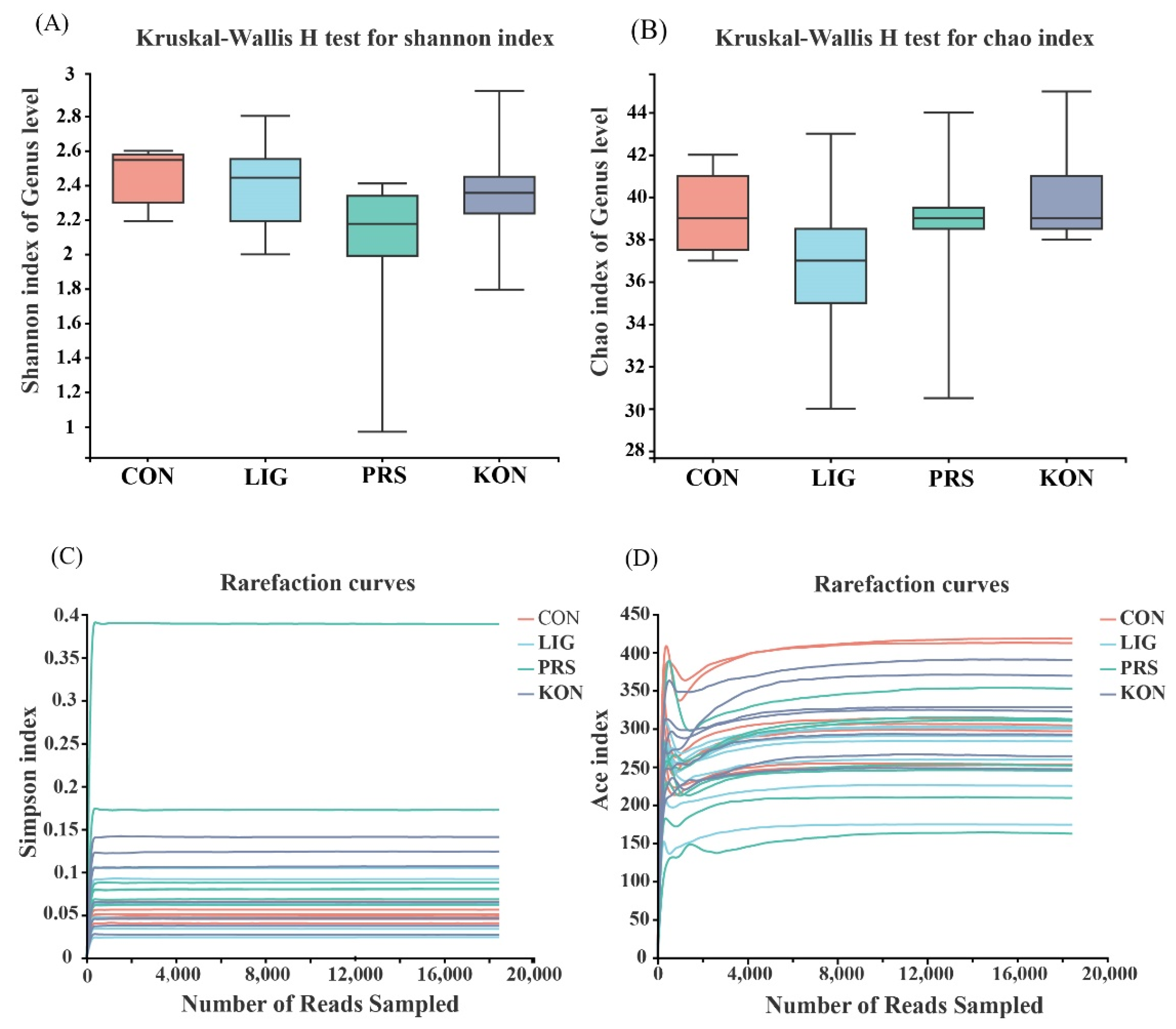
3.4. Effects of Different Fiber Sources on the Composition and Diversity of the Fecal Microbiota of Sows
3.5. Effects of Different Fiber Sources on the Fecal SCFAs Profiles of Sows
3.6. Effects of Different Source Fibers on the Serum Inflammation Factors and Endotoxin of Sows
3.7. Correlation between Defecation Performances, Gut Regulatory Factors, Inflammation Factors, and Differential Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Oliviero, C.; Heinonen, M.; Valros, A.; Peltoniemi, O. Environmental and sow-related factors affecting the duration of farrowing. Anim. Reprod. Sci. 2010, 119, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, T.; Han, L.; Zhao, L.; Niu, Y.; Chen, H. L-glutamine supplementation alleviates constipation during late gestation of mini sows by modifying the microbiota composition in feces. Biomed. Res. Int. 2017, 2017, 4862861. [Google Scholar] [CrossRef] [PubMed]
- Pearodwong, P.; Muns, R.; Tummaruk, P. Prevalence of constipation and its influence on post-parturient disorders in tropical sows. Trop. Anim. Health Prod. 2016, 48, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Tabeling, R.; Schwier, S.; Kamphues, J. Effects of different feeding and housing conditions on dry matter content and consistency of faeces in sows. J. Anim. Physiol. Anim. Nutr. 2003, 87, 116–121. [Google Scholar] [CrossRef]
- Yu, X.; Fu, C.; Cui, Z.; Chen, G.; Xu, Y.; Yang, C. Inulin and isomalto-oligosaccharide alleviate constipation and improve reproductive performance by modulating motility-related hormones, short-chain fatty acids, and feces microflora in pregnant sows. J. Anim. Sci. 2021, 99, skab257. [Google Scholar] [CrossRef]
- Kuronen, M.; Hantunen, S.; Alanne, L.; Kokki, H.; Saukko, C.; Sjovall, S.; Vesterinen, K.; Kokki, M. Pregnancy, puerperium and perinatal constipation—An observational hybrid survey on pregnant and postpartum women and their age-matched non-pregnant controls. BJOG 2021, 128, 1057–1064. [Google Scholar] [CrossRef]
- Bradley, C.S.; Kennedy, C.M.; Turcea, A.M.; Rao, S.S.; Nygaard, I.E. Constipation in pregnancy: Prevalence, symptoms, and risk factors. Obstet. Gynecol. 2007, 110, 1351–1357. [Google Scholar] [CrossRef]
- Wald, A. Constipation: Advances in diagnosis and treatment. JAMA 2016, 315, 185–191. [Google Scholar] [CrossRef]
- Derbyshire, E.; Davies, J.; Costarelli, V.; Dettmar, P. Diet, physical inactivity and the prevalence of constipation throughout and after pregnancy. Matern. Child Nutr. 2006, 2, 127–134. [Google Scholar] [CrossRef]
- Tigchelaar, E.F.; Bonder, M.J.; Jankipersadsing, A.; Fu, J.; Wijmenga, C.; Zhernakova, A. Gut microbiota composition associated with stool consistency. Gut 2016, 65, 540–542. [Google Scholar] [CrossRef]
- Muller, M.; Hermes, G.D.A.; Canfora, E.E.; Smidt, H.; Masclee, A.A.M.; Zoetendal, E.G.; Blaak, E.E. Distal colonic transit is linked to gut microbiota diversity and microbial fermentation in humans with slow colonic transit. Am. J. Physiol-Gastr. Liver Physiol. 2020, 318, G361–G369. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.L.; Ding, C.; Gong, J.F.; Li, N.; Li, J.S. Fecal microbiota transplantation in combination with soluble dietary fiber for the chronic constipation. Am. J. Gastroenterol. 2016, 111, S1251. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Chen, J.; Chen, X.F.; Chia, N.; O’Connor, H.M.; Wolf, P.G.; Gaskins, H.R.; Bharucha, A.E. Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology 2016, 150, 367–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.Q.; Wei, H.K.; Sun, H.Q.; Long, G.; Ao, J.T.; Jiang, S.W.; Peng, J. Effects of supplementing sow diets during two gestations with konjac flour and Saccharomyces boulardii on constipation in peripartal period, lactation feed intake and piglet performance. Anim. Feed Sci. Technol. 2015, 210, 254–262. [Google Scholar] [CrossRef]
- Roager, H.M.; Hansen, L.B.S.; Bahl, M.I.; Frandsen, H.L.; Carvalho, V.; Gobel, R.J.; Dalgaard, M.D.; Plichta, D.R.; Sparholt, M.H.; Vestergaard, H.; et al. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat. Microbiol. 2016, 1, 16093. [Google Scholar] [CrossRef] [PubMed]
- Jonathan, M.C.; van den Borne, J.J.G.C.; van Wiechen, P.; da Silva, C.S.; Schols, H.A.; Gruppen, H. In vitro fermentation of 12 dietary fibres by faecal inoculum from pigs and humans. Food Chem. 2012, 133, 889–897. [Google Scholar] [CrossRef]
- Shang, Q.H.; Liu, S.J.; Liu, H.S.; Mahfuz, S.; Piao, X.S. Impact of sugar beet pulp and wheat bran on serum biochemical profile, inflammatory responses and gut microbiota in sows during late gestation and lactation. J. Anim. Sci. Biotechnol. 2021, 12, 54. [Google Scholar] [CrossRef]
- Pi, Y.; Hu, J.; Bai, Y.; Wang, Z.B.; Wu, Y.J.; Ye, H.; Zhang, S.Y.; Tao, S.Y.; Xiao, Y.P.; Han, D.D.; et al. Effects of dietary fibers with different physicochemical properties on fermentation kinetics and microbial composition by fecal inoculum from lactating sows in vitro. J. Sci. Food Agric. 2021, 101, 907–917. [Google Scholar] [CrossRef]
- Sasaki, D.; Sasaki, K.; Kondo, A. Glycosidic linkage structures influence dietary fiber fermentability and propionate production by human colonic mcrobiota in vitro. Biotechnol. J. 2020, 15, e1900523. [Google Scholar] [CrossRef]
- Jian, W.J.; Wu, H.Y.; Wu, L.L.; Wu, Y.H.; Jia, L.N.; Pang, J.; Sun, Y.M. Effect of molecular characteristics of Konjac glucomannan on gelling and rheological properties of Tilapia myofibrillar protein. Carbohyd. Polym. 2016, 150, 21–31. [Google Scholar] [CrossRef]
- Haralampu, S.G. Resistant starch—A review of the physical properties and biological impact of RS3. Carbohyd. Polym. 2000, 41, 285–292. [Google Scholar] [CrossRef]
- da Silva, C.S.; Bolhuis, J.E.; Gerrits, W.J.J.; Kemp, B.; van den Borne, J.J.G.C. Effects of dietary fibers with different fermentation characteristics on feeding motivation in adult female pigs. Physiol. Behav. 2013, 110, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Barszcz, M.; Taciak, M.; Tusnio, A.; Swiech, E.; Skomial, J.; Cobanobva, K.; Gresakova, L. The effect of organic and inorganic zinc source, used with lignocellulose or potato fiber, on microbiota composition, fermentation, and activity of enzymes involved in dietary fiber breakdown in the large intestine of pigs. Livest. Sci. 2021, 245, 104429. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016, 65, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zhang, X.; Han, D.; Ye, H.; Tao, S.; Pi, Y.; Zhao, J.; Chen, L.; Wang, J. Short administration of combined prebiotics improved microbial colonization, gut barrier, and growth performance of neonatal piglets. ACS Omega 2020, 5, 20506–20516. [Google Scholar] [CrossRef]
- Tateyama, I.; Hashii, K.; Johno, I.; Iino, T.; Hirai, K.; Suwa, Y.; Kiso, Y. Effect of xylooligosaccharide intake on severe constipation in pregnant women. J. Nutr. Sci. Vitaminol. 2005, 51, 445–448. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhong, D.; Sun, R.; Zhang, Y.; Pegg, R.B.; Zhong, G. Prevention of loperamide induced constipation in mice by KGM and the mechanisms of different gastrointestinal tract microbiota regulation. Carbohyd. Polym. 2021, 256, 117418. [Google Scholar] [CrossRef]
- Neri, I.; Blasi, I.; Castro, P.; Grandinetti, G.; Ricchi, A.; Facchinetti, F. Polyethylene glycol electrolyte solution (Isocolan) for constipation during pregnancy: An observational open-label study. J. Midwifery Womens Health 2004, 49, 355–358. [Google Scholar] [CrossRef]
- Hu, T.G.; Wen, P.; Fu, H.Z.; Lin, G.Y.; Liao, S.T.; Zou, Y.X. Protective effect of mulberry (Morus atropurpurea) fruit against diphenoxylate-induced constipation in mice through the modulation of gut microbiota. Food Funct. 2019, 10, 1513–1528. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, X.; Kan, J. Preventive effect of resistant starch on activated carbon-induced constipation in mice. Exp. Ther. Med. 2013, 6, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Gershon, M.D. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 14–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S. 5-Hydroxytryptamine4 receptor agonists and colonic motility. J. Smooth Muscle Res. 2009, 45, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Luo, H.; Liu, Y.; Cao, J.; Xia, H. Plasma hormones facilitated the hypermotility of the colon in a chronic stress rat model. PLoS ONE 2012, 7, e31774. [Google Scholar] [CrossRef] [PubMed]
- Hadizadeh, F.; Walter, S.; Belheouane, M.; Bonfiglio, F.; Heinsen, F.A.; Andreasson, A.; Agreus, L.; Engstrand, L.; Baines, J.F.; Rafter, J.; et al. Stool frequency is associated with gut microbiota composition. Gut 2017, 66, 559–560. [Google Scholar] [CrossRef]
- Fu, J.F.; Wang, Y.T.; Tan, S.M.; Wang, J. Effects of banana resistant starch on the biochemical indexes and intestinal flora of obese rats induced by a high-fat diet and their correlation analysis. Front. Bioeng. Biotechnol. 2021, 9, 575724. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Fung, T.C.; Vuong, H.E.; Luna, C.D.G.; Pronovost, G.N.; Aleksandrova, A.A.; Riley, N.G.; Vavilina, A.; McGinn, J.; Rendon, T.; Forrest, L.R.; et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat. Microbiol. 2019, 4, 2064–2073. [Google Scholar] [CrossRef]
- Wang, Y.J.; Xu, X.J.; Zhou, N.; Sun, Y.T.; Liu, C.; Liu, S.J.; You, X. Parabacteroides acidifaciens sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2019, 69, 761–766. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut microbiota and short chain fatty acids: Implications in glucose homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Choi, C.H.; Chang, S.K. Alteration of gut microbiota and efficacy of probiotics in functional constipation. J. Neurogastroenterol. 2015, 21, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.E.; Choi, S.C.; Park, K.S.; Park, M.I.; Shin, J.E.; Lee, T.H.; Jung, K.W.; Koo, H.S.; Myung, S.J.; Constipation Research group of Korean Society of Neurogastroenterology and Motility; et al. Change of fecal flora and effectiveness of the short-term vsl#3 probiotic treatment in patients with functional constipation. J. Neurogastroenterol. Motil. 2015, 21, 111–120. [Google Scholar] [PubMed] [Green Version]
- Xu, P.; Hong, F.; Wang, J.; Cong, Y.; Dai, S.; Wang, S.; Wang, J.; Jin, X.; Wang, F.; Liu, J.; et al. Microbiome remodeling via the montmorillonite adsorption-excretion axis prevents obesity-related metabolic disorders. Ebiomedicine 2017, 16, 251–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, L.M.; Mendes, M.M.; Oliveira, A.C.; Magalhaes, K.G.; Shivappa, N.; Hebert, J.R.; da Costa, T.H.M.; Botelho, P.B. Dietary inflammatory index and its relationship with gut microbiota in individuals with intestinal constipation: A cross-sectional study. Eur. J. Nutr. 2022, 61, 341–355. [Google Scholar] [CrossRef]
- Zhuang, M.; Shang, W.; Ma, Q.; Strappe, P.; Zhou, Z. Abundance of probiotics and butyrate-production microbiome manages constipation via short-chain fatty acids production and hormones secretion. Mol. Nutr. Food Res. 2019, 63, e1801187. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Yu, S.; Su, Y.; Zhu, W. Effects of early intervention with sodium butyrate on gut microbiota and the expression of inflammatory cytokines in neonatal piglets. PLoS ONE 2016, 11, e0162461. [Google Scholar] [CrossRef] [PubMed]
- Deehan, E.C.; Yang, C.; Perez-Munoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe 2020, 27, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, B.R.; Tuncil, Y.E. A Perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef]
- Albrecht, S.; van Muiswinkel, G.C.J.; Schols, H.A.; Voragen, A.G.J.; Gruppen, H. Introducing capillary electrophoresis with laser-induced fluorescence detection (CE-LIF) for the characterization of konjac glucomannan oligosaccharides and their in vitro fermentation behavior. J. Agric. Food Chem. 2009, 57, 3867–3876. [Google Scholar] [CrossRef]
- Dong, J.L.; Yu, X.; Dong, L.E.; Shen, R.L. In vitro fermentation of oat beta-glucan and hydrolysates by fecal microbiota and selected probiotic strains. J. Sci. Food Agric. 2017, 97, 4198–4203. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Zhang, H.; Lee, Y.K.; Zhai, Q.; Chen, W. Roles of intestinal Bacteroides in human health and diseases. Crit. Rev. Food Sci. Nutr. 2020, 61, 3518–3536. [Google Scholar] [CrossRef]
- Arp, C.G.; Correa, M.J.; Ferrero, C. Resistant starches: A smart alternative for the development of functional bread and other starch-based foods. Food Hydrocolloid. 2021, 121, 106949. [Google Scholar] [CrossRef]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio 2019, 10, e02566-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, F.J.; Fukuma, N.M.; Mikkelsen, D.; Flanagan, B.M.; Williams, B.A.; Lisle, A.T.; Cuív, P.Ó.; Morrison, M.; Gidley, M.J. Food starch structure impacts gut microbiome composition. Msphere 2018, 3, e00086-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, J.; Li, Y.; Li, T.; Zhang, W.; Fan, M.; Zhang, K.; Qian, H.; Zhang, H.; Qi, X.; Wang, L. Comparison of different soluble dietary fibers during the in vitro fermentation process. J. Agric. Food. Chem. 2021, 69, 7446–7457. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, T.T.; Wang, H.W.; Chen, L.; Zhou, Z.K. Studies on nutritional intervention of rice starch- oleic acid complex (resistant starch type V) in rats fed by high-fat diet. Carbohyd. Polym. 2020, 246, 116637. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Su, Y.; Zhu, W.Y. Microbiome-metabolome responses in the cecum and colon of pig to a high resistant starch diet. Front. Microbiol. 2016, 7, 779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soret, R.; Chevalier, J.; De Coppet, P.; Poupeau, G.; Derkinderen, P.; Segain, J.P.; Neunlist, M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 2010, 138, 1772–1782. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, J.B.; Tao, S.Y.; Zhou, X.J.; Pi, Y.; Gerrits, W.J.J.; Johnston, L.J.; Zhang, S.Y.; Yang, H.J.; Liu, L.; et al. Effect of dietary fiber fermentation on short-chain fatty acid production and microbial composition in vitro. J. Sci. Food Agric. 2020, 100, 4282–4291. [Google Scholar] [CrossRef]
- Fukumoto, S.; Tatewaki, M.; Yamada, T.; Fujimiya, M.; Mantyh, C.; Voss, M.; Eubanks, S.; Harris, M.; Pappas, T.N.; Takahashi, T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1269–R1276. [Google Scholar] [CrossRef] [Green Version]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef] [Green Version]
- Regmi, P.R.; van Kempen, T.A.; Matte, J.J.; Zijlstra, R.T. Starch with high amylose and low in vitro digestibility increases short-chain fatty acid absorption, reduces peak insulin secretion, and modulates incretin secretion in pigs. J. Nutr. 2011, 141, 398–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.X.; Lee, J.S.; Campbell, E.L.; Colgan, S.P. Microbiota-derived butyrate dynamically regulates intestinal homeostasis through regulation of actin-associated protein synaptopodin. Proc. Natl. Acad. Sci. USA 2020, 117, 11648–11657. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.W.; Shen, L.Y.; Niu, L.L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.X.; Li, X.W.; et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhu, X.; Cui, Y.; Wang, W.; Liu, H.; Li, Z.; Guo, Z.; Ma, S.; Li, D.; Wang, C.; et al. Consumption of dietary fiber from different sources during pregnancy alters sow gut microbiota and improves performance and reduces inflammation in sows and piglets. mSystems 2021, 6, e00591-20. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018, 9, 3555. [Google Scholar] [CrossRef] [Green Version]
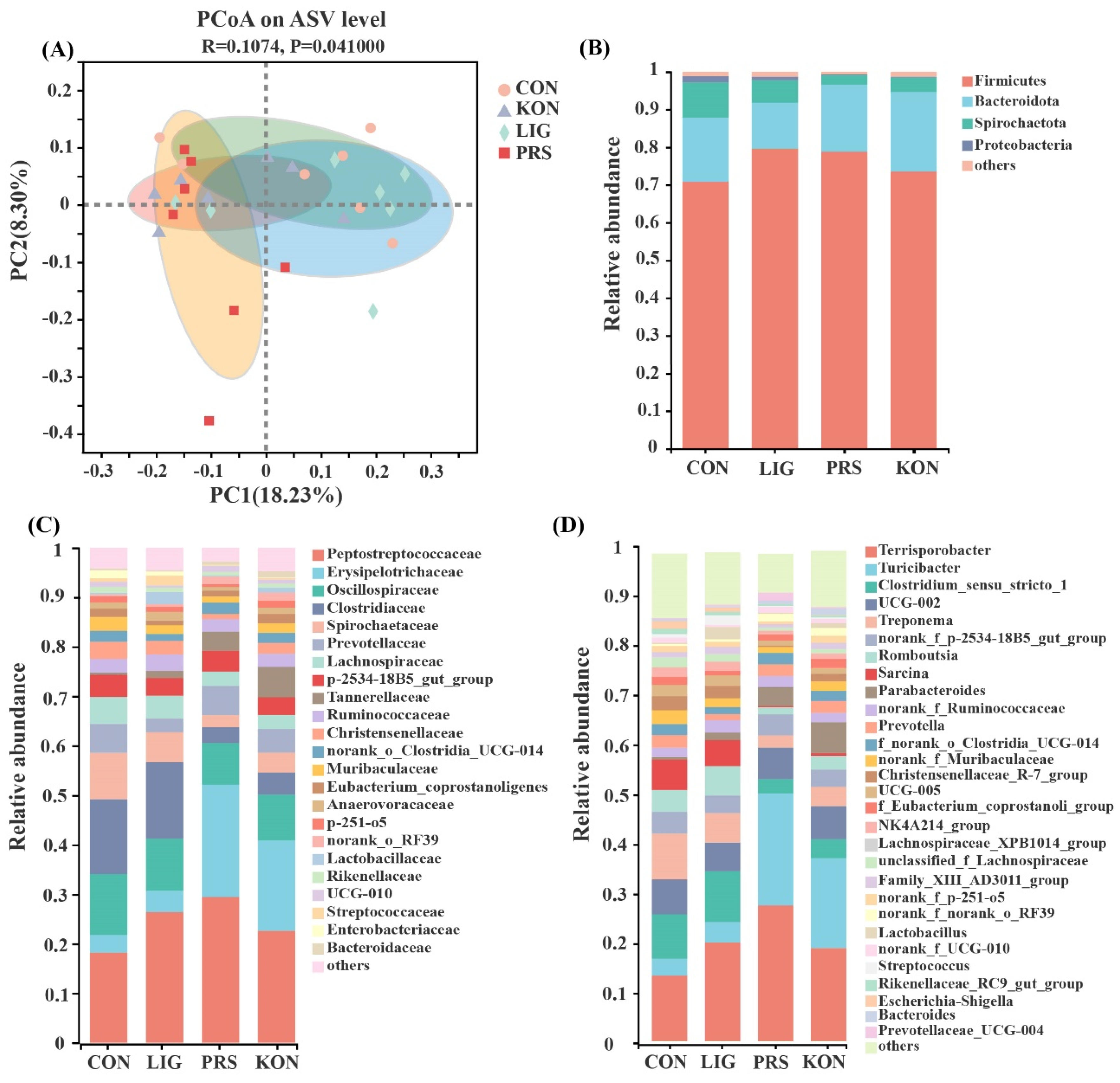
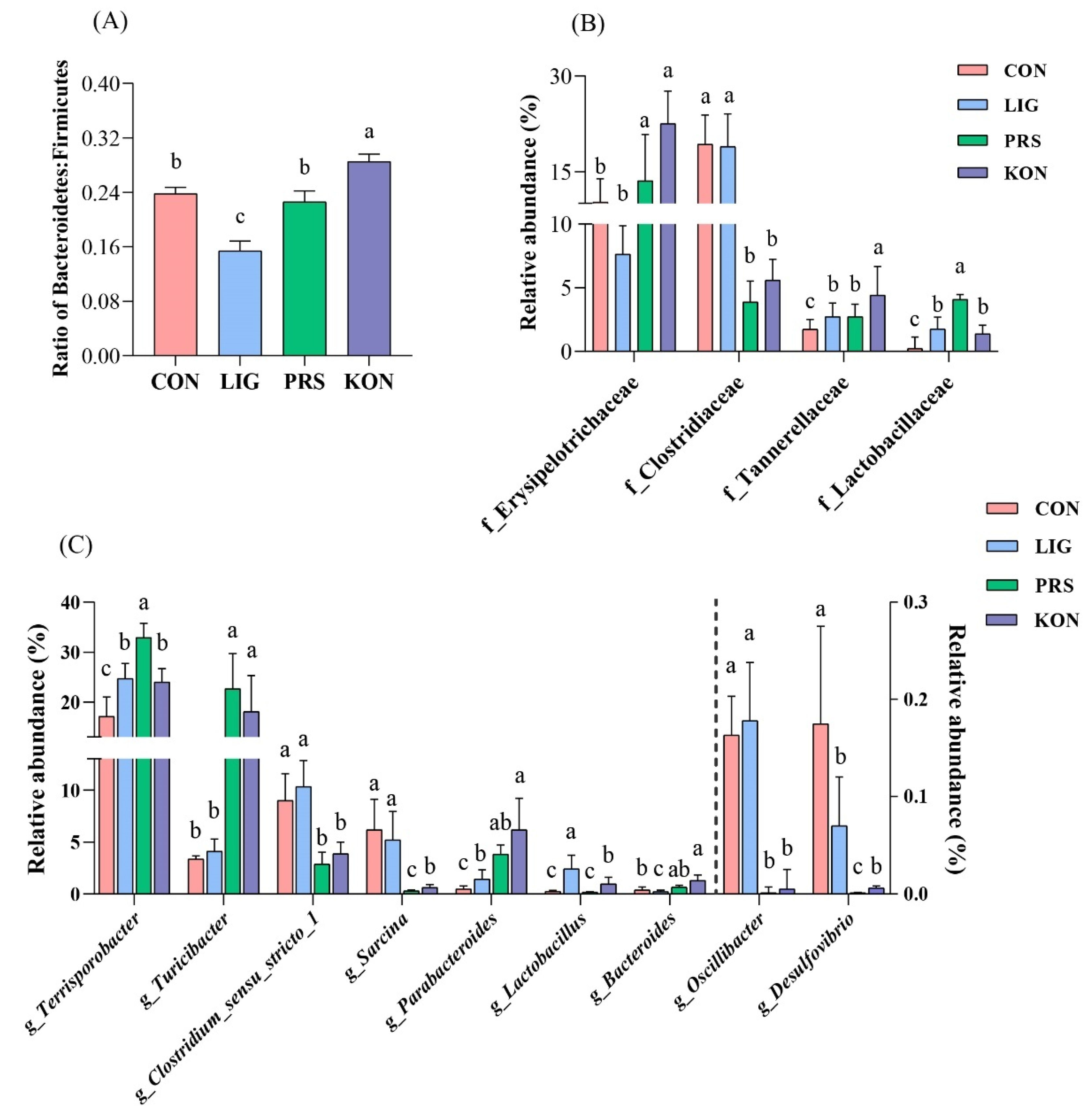
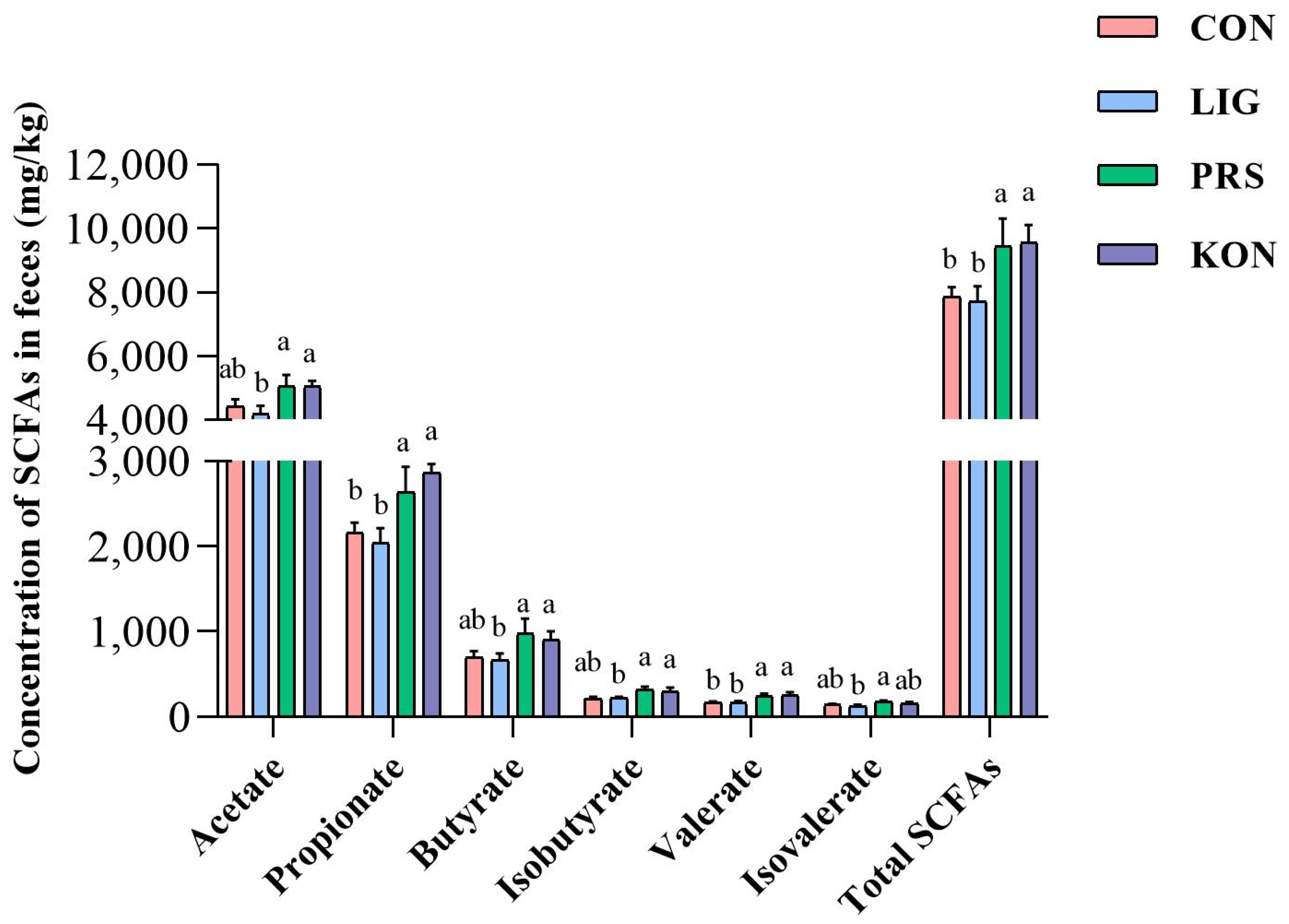
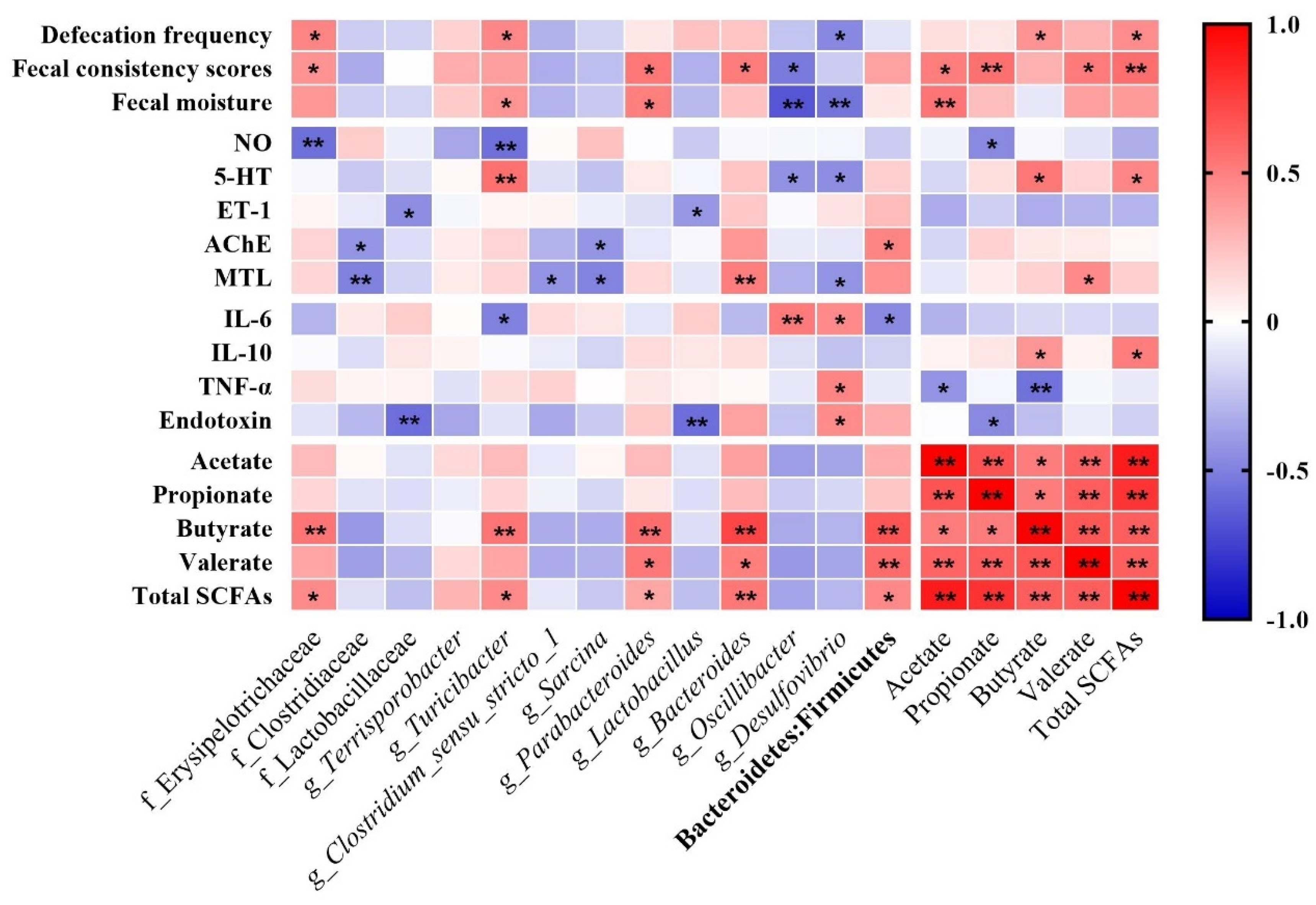
| Items | CON 1 | LIG 1 | PRS 1 | KON 1 |
|---|---|---|---|---|
| Ingredient (%) | ||||
| Corn | 53.50 | 53.50 | 53.50 | 53.50 |
| Soybean meal | 13.00 | 13.50 | 13.00 | 13.00 |
| Soybean oil | 2.00 | 2.00 | 2.00 | 2.00 |
| Wheat bran | 28.00 | 26.00 | 26.00 | 26.00 |
| Lignocellulose | 1.50 | |||
| Resistant starch | 2.00 | |||
| Konjaku flour | 2.00 | |||
| Limestone | 1.25 | 1.25 | 1.25 | 1.25 |
| Dicalcium phosphate | 1.10 | 1.10 | 1.10 | 1.10 |
| NaCl | 0.50 | 0.50 | 0.50 | 0.50 |
| L-Lysine HCl | 0.05 | 0.05 | 0.05 | 0.05 |
| Methionine | 0.04 | 0.04 | 0.04 | 0.04 |
| Threonine | 0.05 | 0.05 | 0.05 | 0.05 |
| Tryptophan | 0.01 | 0.01 | 0.01 | 0.01 |
| Premix 2 | 0.50 | 0.50 | 0.50 | 0.50 |
| Total (%) | 100 | 100 | 100 | 100 |
| Nutrient composition | ||||
| DE 3 (Mcal/kg) | 3.29 | 3.25 | 3.24 | 3.24 |
| CP 3 (%) | 15.50 | 15.38 | 15.29 | 15.33 |
| Ca 3 (%) | 0.78 | 0.78 | 0.78 | 0.78 |
| P 3 (%) | 0.75 | 0.73 | 0.73 | 0.73 |
| Lys 3 (%) | 0.73 | 0.73 | 0.72 | 0.72 |
| Met 3 (%) | 0.30 | 0.30 | 0.29 | 0.29 |
| Thr 3 (%) | 0.58 | 0.58 | 0.57 | 0.57 |
| Trp 3 (%) | 0.17 | 0.17 | 0.17 | 0.17 |
| NDF 3 (%) | 15.64 | 16.23 | 16.12 | 16.48 |
| ADF 3 (%) | 4.92 | 5.76 | 4.77 | 4.93 |
| TDF 3 (%) | 17.89 | 18.86 | 18.69 | 18.72 |
| Items | CON 1 | LIG 1 | PRS 1 | KON 1 | SEM | p-Value |
|---|---|---|---|---|---|---|
| Total piglets (n) | 15.78 | 14.78 | 15.11 | 14.33 | 0.33 | 0.46 |
| Born alive (n) | 14.26 | 13.78 | 14.11 | 13.88 | 0.32 | 0.96 |
| Stillborn number (n) | 1.81 | 1.00 | 0.61 | 0.68 | 0.14 | 0.08 |
| Stillborn rate (%) | 11.29 a | 8.47 ab | 3.94 b | 4.49 b | 0.94 | 0.02 |
| Litter weight (kg) | 19.87 | 19.38 | 19.26 | 19.07 | 0.36 | 0.88 |
| Average bodyweight (kg) | 1.40 | 1.45 | 1.39 | 1.46 | 0.02 | 0.62 |
| Duration of parturition (min) | 397.69 b | 399.07 b | 286.47 a | 282.85 a | 19.35 | 0.03 |
| Item | CON 1 | LIG 1 | PRS 1 | KON 1 | SEM | p-Value |
|---|---|---|---|---|---|---|
| IL-6 (pg/mL) 2 | 12.71 | 12.10 | 11.32 | 10.77 | 1.32 | 0.11 |
| IL-10 (pg/mL) 2 | 18.55 c | 19.81 b | 23.55 a | 21.06 b | 1.05 | 0.04 |
| TNF-α (pg/mL) 2 | 132.11a | 115.33 b | 109.52 c | 115.34 b | 6.88 | 0.01 |
| Endotoxin (EU/L) 2 | 36.42 a | 34.31 b | 31.03 c | 31.71 c | 1.26 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, D.; Pi, Y.; Ye, H.; Wu, Y.; Bai, Y.; Lian, S.; Han, D.; Ni, D.; Zou, X.; Zhao, J.; et al. Consumption of Dietary Fiber with Different Physicochemical Properties during Late Pregnancy Alters the Gut Microbiota and Relieves Constipation in Sow Model. Nutrients 2022, 14, 2511. https://doi.org/10.3390/nu14122511
Lu D, Pi Y, Ye H, Wu Y, Bai Y, Lian S, Han D, Ni D, Zou X, Zhao J, et al. Consumption of Dietary Fiber with Different Physicochemical Properties during Late Pregnancy Alters the Gut Microbiota and Relieves Constipation in Sow Model. Nutrients. 2022; 14(12):2511. https://doi.org/10.3390/nu14122511
Chicago/Turabian StyleLu, Dongdong, Yu Pi, Hao Ye, Yujun Wu, Yu Bai, Shuai Lian, Dandan Han, Dongjiao Ni, Xinhua Zou, Jinbiao Zhao, and et al. 2022. "Consumption of Dietary Fiber with Different Physicochemical Properties during Late Pregnancy Alters the Gut Microbiota and Relieves Constipation in Sow Model" Nutrients 14, no. 12: 2511. https://doi.org/10.3390/nu14122511
APA StyleLu, D., Pi, Y., Ye, H., Wu, Y., Bai, Y., Lian, S., Han, D., Ni, D., Zou, X., Zhao, J., Zhang, S., Kemp, B., Soede, N., & Wang, J. (2022). Consumption of Dietary Fiber with Different Physicochemical Properties during Late Pregnancy Alters the Gut Microbiota and Relieves Constipation in Sow Model. Nutrients, 14(12), 2511. https://doi.org/10.3390/nu14122511