Sustained Consumption of a Decaffeinated Green Coffee Nutraceutical Has Limited Effects on Phenolic Metabolism and Bioavailability in Overweight/Obese Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Phenolic Composition of the GCPE
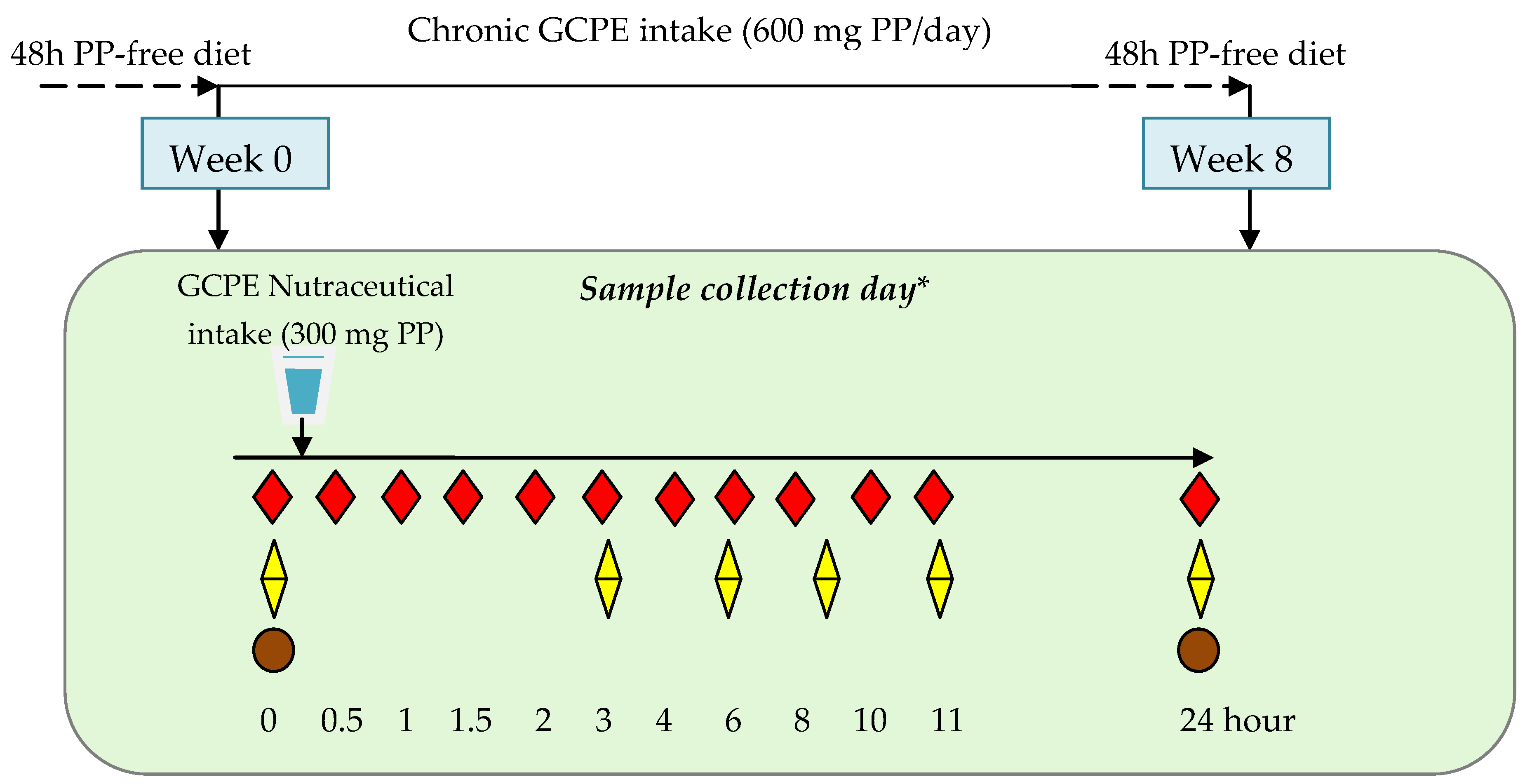
2.3. Participants, Study Design, and Sample Collection
2.4. Plasma, Urine and Fecal Samples Processing and Analysis by HPLC-ESI-QToF
2.5. Pharmacokinetic and Statistical Analysis
3. Results
3.1. Identification and Quantification of Plasma Metabolites
3.2. Identification and Quantification of Urinary Metabolites
3.3. Identification and Quantification of Fecal Metabolites
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baeza, G.; Sarriá, B.; Bravo, L.; Mateos, R. Exhaustive qualitative LC-DAD-MSn analysis of Arabica green coffee beans: Cinnamoyl-glycosides and cinnamoylshikimic acids as new polyphenols in green coffee. J. Agric. Food Chem. 2016, 64, 9663–9674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastian, F.; Hutabarat, O.S.; Dirpan, A.; Nainu, F.; Harapan, H.; Emran, T.B.; Simal-Gandara, J. From plantation to cup: Changes in bioactive compounds during coffee processing. Foods 2021, 10, 2827. [Google Scholar] [CrossRef] [PubMed]
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P.S. The chemistry of chlorogenic acid from green coffee and its role in attenuation of obesity and diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Roshan, H.; Nikpayam, O.; Sedaghat, M.; Sohrab, G. Effects of green coffee extract supplementation on anthropometric indices, glycaemic control, blood pressure, lipid profile, insulin resistance and appetite in patients with the metabolic syndrome: A randomised clinical trial. Br. J. Nutr. 2018, 119, 250–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haidari, F.; Samadi, M.; Mohammadshahi, M.; Jalali, M.T.; Engali, K.A. Energy restriction combined with green coffee bean extract affects serum adipocytokines and the body composition in obese women. Asia Pac. J. Clin. Nutr. 2017, 26, 1048–1054. [Google Scholar] [PubMed]
- Gorji, Z.; Varkaneh, H.K.; Talaei, S.; Nazary-Vannani, A.; Clark, C.; Fatahi, S.; Rahmani, J.; Salamat, S.; Zhang, Y. The effect of green-coffee extract supplementation on obesity: A systematic review and dose-response meta-analysis of randomized controlled trials. Phytomedicine 2019, 63, 153018. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Ahmad, W.; Budin, S.B.; Zainalabidin, S. Implication of dietary phenolic acids on inflammation in cardiovascular disease. Rev. Cardiovasc. Med. 2020, 21, 225–240. [Google Scholar] [CrossRef]
- Chen, H.; Huang, W.; Huang, X.; Liang, S.; Gecceh, E.; O Santos, H.; Khani, V.; Jiang, X. Effects of green coffee bean extract on C-reactive protein levels: A systematic review and meta-analysis of randomized controlled trials. Complementary Ther. Med. 2020, 52, 102498. [Google Scholar] [CrossRef]
- Amigo-Benavent, M.; Wang, S.; Mateos, R.; Sarriá, B.; Bravo, L. Antiproliferative and cytotoxic effects of green coffee and yerba mate extracts, their main hydroxycinnamic acids, methylxanthine and metabolites in different human cell lines. Food Chem. Toxicol. 2017, 106, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.; Dieminger, N.; Beusch, A.; Lee, Y.M.; Dunkel, A.; Suess, B.; Skurk, T.; Wahl, A.; Hauner, H.; Hofmann, T. Bioappearance and pharmacokinetics of bioactives upon coffee consumption. Anal. Bioanal. Chem. 2013, 405, 8487–8503. [Google Scholar] [CrossRef]
- Marmet, C.; Actis-Goretta, L.; Renouf, M.; Giuffrida, F. Quantification of phenolic acids and their methylates, glucuronides, sulfates and lactones metabolites in human plasma by LC-MS/MS after oral ingestion of soluble coffee. J. Pharm. Biomed. Anal. 2014, 88, 617–625. [Google Scholar] [CrossRef]
- Clifford, M.N.; Kerimi, A.; Williamson, G. Bioavailability and metabolism of chlorogenic acids (acyl-quinic acids) in humans. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1299–1352. [Google Scholar] [CrossRef]
- Gómez-Juaristi, M.; Martínez-López, S.; Sarria, B.; Bravo, L.; Mateos, R. Bioavailability of hydroxycinnamates in an instant green/roasted coffee blend in humans. Identification of novel colonic metabolites. Food Funct. 2018, 9, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to look into the puzzle of polyphenols and health? The postbiotics and gut microbiota associated with human metabotypes. Mol. Nutr. Food Res. 2020, 64, e1900952. [Google Scholar] [CrossRef]
- Faraldo, T.A.; Macedo, M.; Aymoto, N.M.; Maria, F. The two-way polyphenols-microbiota interactions and their effects on obesity and related metabolic diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef]
- Mena, P.; Bresciani, L.; Tassotti, M.; Rosi, A.; Martini, D.; Antonini, M.; Cas, A.D.; Bonadonna, R.; Brighenti, F.; Del Rio, D. Effect of different patterns of consumption of coffee and a cocoa-based product containing coffee on the nutrikinetics and urinary excretion of phenolic compounds. Am. J. Clin. Nutr. 2021, 114, 2107–2118. [Google Scholar] [CrossRef]
- Mena, P.; Ludwig, I.A.; Tomatis, V.B.; Acharjee, A.; Calani, L.; Rosi, A.; Brighenti, F.; Ray, S.; Griffin, J.L.; Bluck, L.J.; et al. Inter-individual variability in the production of flavan-3-ol colonic metabolites: Preliminary elucidation of urinary metabotypes. Eur. J. Nutr. 2019, 58, 1529–1543. [Google Scholar] [CrossRef] [Green Version]
- García-Cordero, J.; Sierra-Cinos, J.L.; Seguido, M.A.; González-Rámila, S.; Mateos, R.; Bravo-Clemente, L.; Sarriá, B. Regular consumption of green coffee phenol, oat β-glucan and green coffee phenol/oat β-glucan supplements does not change body composition in subjects with overweight and obesity. Foods 2022, 11, 679. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Williamson, G.; Crozier, A. Impact of dose on the bioavailability of coffee chlorogenic acids in humans. Food Funct. 2014, 5, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Bridge, B.; Renouf, M.; Sauser, J.; Beaumont, M.; Actis-Goretta, L. The roasting process does not influence the extent of conjugation of coffee chlorogenic and phenolic acids. BioFactors 2016, 42, 259–267. [Google Scholar] [CrossRef]
- Manach, C.; Milenkovic, D.; Van de Wiele, T.; Rodriguez-Mateos, A.; de Roos, B.; Garcia-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomás-Barberán, F.; et al. Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction. Mol. Nutr. Food Res. 2017, 61, 1600557. [Google Scholar] [CrossRef] [Green Version]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef]
- Ludwig, I.A.; de Peña, M.P.; Cid, C.; Crozier, A. Catabolism of coffee chlorogenic acids by human colonic microbiota. BioFactors 2013, 39, 623–632. [Google Scholar] [CrossRef]



| Cmax (µM) | Tmax (h) or Range a | AUC0–24h (µM min−1) | ||||
|---|---|---|---|---|---|---|
| Metabolite | Week 0 | Week 8 | Week 0 | Week 8 | Week 0 | Week 8 |
| Intestinal absorption | ||||||
| 5-Caffeoylquinic acid | 0.023 ± 0.001 | 0.022 ± 0.001 | 1.3 ± 0.2 ** | 0.7 ± 0.1 ** | 0.08 ± 0.02 | 0.062 ± 0.009 |
| 5-Feruloylquinic acid | 0.032 ± 0.004 | 0.034 ± 0.005 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.09 ± 0.02 | 0.08 ± 0.02 |
| 4-Feruloylquinic acid | 0.029 ± 0.004 | 0.031 ± 0.004 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.06 ± 0.01 | 0.07 ± 0.01 |
| 4′-Hydroxy-3′-methoxycinnamic acid (Ferulic acid, FA) | Traces b,** | 0.008 ± 0.001 ** | 1.8 ± 0.6 * | 0.6 ± 0.1 * | 0.034 ± 0.006 ** | 0.09 ± 0.02 ** |
| 3′-Hydroxy-4′-methoxycinnamic acid (isoFerulic acid, iFA) | 0.023 ± 0.006 | 0.032 ± 0.007 | 0.5 ± 0.1 | 0.5 ± 0 | 0.025 ± 0.007 ** | 0.043 ± 0.008 ** |
| 3′,4′-Dimethoxycinnamic acid | Traces b | Traces b | (2–4) a | (2–4) a | 0.007 ± 0.003 | 0.01 ± 0.006 |
| 3′-Methoxycinnamic acid-4′-glucuronide (FA-4′-glucuronide) | 0.016 ± 0.009 | 0.009 ± 0.002 | 1.2 ± 0.3 and 4.7 ± 0.3 | 1.2 ± 0.3 and 6.0 ± 0.7 | 0.06 ± 0.01 | 0.063 ± 0.02 |
| 3′-Methoxycinnamic acid-4′-sulfate (FA-4′-sulfate) | 0.017 ± 0.004 | 0.017 ± 0.004 | 0.78 ± 0.09 and 6.8 ± 0.7 | 0.56 ± 0.06 and 7.2 ± 0.6 | 0.11 ± 0.03 | 0.13 ± 0.02 |
| Cinnamic acid-4′-glucuronide (CoA-4′-glucuronide) | 0.039 ± 0.002 | 0.038 ± 0.002 | 1.4 ± 0.3 and 7.0 ± 0.9 | 2.1 ± 0.2 and 9 ± 2 | 0.72 ± 0.08 | 0.71 ± 0.07 |
| Cinnamic acid-4′-sulfate (CoA-4′-sulfate) | 0.02 ± 0.02 | 0.03 ± 0.01 | 1.7 ± 0.9 and 6 ± 3 | 0 ± 0 and 11 ± 3 | 0.2 ± 0.2 | 0.4 ± 0.2 |
| Colonic absorption | ||||||
| 3-(3′,4′-Dihydroxyphenyl)propanoic acid (Dihydrocaffeic acid, DHCA) | 0.22 ± 0.06 | 0.22 ± 0.05 | 10.2 ± 0.3 | 9.4 ± 0.5 | 2.0 ± 0.6 | 2.1 ± 0.5 |
| 3-(4′-Hydroxy-3′-methoxyphenyl)propanoic acid (Dihydroferulic acid, DHFA) | 0.3 ± 0.1 | 0.25 ± 0.06 | 7.6 ± 0.4 | 8.4 ± 0.6 | 2.2 ± 0.6 | 1.8 ± 0.3 |
| 3-(3′-Hydroxy-4′-methoxyphenyl) propanoic acid (Dihydroisoferulic acid, DHiFA) | 0.07 ± 0.02 | 0.07 ± 0.02 | 7 ± 1 | 7 ± 1 | 0.5 ± 0.2 | 0.6 ± 0.2 |
| 3-(3′,4′-Dimethoxyphenyl)propanoic acid (Dihydrodimethoxycinnamic acid) | 0.11 ± 0.02 | 0.12 ± 0.01 | 4 ± 1 | 10 ± 4 | 1.2 ± 0.1 | 1.3 ± 0.1 |
| 3-(3′-Hydroxyphenyl)propanoic acid-4′-sulfate (DHCA-4′-sulfate) | 0.34 ± 0.09 | 0.33 ± 0.07 | 7.9 ± 0.5 | 8.4 ± 0.6 | 2.3 ± 0.5 | 2.1 ± 0.4 |
| 3-(3′-Methoxyphenyl)propanoic acid-4′-glucuronide (DHFA-4′-glucuronide) | 0.13 ± 0.03 | 0.12 ± 0.02 | 8.7 ± 0.6 | 8.7 ± 0.6 | 1.1 ± 0.3 | 0.8 ± 0.1 |
| 3-(4′-Methoxyphenyl)propanoic acid-3′-glucuronide (DHiFA-3′-glucuronide) | 0.06 ± 0.02 | 0.07 ± 0.03 | 9.0 ± 0.6 | 8.1 ± 0.8 | 0.6 ± 0.4 | 0.7 ± 0.4 |
| 3-(3′-Methoxyphenyl)propanoic acid-4′-sulfate(DHFA-4′-sulfate) | 0.19 ± 0.08 | 0.17 ± 0.05 | 8.1 ± 0.4 | 8.4 ± 0.6 | 1.5 ± 0.7 | 1.1 ± 0.3 |
| 3-(4′-Methoxyphenyl)propanoic acid-3′-sulfate(DHiFA-3′-sulfate) | 0.04 ± 0.01 | 0.11 ± 0.06 | 7 ± 1 | 11.6 ± 3.2 | 0.3 ± 0.1 | 0.8 ± 0.5 |
| Feruloylglycine | Traces b | Traces b | 7 ± 1 | 8.4 ± 0.6 | 0.029 ± 0.007 | 0.026 ± 0.004 |
| Other microbial metabolites | ||||||
| 4′-Hydroxy-3′-methoxyphenylacetic acid | 0.14 ± 0.06 | 0.12 ± 0.05 | 7 ± 4 | 4 ± 3 | 1.4 ± 0.9 | 1.5 ± 0.8 |
| 4′-Hydroxyphenylacetic acid | 2 ± 1 | 7 ± 3 | 2 ± 2 | 0.6 ± 0.1 | 2 ± 1 | 36 ± 23 |
| 3′-Hydroxyphenylacetic acid | 0.20 ± 0.07 | 0.15 ± 0.05 | 8 ± 2 | 5 ± 1 | 2.6 ± 0.9 | 1.6 ± 0.6 |
| 4-Hydroxy-3-methoxybenzoic acid | 1.1 ± 0.2 | 1.6 ± 0.4 | 7 ± 3 | 7 ± 2 | 9 ± 2 | 14 ± 4 |
| 4-Hydroxybenzoic acid | 0.09 ± 0.01 | 0.08 ± 0.01 | 7 ± 2 | 5 ± 3 | 0.9 ± 0.2 | 0.5 ± 0.2 |
| 3-Hydroxybenzoic acid | 0.08 ± 0.02 | 0.10 ± 0.03 | 8 ± 1 * | 4 ± 1 * | 0.7 ± 0.3 | 0.8 ± 0.3 |
| 4′-Hydroxyhippuric acid | 0.08 ± 0.01 | 0.09 ± 0.01 | 12 ± 3 | 8 ± 2 | 1.2 ± 0.2 | 1.3 ± 0.2 |
| 3′-Hydroxyhippuric acid | 1.1 ± 0.3 | 1.3 ± 0.2 | 10.0 ± 0.4 | 9.2 ± 0.5 | 10 ± 3 | 13 ± 3 |
| Total 0–24 h (µmol) | ||
|---|---|---|
| Metabolite | Week 0 | Week 8 |
| Intestinal absorption | ||
| 3-Caffeoylquinic acid | 0.21 ± 0.05 | 0.18 ± 0.03 |
| 5-Caffeoylquinic acid | 0.33 ± 0.06 | 0.33 ± 0.04 |
| 4-Caffeoylquinic acid | 0.13 ± 0.07 | 0.15 ± 0.07 |
| 3-Feruloylquinic acid | 0.29 ± 0.07 | 0.34 ± 0.07 |
| 5-Feruloylquinic acid | 1.5 ± 0.2 | 1.5 ± 0.2 |
| 4-Feruloylquinic acid | 0.4 ± 0.2 | 0.4 ± 0.1 |
| Coumaroylquinic acid | 0.14 ± 0.03 | 0.10 ± 0.02 * |
| 3′,4′-Dihydroxycinnamic acid (Caffeic acid, CA) | 0.42 ± 0.06 | 0.45 ± 0.09 |
| 4′Hydroxycinnamic acid-3′-sulfate (CA-3′-sulfate) | 3.7 ± 0.5 | 4.4 ± 0.8 |
| 3′-Hydroxy-4′-methoxycinnamic acid (isoFerulic acid, iFA) | 1.7 ± 0.2 | 2.1 ± 0.2 |
| 3′-Methoxycinnamic acid-4′-glucuronide (FA-4′-glucuronide) | 3.4 ± 0.4 | 3.7 ± 0.5 |
| 4′-Methoxycinnamic acid-3′-glucuronide (iFA-3′-glucuronide) | 4.4 ± 0.5 | 6 ± 1 |
| 3′-Methoxycinnamic acid-4′-sulfate (FA-4′-sulfate) | 32 ± 5 | 33 ± 5 |
| 4′-Methoxycinnamic acid-3′-sulfate (iFA-3′-sulfate) | 1.1 ± 0.3 | 2.1 ± 0.9 |
| TOTAL—Intestinal metabolites | 50 ± 6 | 59 ± 8 |
| Colonic absorption | ||
| 3-(3′,4′-Dihydroxyphenyl)propanoic acid (Dihydrocaffeic acid, DHCA) | 16 ± 2 | 31 ± 5 ** |
| 3-(4′-Hydroxy-3′-methoxyphenyl) propanoic acid (Dihydroferulic, DHFA) | 0.5 ± 0.2 | 1.1 ± 0.4 |
| 3-(3′-Hydroxy-4′-methoxyphenyl) propanoic acid (Dihydroisoferulic, DHiFA) | 3.7 ± 0.4 | 3.2 ± 0.2 |
| 3-(4′-Hydroxyphenyl)propanoic acid (Dihydrocoumaric acid, DHCoA) | 4 ± 1 | 6 ± 2 ** |
| 3-(3′,4′-Dimethoxyphenyl)propanoic acid (Dihydrodimethoxycinnamic acid) | 0.7 ± 0.2 | 0.63 ± 0.08 |
| 3-(4′-Hydroxyphenyl)propanoic acid-3′-glucuronide (DHCA-3′-glucuronide) | 0.6 ± 0.2 | 0.5 ± 0.1 |
| 3-(3′-Hydroxyphenyl)propanoic acid-4′-sulfate (DHCA-4′-sulfate) | 8 ± 2 | 10 ± 2* |
| 3-(4′-Hydroxyphenyl)propanoic acid-3′-sulfate (DHCA-3′-sulfate) | 9 ± 2 | 9 ± 3 |
| 3-(3′-Methoxyphenyl)propanoic acid-4′-glucuronide (DHFA- 4′-glucuronide) | 5 ± 1 | 7 ± 1* |
| 3-(4′-Methoxyphenyl)propanoic acid-3′-glucuronide (DHiFA- 3′-glucuronide) | 2.9 ± 0.5 | 3.1 ± 0.7 |
| 3-(3′-Methoxyphenyl)propanoic acid-4′-sulfate (DHFA- 4′-sulfate) | 10 ± 2 | 9 ± 2 |
| 3-(4′-Methoxyphenyl)propanoic acid-3′-sulfate (DHiFA- 3′-sulfate) | 3 ± 1 | 2.6 ± 0.8 |
| 3-(Phenyl)propanoic acid-4′-glucuronide (DHCoA-4′-glucuronide) | 2.1 ± 0.3 | 1.9 ± 0.3 |
| 3-(Phenyl)propanoic acid-4′-sulfate (DHCoA-4′-sulfate) | 21 ± 6 | 21 ± 4 |
| 3-Dihydrocaffeoylquinic acid | 0.35 ± 0.07 | 0.27 ± 0.08 ** |
| 5-Dihydrocaffeoylquinic acid | 0.03 ± 0.02 | 0.03 ± 0.01 |
| 4-Dihydrocaffeoylquinic acid | 0.06 ± 0.02 | 0.04 ± 0.02 |
| 3-Dihydroferuloylquinic acid | 0.3 ± 0.1 | 0.5 ± 0.07 |
| 5-Dihydroferuloylquinic acid | 0.3 ± 0.1 | 0.18 ± 0.07 |
| 4-Dihydroferuloylquinic acid | 0.06 ± 0.02 | 0.05 ± 0.02 |
| Dihydrocoumaroylquinic acid | 0.5 ± 0.1 | 0.4 ± 0.1 |
| Dihydrocoumaroylquinic acid | 0.22 ± 0.08 | 0.17 ± 0.07 * |
| Feruloylglycine | 19 ± 5 | 25 ± 5 |
| IsoFeruloylglicine | 0.44 ± 0.04 | 0.6 ± 0.1 |
| TOTAL—Colonic metabolites | 106 ± 16 | 132 ± 17 |
| Other microbial metabolites | ||
| 3′,4′-Dihydroxyphenylacetic acid | 1.2 ± 0.2 | 1.3 ± 0.2 * |
| 4′-Hydroxy-3′-methoxyphenylacetic acid | 13.2 ± 0.9 | 12 ± 1 |
| 3′-Hydroxyphenylacetic acid | 12 ± 3 | 9 ± 2 |
| 3,4-Dihydroxybenzoic acid | 0.13 ± 0.02 | 0.18 ± 0.04 |
| 4-Hydroxybenzoic acid | 1.3 ± 0.2 | 1.01 ± 0.09 * |
| 3-Hydroxybenzoic acid | 0.91 ± 0.07 | 1.0 ± 0.1 |
| 4′-Hydroxyhippuric acid | 14 ± 2 | 14 ± 2 |
| 3′-Hydroxyhippuric acid | 32 ± 5 | 46 ± 8 ** |
| TOTAL—Other microbial metabolites | 75 ± 7 | 85 ± 8 |
| TOTAL Colonic + other microbial met. | 181 ± 21 | 217 ± 21 |
| TOTAL INTESTINAL + COLONIC + OTHERS | 231 ± 26 | 274 ± 30 |
| 0 h (µmol/g) | 24 h (µmol/g) | |||
|---|---|---|---|---|
| Metabolite | Week 0 | Week 8 | Week 0 | Week 8 |
| Intestinal absorption | ||||
| 5-Feruloylquinic acid | 0.0009 ± 0.0007 | 0.002 ± 0.002 | 0.002 ± 0.001 | 0.0003 ± 0.0003 |
| 3-Feruloylquinic acid | 0.003 ± 0.003 | 0.0010 ± 0.0007 | 0.003 ± 0.003 | 0.003 ± 0.002 |
| 4-Feruloylquinic acid | N.D. | 0.001 ± 0.001 | N.D. | 0.0004 ± 0.0004 |
| 3′,4′-Dihydroxycinnamic acid (Caffeic acid, CA) | N.D. | 0.003 ± 0.002 | 0.0003 ± 0.0003 | 0.003 ± 0.003 |
| 4′-Hydroxy-3′-methoxycinnamic acid (Ferulic acid, FA) | 0.005 ± 0.002 | 0.009 ± 0.003 | 0.011 ± 0.007 | 0.019 ± 0.007 |
| 4′-Hydroxycinnamic acid (Coumaric acid, CoA) | N.D. | 0.006 ± 0.004 | 0.002 ± 0.002 | 0.003 ± 0.001 |
| TOTAL—Intestinal metabolites | 0.009 ± 0.004 | 0.022 ± 0.007 | 0.017 ± 0.008 | 0.028 ± 0.009 |
| Colonic absorption | ||||
| 3-(3′,4′-Dihydroxyphenyl)propanoic acid (Dihydrocaffeic acid, DHCA) | 0.011 ± 0.007 | 0.005 ± 0.002 | 0.008 ± 0.006 | 0.0001 ± 0.0001 |
| 3-(4′-Hydroxy-3′-methoxyphenyl)propanoic acid (Dihydroferulic, DHFA) | 0.013 ± 0.004 | 0.018 ± 0.003 | 0.019 ± 0.007 | 0.014 ± 0.003 |
| 3-(4′-Hydroxyphenyl)propanoic acid (Dihydrocoumaric acid, DHCoA) | 0.005 ± 0.003 | 0.006 ± 0.006 | 0.005 ± 0.005 | 0.014 ± 0.005 |
| 3-(3′-Hydroxyphenyl)propanoic acid (Dihydroisocoumaric acid, DHiCoA) | 0.21 ± 0.07 | 0.4 ± 0.1 | 0.24 ± 0.07 | 0.25 ± 0.07 |
| 5-Dihydrocaffeoylquinic acid | 0.04 ± 0.01 | 0.06 ± 0.02 | 0.05 ± 0.01 | 0.08 ± 0.02 |
| TOTAL—Colonic metabolites | 0.28 ± 0.07 | 0.5 ± 0.1 | 0.33 ± 0.08 | 0.36 ± 0.08 |
| Other microbial metabolites | ||||
| 3′,4′-Dihydroxyphenylacetic acid | N.D. | N.D. | N.D. | 0.010 ± 0.008 |
| 4′-Hydroxy-3′-methoxyphenylacetic acid | 0.007 ± 0.005 | 0.0006 ± 0.0006 | 0.02 ± 0.01 | 0.003 ± 0.003 |
| 3′-Hydroxyphenylacetic acid | 0.31 ± 0.09 | 0.18 ± 0.08 | 0.21 ± 0.08 | 0.11 ± 0.04 |
| 3,4-Dihydroxybenzoic acid | 0.016 ± 0.006 * | 0.026 ± 0.008 * | 0.018 ± 0.005 | 0.04 ± 0.01 |
| 4-Hydroxy-3-methoxybenzoic acid | 0.06 ± 0.03 | 0.006 ± 0.006 | 0.09 ± 0.05 | N.D. |
| 4-Hydroxybenzoic acid | 0.004 ± 0.004 | 0.006 ± 0.004 | 0.0006 ± 0.0004 | 0.01 ± 0.01 |
| 3-Hydroxybenzoic acid | 0.03± 0.02 | 0.020 ± 0.008 | 0.03 ± 0.01 | 0.020 ± 0.007 |
| TOTAL—Other microbial metabolites | 0.4 ± 0.1 | 0.24 ± 0.09 | 0.4 ± 0.1 | 0.19 ± 0.07 |
| TOTAL Colonic + other microbial met. | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.7 ± 0.1 | 0.6 ± 0.1 |
| TOTAL INTESTINAL + COLONIC + OTHERS | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.7 ± 0.1 | 0.6 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seguido, M.Á.; Tarradas, R.M.; González-Rámila, S.; García-Cordero, J.; Sarriá, B.; Bravo-Clemente, L.; Mateos, R. Sustained Consumption of a Decaffeinated Green Coffee Nutraceutical Has Limited Effects on Phenolic Metabolism and Bioavailability in Overweight/Obese Subjects. Nutrients 2022, 14, 2445. https://doi.org/10.3390/nu14122445
Seguido MÁ, Tarradas RM, González-Rámila S, García-Cordero J, Sarriá B, Bravo-Clemente L, Mateos R. Sustained Consumption of a Decaffeinated Green Coffee Nutraceutical Has Limited Effects on Phenolic Metabolism and Bioavailability in Overweight/Obese Subjects. Nutrients. 2022; 14(12):2445. https://doi.org/10.3390/nu14122445
Chicago/Turabian StyleSeguido, Miguel Ángel, Rosa Maria Tarradas, Susana González-Rámila, Joaquín García-Cordero, Beatriz Sarriá, Laura Bravo-Clemente, and Raquel Mateos. 2022. "Sustained Consumption of a Decaffeinated Green Coffee Nutraceutical Has Limited Effects on Phenolic Metabolism and Bioavailability in Overweight/Obese Subjects" Nutrients 14, no. 12: 2445. https://doi.org/10.3390/nu14122445
APA StyleSeguido, M. Á., Tarradas, R. M., González-Rámila, S., García-Cordero, J., Sarriá, B., Bravo-Clemente, L., & Mateos, R. (2022). Sustained Consumption of a Decaffeinated Green Coffee Nutraceutical Has Limited Effects on Phenolic Metabolism and Bioavailability in Overweight/Obese Subjects. Nutrients, 14(12), 2445. https://doi.org/10.3390/nu14122445








