Oral Administration of East Asian Herbal Medicine for Inflammatory Skin Lesions in Plaque Psoriasis: A Systematic Review, Meta-Analysis, and Exploration of Core Herbal Materials
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.2.1. Type of Studies
2.2.2. Type of Participants
2.2.3. Type of Interventions
2.2.4. Type of Outcome Measures
2.2.5. Data Extraction
2.2.6. Methodological Quality Assessment
2.2.7. Statistical Analysis
Evidence Synthesis
Hierarchical Agglomerative Clustering
Social Network Analysis
2.2.8. Quality of Evidence According to Outcome Measurements
3. Results
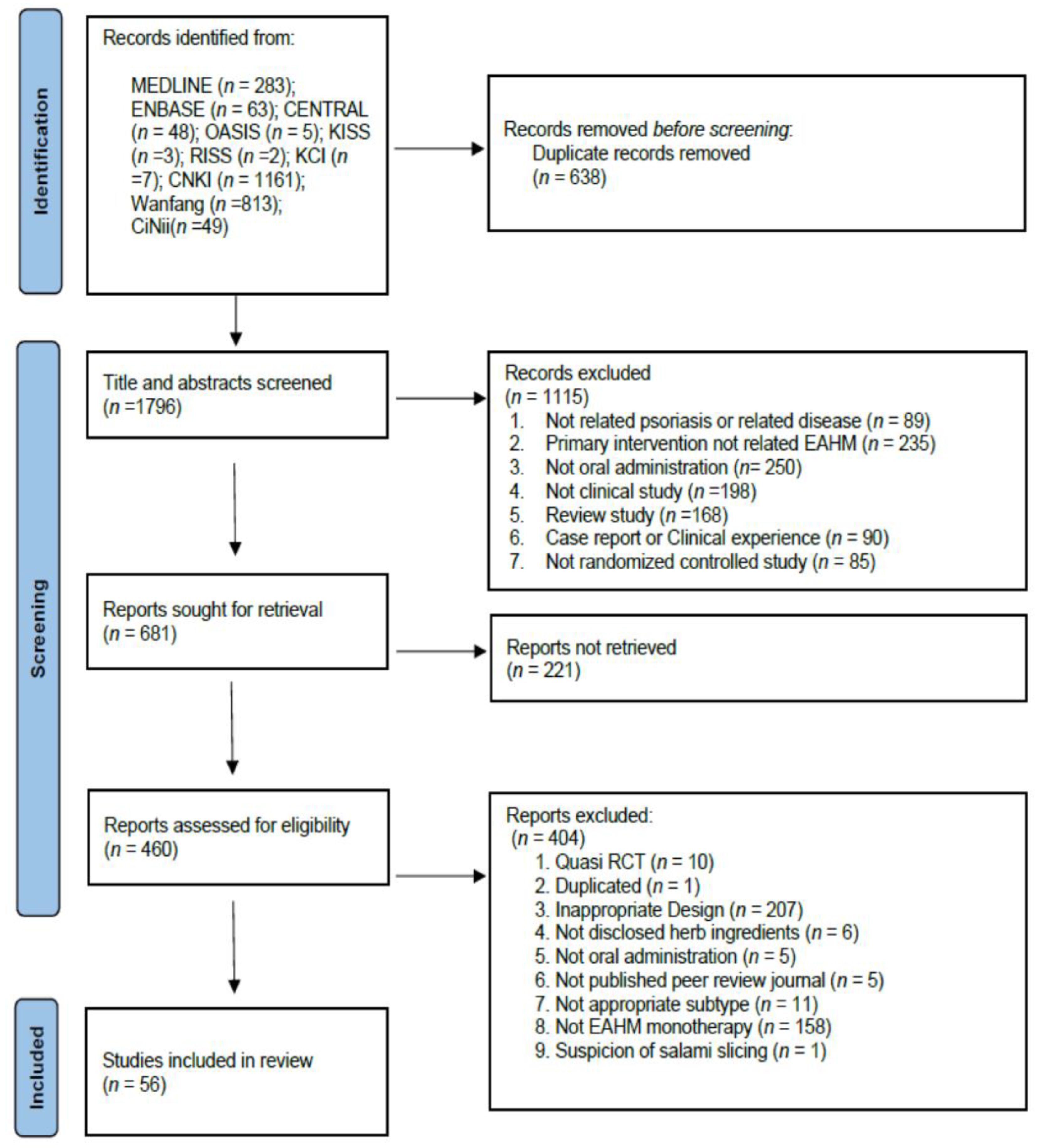
3.1. Study Identification
3.2. Study Characteristics
3.3. Risk of Bias
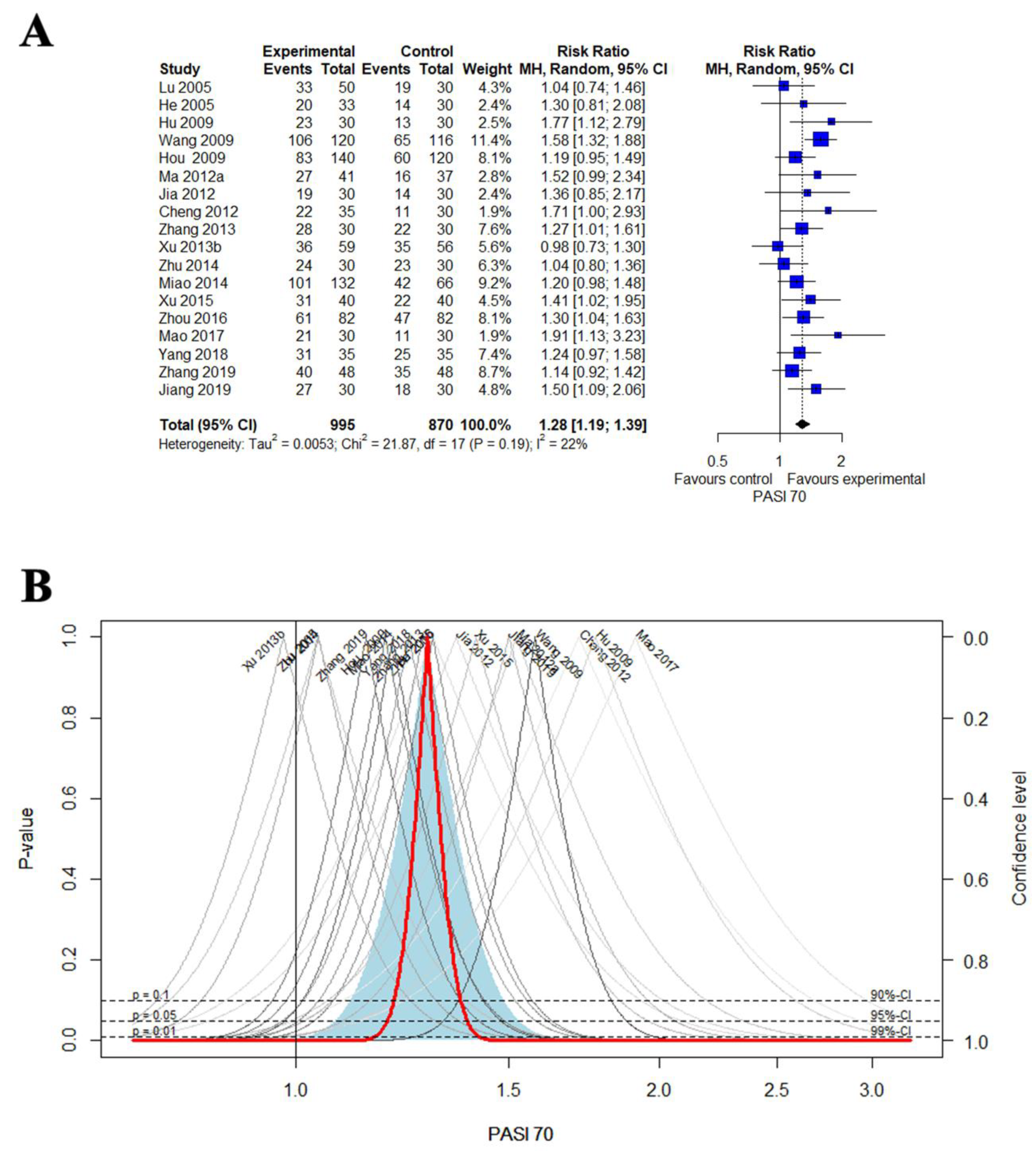
3.4. Primary Outcomes
3.4.1. PASI 70
3.4.2. PASI 60
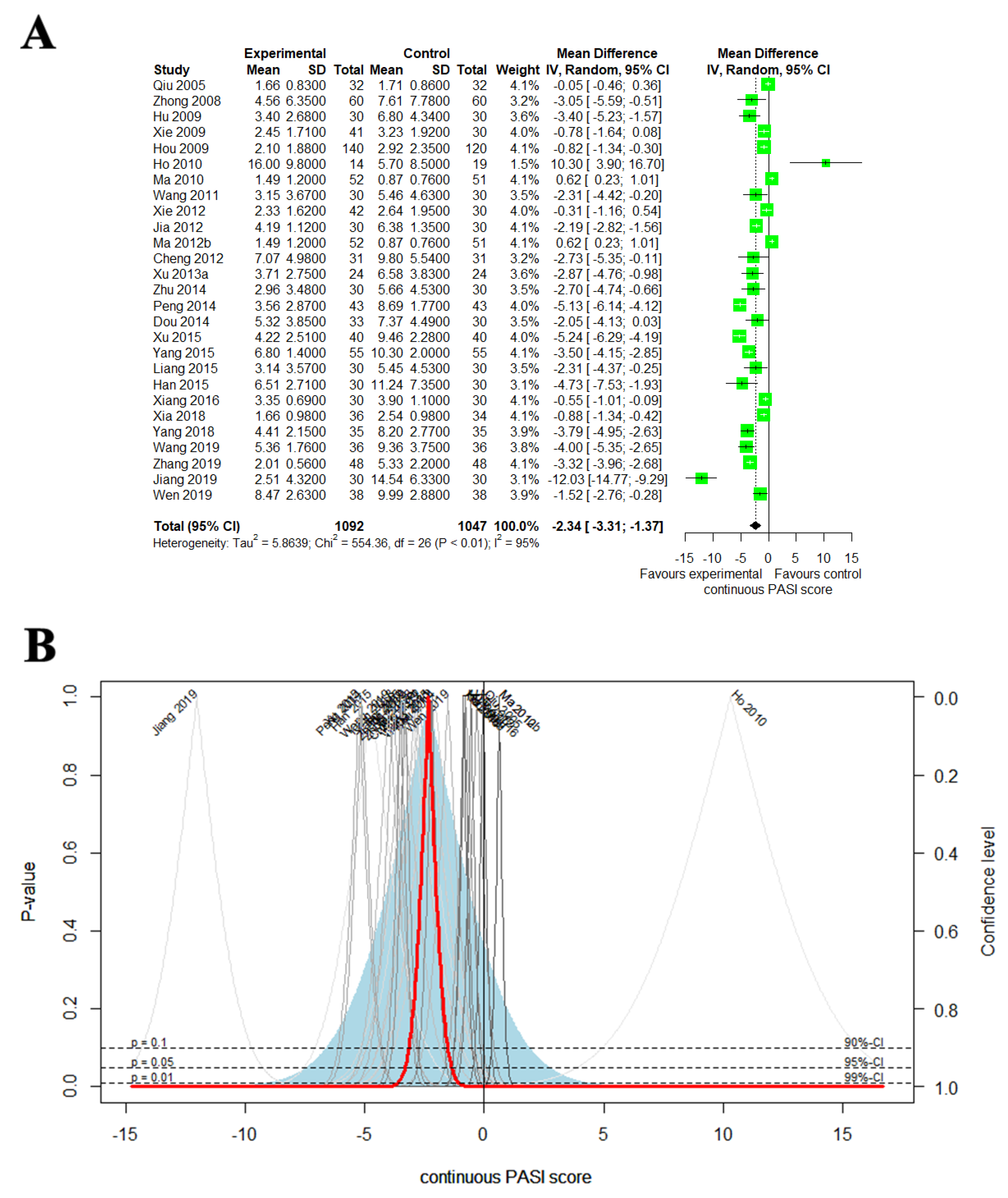
3.4.3. Continuous PASI Score
3.5. Secondary Outcomes
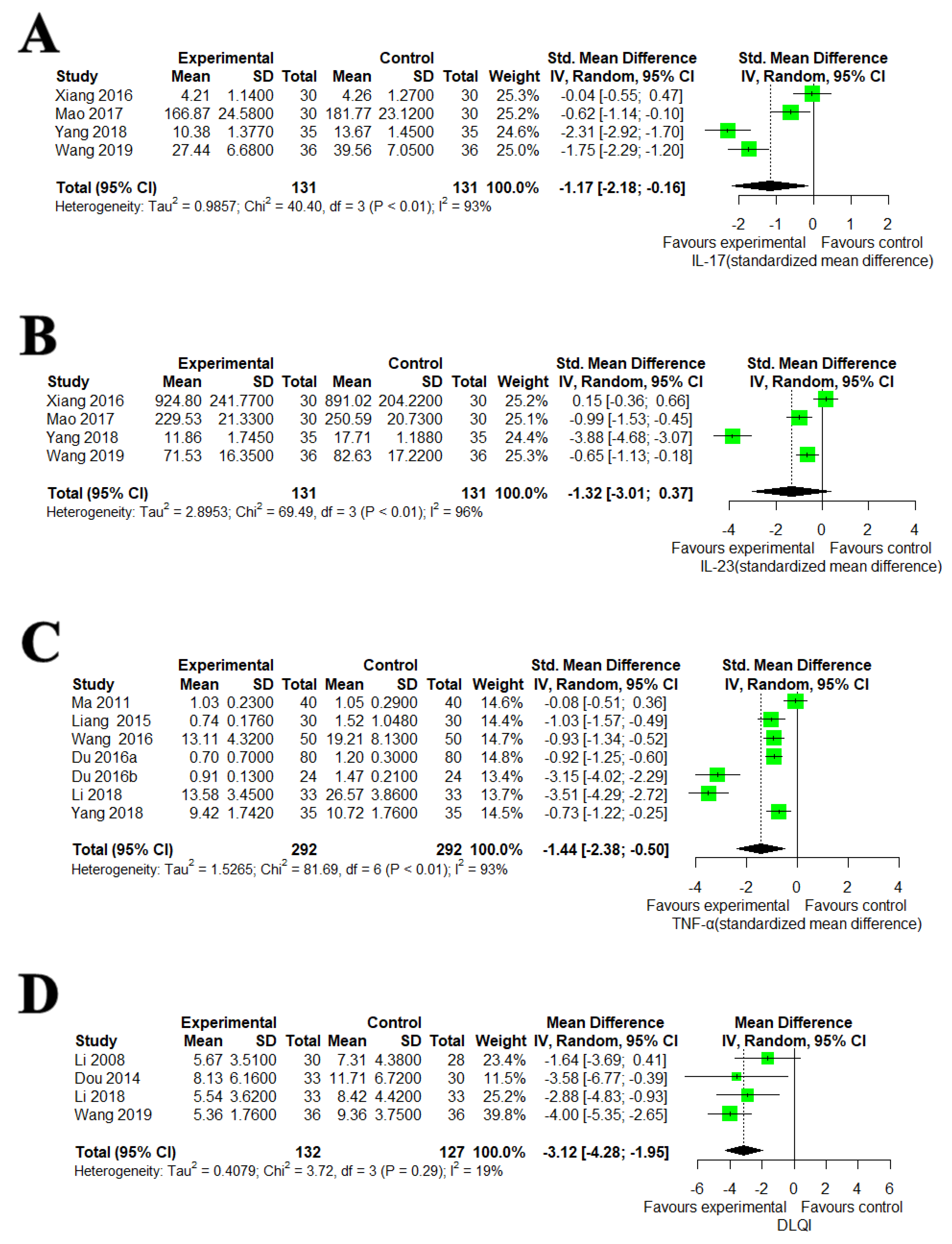
3.5.1. IL-17, IL-23, TNF-α and DLQI
3.5.2. AEs
3.6. Assessing Heterogeneity
3.6.1. Sensitivity Analysis
3.6.2. Meta-Regression and Subgroup Analysis
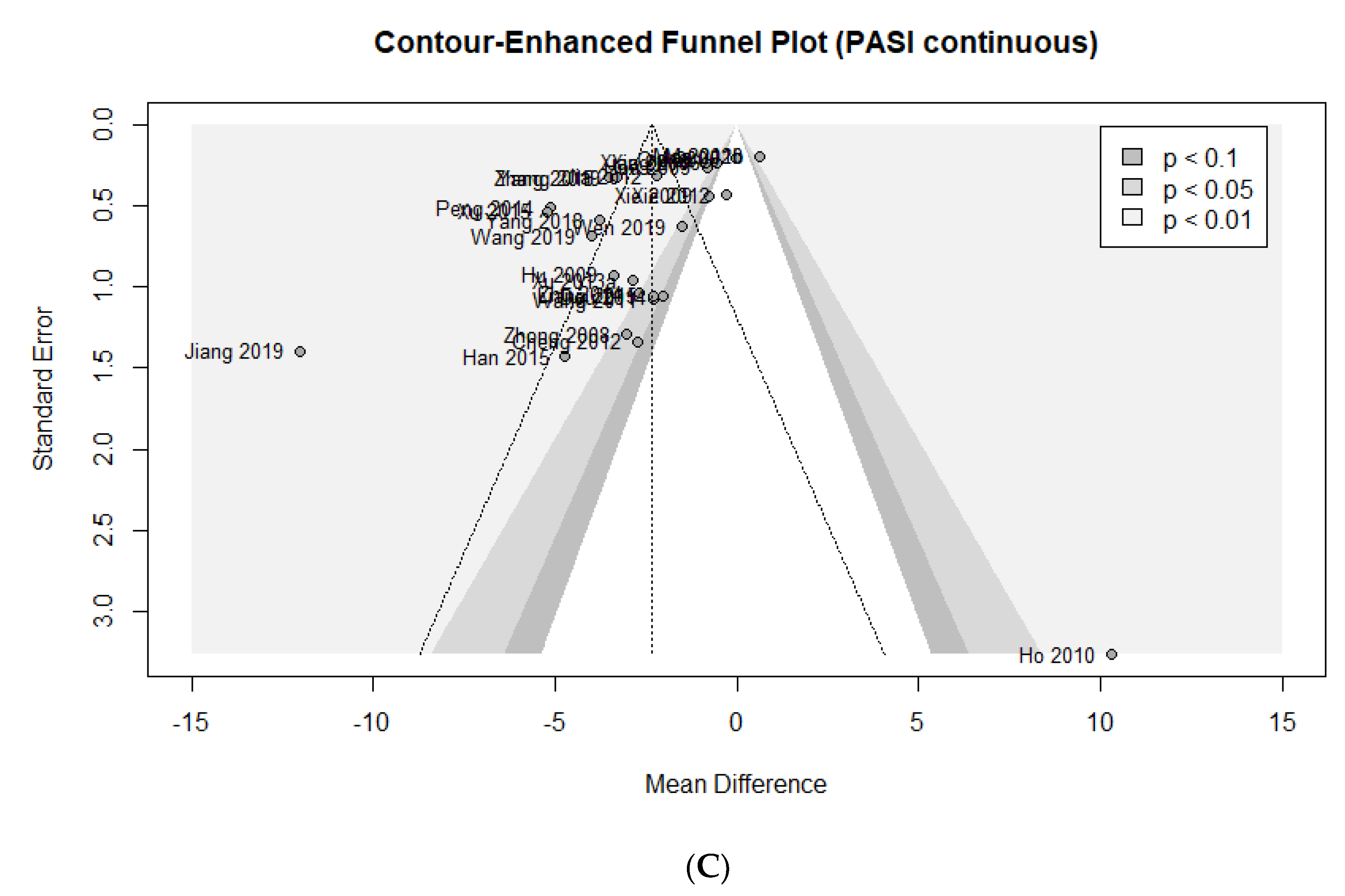
3.7. Assessing Publication Bias
3.8. Quality of Evidence According to Outcome Measures
3.9. Data Mining of EAHM Ingredients
3.9.1. Detailed Information and Distribution of EAHM Ingredients
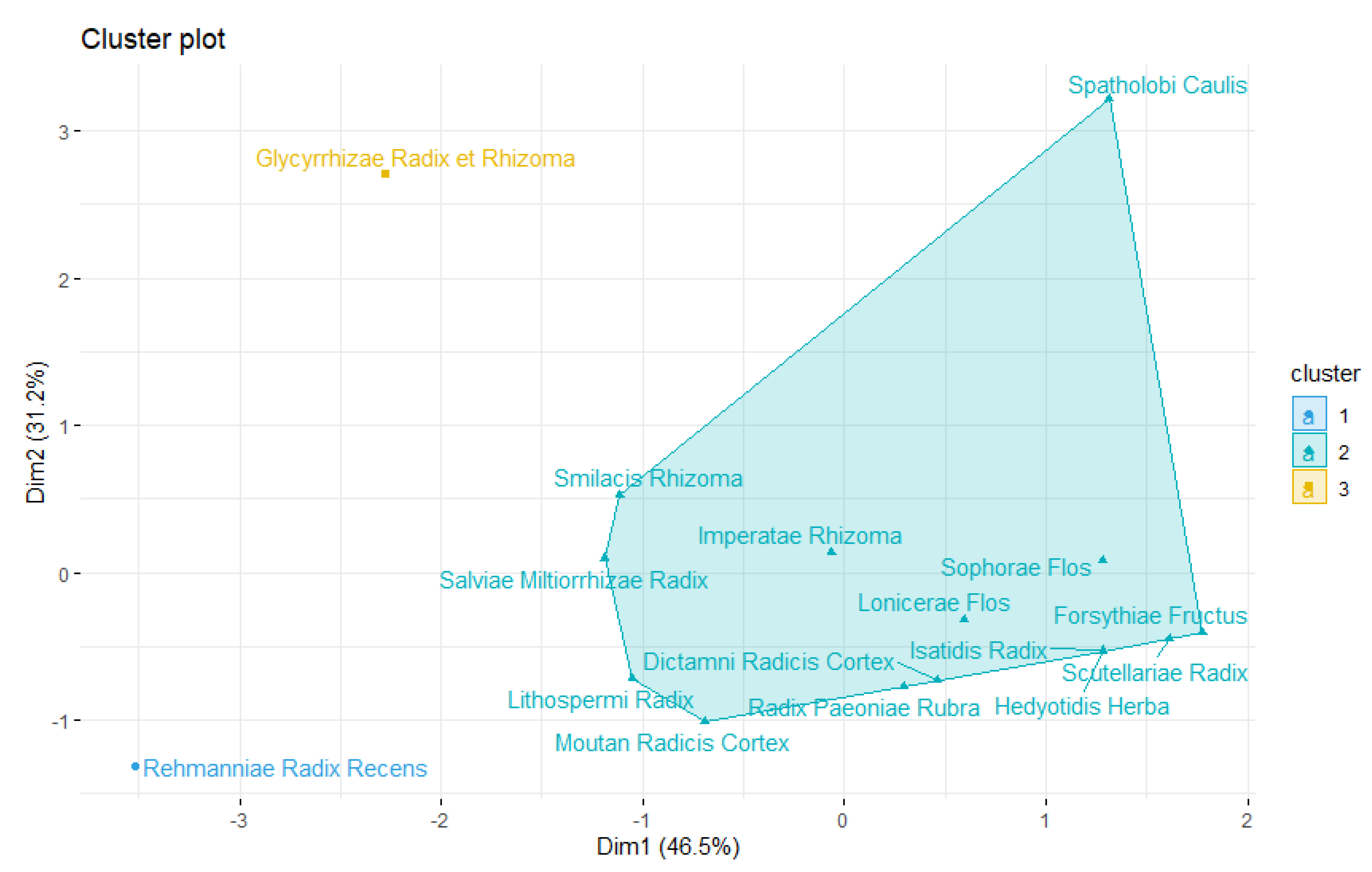
3.9.2. Hierarchical Agglomerative Clustering
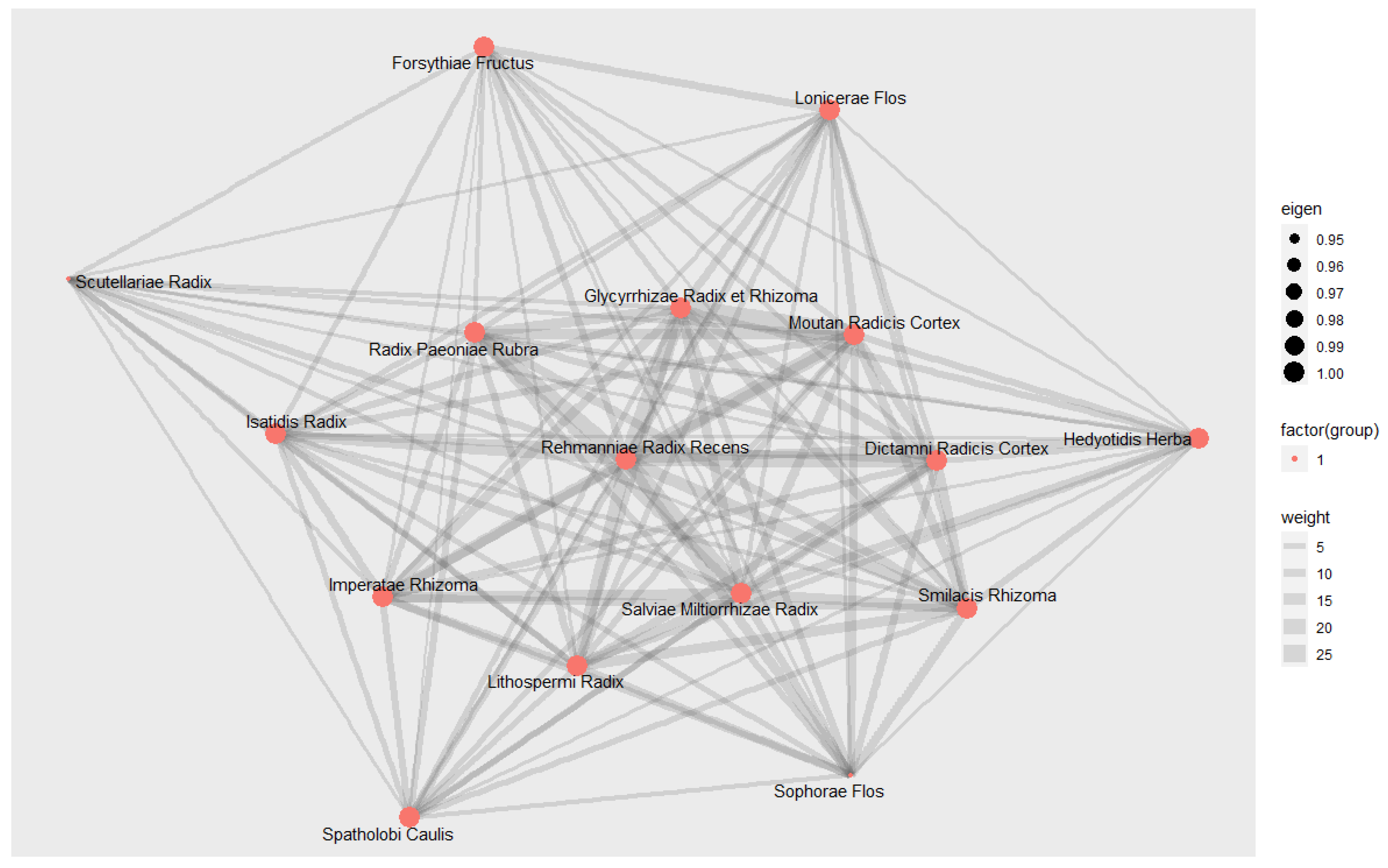
3.9.3. Social Network Analysis
4. Discussion
4.1. Summary of the Main Finding
4.2. Strength and Implications of Clinical Practice
4.3. Implications of Core Material Exploration
4.4. Limitations and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M. Global Psoriasis Atlas National, Regional, and Worldwide Epidemiology of Psoriasis: Systematic Analysis and Modelling Study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef] [PubMed]
- Mattei, P.L.; Corey, K.C.; Kimball, A.B. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): The Correlation between Disease Severity and Psychological Burden in Patients Treated with Biological Therapies. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 333–337. [Google Scholar] [CrossRef]
- Singh, S.; Taylor, C.; Kornmehl, H.; Armstrong, A.W. Psoriasis and Suicidality: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2017, 77, 425–440. [Google Scholar] [CrossRef]
- Ryan, C.; Sadlier, M.; De Vol, E.; Patel, M.; Lloyd, A.A.; Day, A.; Lally, A.; Kirby, B.; Menter, A. Genital Psoriasis Is Associated with Significant Impairment in Quality of Life and Sexual Functioning. J. Am. Acad. Dermatol. 2015, 72, 978–983. [Google Scholar] [CrossRef]
- Semenov, Y.R.; Herbosa, C.M.; Rogers, A.T.; Huang, A.; Kwatra, S.G.; Cohen, B.; Anadkat, M.J.; Silverberg, J.I. Psoriasis and Mortality in the United States: Data from the National Health and Nutrition Examination Survey. J. Am. Acad. Dermatol. 2021, 85, 396–403. [Google Scholar] [CrossRef]
- Amin, M.; Lee, E.B.; Tsai, T.-F.; Wu, J.J. Psoriasis and Co-Morbidity. Acta Derm. Venereol. 2020, 100, adv00033. [Google Scholar] [CrossRef]
- Korman, N.J. Management of Psoriasis as a Systemic Disease: What Is the Evidence? Br. J. Dermatol. 2020, 182, 840–848. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Nast, A.; Smith, C.; Spuls, P.I.; Avila Valle, G.; Bata-Csörgö, Z.; Boonen, H.; De Jong, E.; Garcia-Doval, I.; Gisondi, P.; Kaur-Knudsen, D.; et al. EuroGuiDerm Guideline on the Systemic Treatment of Psoriasis Vulgaris-Part 1: Treatment and Monitoring Recommendations. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2461–2498. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Puig, L.; Joshi, A.; Skup, M.; Williams, D.; Li, J.; Betts, K.A.; Augustin, M. Comparison of Biologics and Oral Treatments for Plaque Psoriasis: A Meta-Analysis. JAMA Dermatol. 2020, 156, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, N.E.G.; Nijhawan, R.I.; Weinberg, J.M. Acitretin. Dermatol. Ther. 2013, 26, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, H.; Liu, L. Side Effects of Methotrexate Therapy for Rheumatoid Arthritis: A Systematic Review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef]
- Yin, C.S.; Ko, S.-G. Introduction to the History and Current Status of Evidence-Based Korean Medicine: A Unique Integrated System of Allopathic and Holistic Medicine. Evid. Based Complement. Altern. Med. 2014, 2014, 740515. [Google Scholar] [CrossRef]
- Shim, J.-M.; Kim, J. Cross-National Differences in the Holistic Use of Traditional East Asian Medicine in East Asia. Health Promot. Int. 2018, 33, 536–544. [Google Scholar] [CrossRef]
- Shim, J.-M.; Lee, Y.-S. The Association between the Use of Biomedical Services and the Holistic Use of Traditional East Asian Medicine: A National Survey of Outpatients in South Korea. BMJ Open 2017, 7, e018414. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-M. The Relationship Between the Use of Complementary and Alternative Medicine and the Use of Biomedical Services: Evidence From East Asian Medical Systems. Asia Pac. J. Public Health 2016, 28, 51–60. [Google Scholar] [CrossRef]
- Kim, H.U.; Ryu, J.Y.; Lee, J.O.; Lee, S.Y. A Systems Approach to Traditional Oriental Medicine. Nat. Biotechnol. 2015, 33, 264–268. [Google Scholar] [CrossRef]
- Coyle, M.E.; Yu, J.J.; Zhang, A.L.; Jones, L.; Xue, C.C.; Lu, C. Patient Experiences of Using Chinese Herbal Medicine for Psoriasis Vulgaris and Chronic Urticaria: A Qualitative Study. J. Dermatol. Treat. 2020, 31, 352–358. [Google Scholar] [CrossRef]
- Weng, S.-W.; Chen, B.-C.; Wang, Y.-C.; Liu, C.-K.; Sun, M.-F.; Chang, C.-M.; Lin, J.-G.; Yen, H.-R. Traditional Chinese Medicine Use among Patients with Psoriasis in Taiwan: A Nationwide Population-Based Study. Evid. Based Complement. Altern. Med. 2016, 2016, 3164105. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, J.; Kuai, L.; Zhang, Y.; Ding, X.; Luo, Y.; Ru, Y.; Xing, M.; Li, H.; Sun, X.; et al. Chinese Herbal Medicine for Psoriasis: Evidence From 11 High-Quality Randomized Controlled Trials. Front. Pharmacol. 2020, 11, 599433. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.; Zhang, C.S.; Yu, J.J.; Lu, C.; Zhang, A.L.; Xue, C.C. Oral Chinese Herbal Medicine versus Placebo for Psoriasis Vulgaris: A Systematic Review. J. Dermatol. Treat. 2017, 28, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, Q.; Peng, L.; Qin, Y.; Jing, M.; Huang, D.; Guo, J.; Xiao, M.; Chen, M. Benefits and Safety of Chinese Herbal Medicine in Treating Psoriasis: An Overview of Systematic Reviews. Front. Pharmacol. 2021, 12, 680172. [Google Scholar] [CrossRef]
- Zhou, X.; Seto, S.W.; Chang, D.; Kiat, H.; Razmovski-Naumovski, V.; Chan, K.; Bensoussan, A. Synergistic Effects of Chinese Herbal Medicine: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2016, 7, 201. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yang, C.; Meng, S.; Li, M.; Wang, X.; Zhu, Y.; Kong, L.; Lv, W.; Qiao, H.; Sun, Y. Deciphering the Mechanism of Fang-Ji-Di-Huang-Decoction in Ameliorating Psoriasis-like Skin Inflammation via the Inhibition of IL-23/Th17 Cell Axis. J. Ethnopharmacol. 2021, 281, 114571. [Google Scholar] [CrossRef]
- Wu, M.; Deng, Y.; Li, S.; Chen, Y.; Guo, D.; Jin, X.; Xu, Q.; Li, B.; Li, F. The Immunoregulatory Effects of Traditional Chinese Medicine on Psoriasis via Its Action on Interleukin: Advances and Considerations. Am. J. Chin. Med. 2018, 46, 739–750. [Google Scholar] [CrossRef]
- Bae, K.-H.; Lee, Y.; Park, K.-H.; Yoon, Y.; Mun, S.; Lee, S. Perception of Cold and Heat Pattern Identification in Diseases: A Survey of Korean Medicine Doctors. Integr. Med. Res. 2017, 6, 26–32. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Y.; Tan, W.; Wu, X.; Chen, R.; Cao, J.; Chen, M.; Wang, Y. Compatibility Art of Traditional Chinese Medicine: From the Perspective of Herb Pairs. J. Ethnopharmacol. 2012, 143, 412–423. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Lortie, C.J.; Filazzola, A. A Contrast of Meta and Metafor Packages for Meta-Analyses in R. Ecol. Evol. 2020, 10, 10916–10921. [Google Scholar] [CrossRef]
- Rücker, G.; Schwarzer, G. Beyond the Forest Plot: The Drapery Plot. Res. Synth. Methods 2021, 12, 13–19. [Google Scholar] [CrossRef]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Contour-Enhanced Meta-Analysis Funnel Plots Help Distinguish Publication Bias from Other Causes of Asymmetry. J. Clin. Epidemiol. 2008, 61, 991–996. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yang, C.-W.; Lin, Y.-H.; Hsueh, J.-Y.; Chen, J.-L.; Yang, S.-H.; Chen, Y.-C.; Chen, H.-Y. Identifying the Chinese Herbal Medicine Network and Core Formula for Allergic Rhinitis on a Real-World Database. Evid. Based Complement. Altern. Med. 2020, 2020, 5979708. [Google Scholar] [CrossRef]
- Yang, D.H.; Kang, J.H.; Park, Y.B.; Park, Y.J.; Oh, H.S.; Kim, S.B. Association Rule Mining and Network Analysis in Oriental Medicine. PLoS ONE 2013, 8, e59241. [Google Scholar] [CrossRef][Green Version]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Yan, W.; Gao, Z.; Wang, F. Clinical Effects of Quyin Decoction on Treating Psoriasis Vulgaris and Detection of Peripheral Blood Th17 Cell Quantity and Related Cytokines. Chin. J. Derm. Venerol. Integ. Trad. W Med. 2010, 9, 149–151. [Google Scholar]
- Zhou, C. Clinical Study on Yuyin Capsule in Treating Psoriasis Vulgaris. New J. Tradit. Chin. Med. 2002, 34, 27–28. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, M. Clinical Observation on 30 Cases of Psoriasis Vulgaris Treated with Traditional Chinese Medicine Xiaoyin Decoction. Inf. Tradit. Chin. Med. 2003, 20, 47. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S. Observation on the Curative Effect of Compound Qingdai Capsule in the Treatment of Psoriasis Vulgaris and Its Effect on Serum IL-2 and IL-8. J. Chin. Med. Mater. 2004, 27, 885–886. [Google Scholar] [CrossRef]
- Lu, T. Clinical Observation of Yinxieling Capsule on Patients with Psoriasis Vulgaris. Hebei J. TCM 2005, 27, 655–656. [Google Scholar]
- Liu, X. Efficacy Observation of Traditional Chinese Medicine Jiedu Liangxue Decoction Combined with Acitretin in the Treatment of Psoriasis Vulgaris. J. Pract. Med. Tech. 2005, 12, 3507–3508. [Google Scholar]
- He, Y. Curative Effect of Antidote Decoction in Treating Psoriasis Vulgaris 63 Cases. Mod. Hosp. 2005, 5, 31–32. [Google Scholar]
- Qiu, Y.; Tan, S.; Sun, Z.; Zhang, J.; Yuan, J.; Liu, P. Clinical Study on the Treatment of Psoriasis Vulgaris with Blood Stasis Syndrome with Huoxue Sanyu Xiaoyin Decoction. J. Chin. Med. Mater. 2005, 28, 442–444. [Google Scholar] [CrossRef]
- Li, C. Observation of Curative Effect of Traditional Chinese Medicine Combined with Narrow-Spectrum UV Rays in the Treatment of Blood-Heat Type Psoriasis Vulgaris. J. Emerg. Tradit. Chin. Med. 2006, 15, 730–731. [Google Scholar]
- Li, F.; Li, B.; Xu, R.; Song, X.; Yu, Y.; Xu, Z. Qinzhu Liangxue Decoction in Treatment of Blood-Heat Type Psoriasis Vulgaris: A Randomized Controlled Trial. J. Chin. Integr. Med. 2008, 6, 586–590. [Google Scholar] [CrossRef]
- Ye, Z. Clinical Observation on Treatment of 56 Cases of Psoriasis Vulgaris with Zhixue Jiedu Xiaoyin Decoction. Guid. J. Tradit. Chin. Med. Pharm. 2008, 14, 60–61. [Google Scholar] [CrossRef]
- Zhong, J.; Li, Y.; He, W.; Li, Y.; Liang, Y. Observation on the Curative Effect of “Xiaoyin Granule” in the Treatment of 60 Cases of White Blood Deficiency and Wind-Dryness Type. Gansu J. TCM 2008, 21, 28–29. [Google Scholar]
- Hu, A. Clinical Observation on 30 Cases of Psoriasis Vulgaris Treated by Liangxue Decoction. Guid. J. Tradit. Chin. Med. Pharm. 2009, 15, 41–42, 47. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, Z. Therapeutic Observation on 120 Cases of Psoriasis Treated by Self-Made Baiji Decoction. Chin. Community Dr. 2009, 11, 156–157. [Google Scholar] [CrossRef]
- Feng, C. Clinical Observation of Self-Made Wushe Decoction in the Treatment of Psoriasis. J. Sichuan Tradit. Chin. Med. 2009, 27, 101–102. [Google Scholar]
- Xie, S.; Yi, X.; Yang, L.; Li, Y. Clinical Observation of Kangyin 1 Decoction on 41 Cases of Psoriasis Vulgaris. Hebei J. Tradit. Chin. Med. 2009, 31, 173–175. [Google Scholar]
- Hou, L. Clinical Observation on the Treatment of Psoriasis Vulgaris with Modified Huoxueliangxue Decoction. J. Liaoning Univ. Tradit. Chin. Med. 2009, 11, 132–133. [Google Scholar] [CrossRef]
- Ho, S.G.Y.; Yeung, C.K.; Chan, H.H.L. Methotrexate versus Traditional Chinese Medicine in Psoriasis: A Randomized, Placebo-Controlled Trial to Determine Efficacy, Safety and Quality of Life. Clin. Exp. Dermatol. 2010, 35, 717–722. [Google Scholar] [CrossRef]
- Si, X.; Tan, J.; Li, B. Observation on the Clinical Efficacy of Jiawei Xiaoyaosan in the Treatment of Psoriasis Vulgaris. Nei Mong. J. Tradit. Chin. Med. 2010, 29, 12–13. [Google Scholar] [CrossRef]
- Ma, W.; Qu, Y.; Pan, H.; Jiang, S. Observation on the Curative Effect of Psoriasis Prescription on 52 Cases of Psoriasis Vulgaris with Blood Heat Syndrome. J. New Chin. Med. 2010, 42, 68–70. [Google Scholar] [CrossRef]
- Ma, X.; Chen, H.; Zhang, P.; Yun, Y.; Wang, J. Effects of KeyinⅠRecipe on IL-2, IL-2R and TNF-α in Serum of Psoriasis. Hebei J. Tradit. Chin. Med. 2011, 33, 1617–1619. [Google Scholar]
- Wang, W.; Liang, Y.; Yan, Z. Clinical Report on 30 Cases of Psoriasis Vulgaris Treated by Tufuling Qingdai Decoction. Liaoning J. Tradit. Chin. Med. 2011, 38, 1135–1136. [Google Scholar] [CrossRef]
- Xie, S.; Wang, Q.; Song, X.; Zhang, L. Clinical Observation of Liangxie Runfu Decoction on 42 Cases of Psoriasis Vulgaris. Chin. J. Derm. 2012, 26, 743–744. [Google Scholar]
- Jia, M.; He, A.; Xia, G.; Tang, T. Observation of Curative Effect of Xiaobi Decoction in Treating Psoriasis Vulgaris. J. Guiyang Coll. Tradit. Chin. Med. 2012, 34, 33–35. [Google Scholar]
- Cheng, Y.; Ding, L. Clinical Observation on Children’s Psoriasis Vulgaris Treated with Traditional Chinese Medicine. Mod. J. Integr. Tradit. Chin. West. Med. 2012, 21, 167–168. [Google Scholar]
- Ma, M.; Ma, H. Clinical Observation on 41 Cases of Acute Psoriasis Treated by Liangxuejiedu Decoction. J. Emerg. Tradit. Chin. Med. 2012, 21, 968. [Google Scholar]
- Ma, W.; Qu, Y.; Pan, H.; Jiang, S. Clinical Observation of Yinxiebingfang Decoction in the Treatment of 52 Cases with Psoriasis Vulgaris Blood-Heat Syndrome and the Effects on Hemorheology. J. Diagn. Derm. Venereol. 2012, 19, 272–275. [Google Scholar]
- Zhang, L.; Wang, L.; Liu, Y. Clinical Observation on the Curative Effect of Blood Cooling Decoction in the Treatment of White Bi Blood- Heat Syndrome. Jilin J. Tradit. Chin. Med. 2013, 33, 269–270. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, J. Clinical Observation of Wanji Decoction in Treating Psoriasis Vulgaris with Blood Heat Intrinsic Type. Heilongjiang J. Tradit. Chin. Med. 2013, 22–23. [Google Scholar]
- Xu, B.; Zeng, X. 24 Cases of Psoriasis Treated by Self-Made Shufeng Yangxue Decoction. Hunan J. Tradit. Chin. Med. 2013, 29, 56–57. [Google Scholar] [CrossRef]
- Xu, M. Clinical Curative Effect Observation of Qingre Liangxue Decoction in Treating Psoriasis Vulgaris with Blood-Heat Syndrome. J. Liaoning Univ. Tradit. Chin. Med. 2013, 15, 177–178. [Google Scholar] [CrossRef]
- Zhu, F.; Zhu, L.; Li, Y.; Luan, L.; Chen, L. Clinical Observation on 30 Cases with Blood Stasis Type Psoriasis Dahuang Zhechong Capsule. Chin. J. Derm. Venerol. 2014, 28, 195–196. [Google Scholar] [CrossRef]
- Chen, M.; Tang, Y. Clinical Observation on 30 Cases of Blood Heat Type Psoriasis Treated by Liangxue No.1 Formula. Hunan J. Tradit. Chin. Med. 2014, 30, 77–78. [Google Scholar] [CrossRef]
- Qian, W. Clinical Value Analysis of Liangxue Runfu Decoction in the Treatment of Psoriasis Vulgaris. Shenzhen J. Integr. Tradit. Chin. West. Med. 2014, 24, 166–167. [Google Scholar] [CrossRef]
- Peng, X. Clinical Observation on 43 Cases of Psoriasis Treated by Liangxuejiedu Decoction. Chin. J. Ethnomed. Ethnopharm. 2014, 58, 60. [Google Scholar]
- Dou, H.; Yang, B.; Liu, L.; Wang, D.; Li, W.; Zhou, G. Blood Stasis and Wind Dry-Type Psoriasis Clinical Studies Wuteng Xiaoyin Feng Treatment. China J. Chin. Med. 2014, 29, 761–763. [Google Scholar] [CrossRef]
- Miao, S. Clinical Observation on 198 Cases of Psoriasis Vulgaris Treated by Self-Made Quyin Decoction. Strait Pharm. J. 2014, 26, 95–97. [Google Scholar]
- Xu, R. Clinical Curative Effect of Senile Vulgaris Liangxue Jiedu Decoction in Treating Psoriasis and Effect on Serum Interleukin Levels. J. Liaoning Univ. Tradit. Chin. Med. 2015, 17, 169–172. [Google Scholar] [CrossRef]
- Zhang, T.; Jia, Z. To Observe the Effect of Nourishing Yin and Zinyin Qingre Xiaofeng San Efficacy and Safety in the Treatment of Psoriasis Vulgaris. Clin. J. Chin. Med. 2015, 7, 89–90. [Google Scholar] [CrossRef]
- Yang, F. Clinical Curative Effect Observation of Qingre Liangxue Decoction in Treating Psoriasis Vulgaris With Blood-Heat Syndrome. China Health Stand. Manag. 2015, 6, 123–124. [Google Scholar]
- Liang, Y.; Wang, W.; Yi, J.; Li, Y.; Qiu, L. Clinical Observation of Tufuling Qingdai Decoction in the Treatment of Psoriasis Vulgaris (Blood Heat Type) and Detection of TNF-α and VEGF Levels. Guangming J. Chin. Med. 2015, 30, 2132–2134. [Google Scholar] [CrossRef]
- Han, K.; Dai, Y.; Li, Y.; Deng, H.; Zeng, H.; Xie, Z.; Pan, Q.; Xing, Y. Serum TGF-Β1, MMP-9 and TIMP-1 Change in Patients with Pustules Psoriasis after Treatment of Huayinjiedu Decoction. J. Hainan Med. Univ. 2015, 21, 1140–1142,1145. [Google Scholar] [CrossRef]
- Xiang, Y.; Fan, B.; Xu, B.; Xu, W.; Gu, D.; Zhou, M. Influences of Qinzhu Liangxue Decoction on Serum IL-17, IL-23 and Related Transcription Factors of Skin Lesion in Patients with Psoriasis of Blood-Heat Syndrome. J. Nanjing Univ. Tradit. Chin. Med. 2016, 32, 326–329. [Google Scholar] [CrossRef]
- Wang, L. Observation on Curative Effect of Liangxue Runfu Decoction in Treating Psoriasis Vulgaris. Contemp. Med. 2016, 22, 155–156. [Google Scholar] [CrossRef]
- Zhou, L. Clinical Study on 82 Cases of Psoriasis Vulgaris Treated with Shufengjiedu Capsules. Contemp. Med. 2016, 22, 154–155. [Google Scholar] [CrossRef]
- Du, X.; Yang, X.; Fu, Y.; Li, L.; Jiang, G.; Xiao, J.; Gong, A. Clinical Effect of Shengdi Baimao Decoction on Blood Heat Type Psoriasis and Its Effect on the Expression Levels of Serum Interleukin-6 and Tumor Necrosis Factor-α. Glob. Tradit. Chin. Med. 2016, 9, 865–867. [Google Scholar]
- Du, J. Effect of Heat-Clearing and Detoxicating Oral Liquid Combined with Acitretin Capsule and Safety in Patients with Refractory Psoriasis. Chin. Arch. Tradit. Chin. Med. 2016, 34, 1517–1519. [Google Scholar] [CrossRef]
- Mao, R.; Zhang, G.; Ma, X. Evaluation of Miao Medicine Xiaoyinfang Combined with Calcipotriol Ointment in Treatment of Psoriasis Vulgaris and Its Influence on Th17 and Cytokines. Med. Forum 2017, 21, 3313–3315. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, Q.; Tan, C. Clinical Observation on the Treatment of Flaming and Soil Coke Syndrome of Psoriasis by Kanli-Fang. Lishizhen Med. Mater. Med. Res. 2018, 29, 1897–1898. [Google Scholar] [CrossRef]
- Li, Y. Study on the Effect and Mechanism of Jianpi Jiedu Decoction in the Treatment of Psoriasis Due to Spleen Deficiency and Dampness. J. Dermatol. Venereol. 2018, 40, 844–845. [Google Scholar]
- Yang, R.; Yang, B.; Wang, W.; Yang, J.; Guo, J. Effects of Jinyu Xiaoyin Granules on Serum Levels of IL-23, IL-17, IL-22 and TNF-α in Patients with Plaque Psoriasis. J. Ningxia Med. Univ. 2018, 40, 1228–1231. [Google Scholar] [CrossRef]
- Wang, L.; Fang, Y.; Zhou, G.; Wu, P.; Li, Q.; Geng, Q. Study on Clinical Efficacy and Mechanism of Qingying Tang for Treating Psoriatic Blood-Heat Syndrome Based on IL-23/Th17. China J. Chin. Mater. Med. 2019, 44, 175–180. [Google Scholar] [CrossRef]
- Zhang, X. Clinical Effect of Xijiao Dihuang Decoction Combined with Yinqiao Powder in the Treatment of Psoriasis Vulgaris. Clin. Res. Pract. 2019, 4, 110–111. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, K.; Liu, A. Clinical Observation of Xijiao Dihuang Jiedu Decoction in Treating 30 Cases of Guttate Psoriasis. Chin. J. Ethnomed. Ethnopharm. 2019, 28, 95–97. [Google Scholar]
- Wen, C.; Zhang, S.; Jia, M. Clinical Efficacy of Xiaobi Decoction for the Patients in Progressive Stage with Psoriasis Vulgaris. Guizhou Med. J. 2019, 43, 196–198. [Google Scholar]
- Chen, X.; Su, M.; He, J. Clinical Effect of Quyin Decoction in the Treatment of Blood-Heat Psoriasis Vulgaris. Nei Mong. J. Tradit. Chin. Med. 2020, 39, 18–19. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Niu, W.; Olaleye, O.E.; Du, F.-F.; Wang, F.-Q.; Huang, Y.-H.; Yuan, L.; Li, Y.-F.; Liu, G.-P.; Xu, F.; et al. Comparison of Intramuscular and Intravenous Pharmacokinetics of Ginsenosides in Humans after Dosing XueShuanTong, a Lyophilized Extract of Panax Notoginseng Roots. J. Ethnopharmacol. 2020, 253, 112658. [Google Scholar] [CrossRef] [PubMed]
- Wei-Ya, C.; Fei-Fei, Y.; Cui, L.; Wen-Hui, L.; Jie, H.; Yong-Hong, L. Comparison of Plasma and Pulmonary Availability of Chlorogenic Acid, Forsythiaside A and Baicalin after Intratracheal and Intravenous Administration of Shuang-Huang-Lian Injection. J. Ethnopharmacol. 2021, 274, 114082. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhu, S.-J.; Shen, C.; Zhai, R.; Li, D.-D.; Fang, M.; Xu, J.-N.; Gan, Y.-N.; Yang, L.; Ren, Z.-Y.; et al. Clinical Application of Chinese Herbal Injection for Cancer Care: Evidence-Mapping of the Systematic Reviews, Meta-Analyses, and Randomized Controlled Trials. Front. Pharm. 2021, 12, 666368. [Google Scholar] [CrossRef]
- Lu, P.; Xing, Y.; Xue, Z.; Ma, Z.; Zhang, B.; Peng, H.; Zhou, Q.T.; Liu, H.; Liu, Z.; Li, J. Pharmacokinetics of Salvianolic Acid B, Rosmarinic Acid and Danshensu in Rat after Pulmonary Administration of Salvia Miltiorrhiza Polyphenolic Acid Solution. Biomed. Chromatogr. 2019, 33, e4561. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Topically Used Herbal Products for the Treatment of Psoriasis-Mechanism of Action, Drug Delivery, Clinical Studies. Planta Med. 2016, 82, 1447–1455. [Google Scholar] [CrossRef]
- Zhang, C.S.; Yang, L.; Zhang, A.L.; May, B.H.; Yu, J.J.; Guo, X.; Lu, C.; Xue, C.C. Is Oral Chinese Herbal Medicine Beneficial for Psoriasis Vulgaris? A Meta-Analysis of Comparisons with Acitretin. J. Altern. Complement. Med. 2016, 22, 174–188. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, J.A.; Alraek, T.; Bian, Z.-X.; Birch, S.; Goto, H.; Jung, J.; Kao, S.-T.; Moon, S.-K.; Park, B.; et al. Current Research and Future Directions in Pattern Identification: Results of an International Symposium. Chin. J. Integr. Med. 2016, 22, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Lu, C.; Zhang, C.; Yang, J.; Tan, Y.; Lu, A.; Chan, K. Syndrome Differentiation in Modern Research of Traditional Chinese Medicine. J. Ethnopharmacol. 2012, 140, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Li, L.; Wang, M.; Niu, X.; Zhan, J.; He, X.; Yu, C.; Jiang, M.; Lu, A. Molecular Network and Chemical Fragment-Based Characteristics of Medicinal Herbs with Cold and Hot Properties from Chinese Medicine. J. Ethnopharmacol. 2013, 148, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Motoo, Y.; Arai, I.; Tsutani, K. Use of Kampo Diagnosis in Randomized Controlled Trials of Kampo Products in Japan: A Systematic Review. PLoS ONE 2014, 9, e104422. [Google Scholar] [CrossRef]
- Park, S.-M.; Baek, S.-J.; Ban, H.-J.; Jin, H.-J.; Cha, S. Systematic Analysis of the Molecular Mechanisms of Cold and Hot Properties of Herbal Medicines. Plants 2022, 11, 997. [Google Scholar] [CrossRef]
- Liu, J.; Feng, W.; Peng, C. A Song of Ice and Fire: Cold and Hot Properties of Traditional Chinese Medicines. Front. Pharm. 2020, 11, 598744. [Google Scholar] [CrossRef]
- Muluye, R.A.; Bian, Y.; Alemu, P.N. Anti-Inflammatory and Antimicrobial Effects of Heat-Clearing Chinese Herbs: A Current Review. J. Tradit. Complement. Med. 2014, 4, 93–98. [Google Scholar] [CrossRef]
- Guan, F.; Lam, W.; Hu, R.; Kim, Y.K.; Han, H.; Cheng, Y.-C. Majority of Chinese Medicine Herb Category “Qing Re Yao” Have Multiple Mechanisms of Anti-Inflammatory Activity. Sci. Rep. 2018, 8, 7416. [Google Scholar] [CrossRef]
- Sui, Z.; Li, L.; Liu, B.; Gu, T.; Zhao, Z.; Liu, C.; Shi, C.; Yang, R. Optimum Conditions for Radix Rehmanniae Polysaccharides by RSM and Its Antioxidant and Immunity Activity in UVB Mice. Carbohydr. Polym. 2013, 92, 283–288. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, D.; Lou, H.; Sun, L.; Ji, J. Evaluation of the Anti-Inflammatory Activities of Tanshinones Isolated from Salvia Miltiorrhiza Var. Alba Roots in THP-1 Macrophages. J. Ethnopharmacol. 2016, 188, 193–199. [Google Scholar] [CrossRef]
- Yu, H.; Li, H.; Li, Y.; Li, M.; Chen, G. Effect of Isoliquiritigenin for the Treatment of Atopic Dermatitis-like Skin Lesions in Mice. Arch. Dermatol. Res. 2017, 309, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.-S.; Choi, Y.-G.; Jeong, M.-Y.; Lee, J.-H.; Lim, S. Moutan Cortex Radicis Inhibits Inflammatory Changes of Gene Expression in Lipopolysaccharide-Stimulated Gingival Fibroblasts. J. Nat. Med. 2013, 67, 576–589. [Google Scholar] [CrossRef]
- Kim, E.K.; Kim, E.-Y.; Moon, P.-D.; Um, J.-Y.; Kim, H.-M.; Lee, H.-S.; Sohn, Y.; Park, S.K.; Jung, H.-S.; Sohn, N.-W. Lithospermi Radix Extract Inhibits Histamine Release and Production of Inflammatory Cytokine in Mast Cells. Biosci. Biotechnol. Biochem. 2007, 71, 2886–2892. [Google Scholar] [CrossRef] [PubMed]
- Ki, N.Y.; Park, E.-J.; Sung, I.S.; Ju, S.A.; Kim, K.U.; Kim, M.R.; Song, D.Y.; Lee, M.-J.; Kim, H.-S.; Kang, B.-H.; et al. The Hot-Water Extract of Smilacis Chinae Rhizome Suppresses 2,4-Dinitrochlorobenzene and House Dust Mite-Induced Atopic Dermatitis-Like Skin Lesions in Mice. Phytother. Res. 2016, 30, 636–645. [Google Scholar] [CrossRef]
- Zhao, J.; Di, T.; Wang, Y.; Wang, Y.; Liu, X.; Liang, D.; Li, P. Paeoniflorin Inhibits Imiquimod-Induced Psoriasis in Mice by Regulating Th17 Cell Response and Cytokine Secretion. Eur. J. Pharmacol. 2016, 772, 131–143. [Google Scholar] [CrossRef]
- Yang, B.; Lee, H.-B.; Kim, S.; Park, Y.C.; Kim, K.; Kim, H. Decoction of Dictamnus Dasycarpus Turcz. Root Bark Ameliorates Skin Lesions and Inhibits Inflammatory Reactions in Mice with Contact Dermatitis. Pharmacogn. Mag. 2017, 13, 483–487. [Google Scholar] [CrossRef]
- Ruan, J.-Y.; Cao, H.-N.; Jiang, H.-Y.; Li, H.-M.; Hao, M.-M.; Zhao, W.; Zhang, Y.; Han, Y.; Zhang, Y.; Wang, T. Structural Characterization of Phenolic Constituents from the Rhizome of Imperata Cylindrica Var. Major and Their Anti-Inflammatory Activity. Phytochemistry 2022, 196, 113076. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y.; Li, Y.; Li, C. Total Flavonoids of Hedyotis Diffusa Willd Inhibit Inflammatory Responses in LPS-Activated Macrophages via Suppression of the NF-ΚB and MAPK Signaling Pathways. Exp. Ther. Med. 2016, 11, 1116–1122. [Google Scholar] [CrossRef]
- Fan, Z.; Cai, L.; Wang, Y.; Zhu, Q.; Wang, S.; Chen, B. The Acidic Fraction of Isatidis Radix Regulates Inflammatory Response in LPS-Stimulated RAW264.7 Macrophages through MAPKs and NF-ΚB Pathway. Evid. Based Complement. Altern. Med. 2021, 2021, 8879862. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Chen, Y.-J.; Bai, L.; Liu, Y.-X.; Fu, X.-Q.; Zhu, P.-L.; Li, J.-K.; Chou, J.-Y.; Yin, C.-L.; Wang, Y.-P.; et al. Chrysoeriol Ameliorates TPA-Induced Acute Skin Inflammation in Mice and Inhibits NF-ΚB and STAT3 Pathways. Phytomedicine 2020, 68, 153173. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, H.S.; Kim, Y.J.; Kim, J.S.; Park, Y.S.; Kang, J.S.; Yuk, D.Y.; Hong, J.T.; Kim, Y.; Han, S.-B. Sophoricoside Isolated from Sophora Japonica Ameliorates Contact Dermatitis by Inhibiting NF-ΚB Signaling in B Cells. Int. Immunopharmacol. 2013, 15, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xian, Y.-F.; Loo, S.K.F.; Ip, S.P.; Yang, W.; Chan, W.Y.; Lin, Z.-X.; Wu, J.C.Y. Baicalin Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis-like Skin Lesions in Mice through Modulating Skin Barrier Function, Gut Microbiota and JAK/STAT Pathway. Bioorg. Chem. 2022, 119, 105538. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.-Y.; Yoon, T.; Jang, S.; Kim, H.K. Forsythia Suspensa Suppresses House Dust Mite Extract-Induced Atopic Dermatitis in NC/Nga Mice. PLoS ONE 2016, 11, e0167687. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Liu, H.; Lin, B.; Yang, W.; Chen, W.; Lu, Z.; Li, P.; Gui, S.; Zhan, Y.; Lin, B. Spatholobi Caulis Dispensing Granule Reduces Deep Vein Thrombus Burden through Antiinflammation via SIRT1 and Nrf2. Phytomedicine 2020, 77, 153285. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, H.; Chen, L.; Jafari, M.; Tang, J. Network-Based Modeling of Herb Combinations in Traditional Chinese Medicine. Brief Bioinform. 2021, 22, bbab106. [Google Scholar] [CrossRef]
- Wang, S.; Sun, L.; Gu, L.; Zhang, Y.; Zhao, S.; Zhao, L.-S.; Bi, K.-S.; Chen, X. The Comparative Pharmacokinetics of Four Bioactive Ingredients after Administration of Ramulus Cinnamomi-Radix Glycyrrhizae Herb Pair Extract, Ramulus Cinnamomi Extract and Radix Glycyrrhizae Extract. Biomed. Chromatogr. 2016, 30, 1270–1277. [Google Scholar] [CrossRef]
- Li, F.-S.; Weng, J.-K. Demystifying Traditional Herbal Medicine with Modern Approach. Nat. Plants 2017, 3, 17109. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Zhou, W.; Wang, Y.; Yang, L. Systems Approaches and Polypharmacology for Drug Discovery from Herbal Medicines: An Example Using Licorice. J. Ethnopharmacol. 2013, 146, 773–793. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Bai, J.-X.; Li, T.; Fu, X.-Q.; Guo, H.; Zhu, P.-L.; Chan, Y.-C.; Chou, J.-Y.; Yin, C.-L.; Li, J.-K.; et al. A TCM Formula Comprising Sophorae Flos and Lonicerae Japonicae Flos Alters Compositions of Immune Cells and Molecules of the STAT3 Pathway in Melanoma Microenvironment. Pharmacol. Res. 2019, 142, 115–126. [Google Scholar] [CrossRef]
- Zhou, X.; Razmovski-Naumovski, V.; Kam, A.; Chang, D.; Li, C.G.; Chan, K.; Bensoussan, A. Synergistic Study of a Danshen (Salvia Miltiorrhizae Radix et Rhizoma) and Sanqi (Notoginseng Radix et Rhizoma) Combination on Cell Survival in EA.Hy926 Cells. BMC Complement. Altern. Med. 2019, 19, 50. [Google Scholar] [CrossRef]





| First Author (Year) [Reference] | Type of Condition | Trial Design | Number of Participants (Male/Female); Age (Mean ± SD) | Interventions | Morbidity Period (Mean ± SD or Range) | Outcome Index | Course of Treatment | Adverse Event (Case/Symptom) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial | Control | Trial | Control | Trial | Control | ||||||
| Zhou (2002) [40] | Psoriasis vulgaris | Randomized; Single center; Parallel | 61 (37/24) 36.5 y | 36 (21/15) 38.7 y | Yuyin capsule (18 caps, t.i.d) | Compound amino-polypeptide tablets (10 caps, b.i.d) | 4.7 y | 4.3 y | 1.PASI 60 response rate | 8 w | Trial: 1 AE/ Control: 26 AEs/Thirst and xerostomia (8), xeroderma (7), desquamation (11) |
| Zhao (2003) [41] | Psoriasis vulgaris | Randomized; Single center; Parallel | 30 (19/11) Range 19~63 y | 30 (16/14) NR | Xiaoyin decoction (200 mL, t.i.d) | Compound Amino-polypeptide tablets (10 caps, b.i.d) | Range 0.5~45 y | Range 0.5~45 y | 1.PASI 60 response rate | 8 w | Trial: 1 AE/Loose stool Control: 6 AEs/ xerostomia, xeroderma |
| Chen (2004) [42] | Psoriasis vulgaris | Randomized; Single center; Parallel | 61 (40/21) 37.51 ± 11.32 y | 55 (35/20) 35.13 ± 10.91 y | Compound Qingdai pill (12 caps, t.i.d) | Compound Amino-polypeptide tablets (10 caps, q.d or b.i.d) | 5.16 ± 5.02 y | 4.87 ± 4.71 y | 1.PASI 60 response rate | 8 w | Trial: 6 AEs/Nausea and anorexia Control: 21 AEs/including xerostomia, xeroderma |
| Lu (2005) [43] | Psoriasis vulgaris | Randomized; Single center; Parallel | 50 (32/18) 38.2 ± 16.4 y | 30 (19/11) 39.3 ± 17.1 y | Yinxieling capsule (12~18 caps, t.i.d) | Compound Amino-polypeptide tablets (10 caps, b.i.d) | 11.6 ± 8.4 y | 11.3 ± 8.1 y | 1. PASI 70 response rate | 8 w | Trial: 4 AEs/Gastrointestinal reaction Control: 22 AEs/Xerostomia, xeroderma, scale, pruritus |
| Liu (2005) [44] | Psoriasis vulgaris | Randomized; Single center; Parallel | 44 Both group (62/76) Range 17~64 y | 46 Both group (62/76) Range 17~64 y | Jiedulaingxue decoction (b.i.d) | Etretin (30 mg, t.i.d) | NR | NR | 1. PASI 60 response rate | 4 w | Trial: No AE Control: pruritus and thirst (NR) |
| He (2005) [45] | Psoriasis vulgaris | Randomized; Single center; Parallel | 33 (18/15) Range 16~65 y | 30 (19/11) NR | 1. Antidote decoction (b.i.d) 2. Tretinoin | 1. Vitamin A [Retinol] (50,000 U, i.v., q.d) 2. Compound vitamin B tablets (6 caps, t.i.d) 3.Tretinoin | Range 2 m~30 y | Range 40 d~28 y | 1. PASI 70 response rate | 4 w | NR |
| Qiu (2005) [46] | Psoriasis vulgaris | Randomized; Single center; Parallel | 32 (18/14) 30.42 ± 8.57 y | 32 (17/15) 33.34 ± 8.21 y | Huoxuesanyuxiaoyin decoction (b.i.d) | Acitretin (20 mg, b.i.d) | 6.53 ± 2.86 y | 7.04 ± 3.12 y | 1. PASI 60 response rate 2. PASI score | 8 w | Trial: 3 AEs/Diarrhea (2), constipation and vomiting (1) Control: Total AEs NR/Xerostomia, xeroderma, scale, dizziness, headache/AST, ALT elevation (3)/BUN elevation (1)/hyperlipidemia (5) |
| Li (2006) [47] | Psoriasis vulgaris | Randomized; Single center; Parallel | 43 (24/19) Range 13~55 y | 40 (27/13) Range 15~67 y | 1. Oral EAHM decoction (400 mL, b.i.d) 2. NB-UVB (0.3~0.5 J/cm2; 20%; NR; NR; q.o.d) | 1. Compound Amino-polypeptide tablets (15 tabs, t.i.d) 2. NB-UVB (0.3~0.5 J/ cm2; 20%; NR; NR; q.o.d) | Range 1 m~42 y | Range 2 w~36 y | 1. PASI 60 response rate | 40 d | Trial: No AE Control: 5 AEs/Xerostomia, xeroderma, dizziness |
| Li (2008) [48] | Psoriasis vulgaris | Randomized; Single center; Parallel | 30 (19/11) 42.16 ± 11.26 y | 28 (18/10) 38.08 ± 9.64 y | Qinzhu Liangxue decoction (30 mL, b.i.d) | Compound amino-polypeptide tablets (15 tabs, t.i.d) | 5.16 ± 1.34 y | 6.28 ± 1.66 y | 1. PASI 60 response rate 2. DLQI | 4 w | NR |
| Ye (2008) [49] | Psoriasis vulgaris | Randomized; Single center; Parallel | 56 (38/18) Range 8~65 y | 56 (36/20) Range 9~68 y | Zhixuejieduxiaoyin decoction (b.i.d) | Compound amino -polypeptide tablets (15 tabs, t.i.d) | NR | NR | 1. PASI 60 response rate | 8 w | Trial: 8 AEs/ Dizziness, anorexia, abdominal distention, abdominal pain Control: 22 AEs/ xerostomia, hot flush, xeroderma, scale, pruritus |
| Zhong (2008) [50] | Psoriasis vulgaris | Randomized; Single center; Parallel | 60 (39/21) 36.20 ± 10.74 y | 60 (43/17) 36.18 ± 10.82 y | Xiaoyin granule (10.5 g, b.i.d) | Compound amino-polypeptide tablets (15 taps, t.i.d) | 7.08 ± 4.46 y | 7.24 ± 4.33 y | 1. PASI 60 response rate 2. PASI score | 8 w | Trial 12 AEs/Xerostomia, gastrointestinal discomfort, nausea, loose stool (12) Control: Total AEs NR Xerostomia (12), aggravated pruritus (22), dyssebacia (15), scale at hand and foot (9), dermatitis (8),conjunctival injection (2), hypermenorrhea (3) |
| Hu (2009) [51] | Psoriasis vulgaris | Randomized; Single center; Parallel | 30 (16/14) 39.7 ± 11.7 y | 30 (17/13) 37.0 ± 11.7 ye | Liangxue decoction (b.i.d) | Compound Amino-polypeptide tablets (15 tabs, t.i.d) | 6.8 ± 3.4 y | 6.7 ± 3.8 y | 1.PASI 70 response rate 2.PASI score | 8 w | Trial: No AE Control: No AE |
| Wang (2009) [52] | Psoriasis vulgaris | Randomized; Single center; Parallel | 120 (65/55) Range 8~78 y | 116 (62/54) Range 9~75 y | Baibi decoction (300 mL, b.i.d) | Compound Amino-polypeptide tablets (10 tabs, b.i.d) | Range 1 w~40 y | Range 1 w~35 y | 1. PASI 70 response rate | 90 d | NR |
| Feng (2009) [53] | Psoriasis vulgaris | Randomized; Single center; Parallel | 100 (60/40) 43.28 ± 12.01 y | 50 (30/20) 42.31 ± 11.08 y | Wushe decoction (q.d) | Compound econazole nitrate cream (b.i.d) | 8.67 ± 6.5 y | 8.23 ± 7.1 y | 1. PASI 60 response rate | Trial: 30 d Control: 21 d | NR |
| Xie (2009) [54] | Psoriasis vulgaris | Randomized; Single center; Parallel | 41 (21/20) Mean 42.5 y | 30 (16/14) Mean 37.5 y | Kangyin1 decoction (b.i.d) | Acitretin (20 mg, b.i.d) | Range 1 m~28 y | Range 4 m~21 y | 1. PASI score | 8 w | Trial: 2 AEs/Gastrointestinal discomfort (2) Control: 24 AEs/Xeroderma (23), ALT elevation (2), hyperlipidemia (2), Headache with tinnitus (1), gastrointestinal discomfort (2) |
| Hou (2009) [55] | Psoriasis vulgaris | Randomized; Single center; Parallel | 140 (72/68) 32.1 ± 6.6 y | 120 (63/57) 38.4 ± 5.9 y | Huoxueliangxue decoction (b.i.d) | Compound Amino-polypeptide tablets (10 tabs, b.i.d) | Range 20 d~30 y | Range 15 d~32 y | 1. PASI 70 response rate 2. PASI score | 8 w | Trial: 5 AEs/diarrhea Control: 13 AEs/Xerostomia, (13), dizziness and drowsy (2) |
| Ho (2010) [56] | Plaque vulgaris | Randomized; Multi center; Parallel | 21 (14/7) 48.52 y | 20 (18/2) 43.45 y | Wen-tong- hua-yu formulation | 1.Methotrexate (2.5~5 mg 1st week, increased to 10 mg q.w, not to exceed 30 mg q.w) 2.Folic acid (5 mg, q.d) | NR | NR | 1. PASI score | 24 w | Trial: 48% reported/Infection, gastrointestinal side effects, a few developed abnormalities in liver function Control: 65% reported/Nausea, vomiting, increased liver enzyme level |
| Si (2010) [57] | Psoriasis vulgaris | Randomized; Single center; Parallel | 66 (28/38) 37.61 ± 14.43 y | 59 (23/35) 34.25 ± 12.66 y | 1. Jiawei Xiaoyaosan 2. Pulian ointment 3. NB-UVB (0.5 J/cm2; t.i.week) | 1. Acitretin (20 mg, qd) 2. Pulian ointment 3. NB-UVB (0.5 J/cm2; t.i.week) | 4.25 ± 5.06 y | 3.40 ± 4.77 y | 1. PASI 60 response rate | 4 w | NR |
| Yan (2010) [39] | Psoriasis vulgaris | Randomized; Single center; Parallel | 28 (Other information NR) | 28 (Other information NR) | Quyin decoction (300 mL, b.i.d) | Placebo | NR | NR | 1. PASI 60 response rate | 12 w | NR |
| Ma (2010) [58] | Psoriasis vulgaris | Randomized; Single center; Parallel | 52 (28/24) 39.04 ± 18.58 y | 51 (26/25) 40.67 ± 13.64 y | Yinxiebing fang decoction (b.i.d) | Acitretin (30 mg, t.i.d) | 4.82 ± 7.29 y | 2.74 ± 3.32 y | 1. PASI 60 response rate 2. PASI score | 12 w | Trial: 5 AEs/Gastrointestinal discomfort (5) Control: Total AEs NR/Cheilitis (25), headache (6), tinnitus (2)/Gastrointestinal discomfort, liver function abnormality (4), xerostomia and scale (34), hyperlipidemia (8) |
| Ma (2011) [59] | Psoriasis vulgaris | Randomized; Single center; Parallel | 40 (22/18) Mean 35.3 y | 40 (23/17) Mean 37.8 y | Keyin Ⅰ prescription (300 mL, b.i.d) | Acitretin (30 mg, q.d; after 3rd week 60 mg, q.d) | 7.8 y | 8.3 y | 1. PASI 60 response rate 2. TNF-α | 90 d | Trial: No AE Control: 12 AEs/Cheilitis (3), pruritus and scale (7), Nausea with abdominal pain (2) |
| Wang (2011) [60] | Psoriasis vulgaris | Randomized; Single center; Parallel | 30 (17/13) 35.24 ± 10.28 y | 30 (16/14) 33.48 ± 10.02 y | Tufulingqingdai decoction (300 mL, b.i.d) | Compound Amino-polypeptide tablets (10 tabs, b.i.d) | 6.5 y | 5.4 y | 1. PASI 60 response rate 2. PASI score | 4 w | NR |
| Xie (2012) [61] | Psoriasis vulgaris | Randomized; Single center; Parallel | 42 (22/20) Mean 41.5 y | 30 (16/14) Mean 36.5 y | Liangxie Runfu decoction (b.i.d) | Acitretin (20 mg, b.i.d) | Range 1 m~25 y | Range 5 m~22 y | 1. PASI score | 12 w | Trial: 5 AEs/Gastrointestinal discomfort (5) Control: 25 AEs/Gastrointestinal discomfort (3), xerostomia and xeroderma (21), hyperlipidemia (1) |
| Jia (2012) [62] | Psoriasis vulgaris | Randomized; Single center; Parallel | 30 (18/12) 35.67 ± 8.86 y | 30 (16/14) 35.67 ± 8.86 y | Xiaobi decoction (300 mL, t.i.d) | Acitretin (20~30 mg, b.i.d or t.i.d) | 8.13 ± 1.35 y | 7.59 ± 1.46 y | 1. PASI 70 response rate 2. PASI score | 12 w | Trial: 9 AEs/Nausea (5), anorexia (2), loose stool (2) Control: 49 AEs/Nausea (6), anorexia (2), xeroderma (37), hyperlipidemia (4) |
| Cheng (2012) [63] | Psoriasis vulgaris | Randomized; Single center; Parallel | 35 (13/22) Range 3~18 y | 30 (10/20) Range 4~17 y | EAHM prescription for individual clinical trial (b.i.d) | 1. Penicilin 2. Cephalosporin | NR | NR | 1. PASI 70 response rate | 4 w | Trial: No AE Control: No AE |
| Ma (2012a) [64] | Psoriasis vulgaris | Randomized; Single center; Parallel | 41 (23/18) Mean 45.3 y | 37 (21/16) Mean 44.8 y | Liangxue jiedu decoction (300 mL, b.i.d) | Compound Amino-polypeptide tablets (10 tabs, b.i.d) | Range 5 m~5 y | Range 5 m~5 y | 1. PASI 70 response rate | 8 w | NR |
| Ma (2012b) [65] | Psoriasis vulgaris | Randomized; Single center; Parallel | 52 (28/24) 39.04 ± 18.58 y | 51 (26/25) 40.67 ± 13.64 y | Yinxiaobing decoction | Acitretin (30 mg, t.i.d) | 4.82 ± 7.29 y | 2.74 ± 3.32 y | 1. PASI 60 response rate 2. PASI score | 12 w | Trial: 5 AEs/ Gastrointestinal discomfort (5) Control: 77 AEs/ Cheilitis (25), headache (6), tinnitus (2), abnormality of liver function (4), xeroderma and scale (34), hyperlipidemia (8) |
| Zhang (2013) [66] | Psoriasis vulgaris | Randomized; Single center; Parallel | 30 (19/11) 40.83 ± 6.48 y | 30 (18/12) 44.30 ± 5.80 y | Blood cooling decoction (300 mL, b.i.d) | Acitretin (20 mg, b.i.d) | 6.24 ± 1.48 y | 5.49 ± 1.24 y | 1. PASI 70 response rate | 8 w | NR |
| Liu (2013) [67] | Psoriasis vulgaris | Randomized; Single center; Parallel | 31 (18/13) 40.55 ± 12.83 y | 31 (16/15) 38.84 ± 10.57 y | Wanbi decoction (300 mL, b.i.d) | Acitretin (6 caps, b.i.d) | 9.45 ± 5.07 y | 7.80 ± 4.93 y | 1. PASI 60 response rate 2. PASI score | Trial: 56 d Control: 60 d | Trial: No AE Control: No AE |
| Xu (2013a) [68] | Psoriasis vulgaris | Randomized; Single center; Parallel | 24 (15/9) 44.78 ± 4.13 y | 24 (16/8) 44.13 ± 4.46 y | 1. Shufengyangtxue decoction (b.i.d) 2. Calcipotriol ointment (b.i.d) | 1. Metotrexate (5 mg, b.i.d, continuous three days in a week) 2. Calcipotriol ointment (b.i.d) | 101.53 ± 63.01 m | 102.65 ± 63.01 m | 1. PASI 60 response rate 2. PASI score | 8 w | Trial: 1 AE/ Gastrointestinal discomfort (1) Control: 3 AEs/Gastrointestinal discomfort (3), loose stool (1) |
| Xu (2013b) [69] | Psoriasis vulgaris | Randomized; Single center; Parallel | 59 (28/31) 41.26 ± 12.26 y | 56 (26/30) 39.42 ± 10.87 y | Qingre Liangxue decoction (400 mL, b.i.d) | Acitretin (0.5 mg/kg, q.d) | 6.24 ± 1.48 y | 5.49 ± 1.24 y | 1. PASI 70 response rate | 6 w | Trial: 5 AEs/Gastrointestinal discomfort Control: 29 AEs/Gastrointestinal discomfort, cheilitis and scale |
| Zhu (2014) [70] | Psoriasis vulgaris | Randomized; Single center; Parallel | 30 (14/16) 43.53 ± 2.15 y | 30 (15/15) 43.75 ± 2.66 y | 1. Dahuang Zhechong Capsule (4 caps, b.i.d) 2. Vitamin E cream (b.i.d) | 1. Acitretin (30 mg, b.i.d−20 mg, 10 mg) 2. Vitamin E cream (b.i.d) | 19.23 ± 2.33 y | 18.17 ± 3.02 y | 1. PASI 70 response rate 2. PASI score | 12 w | Trial: No AE Control: 4 AEs/Xerostomia and xeroderma (3), elevation of liver enzyme (1) |
| Chen (2014) [71] | Psoriasis vulgaris | Randomized; Single center; Parallel | 30 (16/14) 32.45 ± 24.89 y | 30 (18/12) 31.73 ± 24.65 y | Liangxue No.1 formula (b.i.d) | 1. Clobetasol propionate cream (t.i.d) 2. Loratadine (10 mg, q.d) 3. Acitretin (30 mg, q.d) | 14.12 ± 4.76 y | 14.58 ± 3.73 y | 1. PASI 60 response rate | 4 w | Trial: 1 AE/Nausea with vomiting Control: 12 AEs/Cheilitis, xerostomia, headache |
| Qian (2014) [72] | Psoriasis vulgaris | Randomized; Single center; Parallel | Both group 74 (43/31) 23.1 ± 2.6 y Trial: 38 | Both group 74 (43/31) 23.1 ± 2.6 y Control: 36 | 1. Liangxue Runfu decoction (b.i.d) 2.15% urea cream (b.i.d) | 1. Acitretin (20 mg, b.i.d) 2.15% urea cream (b.i.d) | 5.2 ± 1.8 y (Both group) | 5.2 ± 1.8 y (Both group) | 1. PASI 60 response rate | 8 w | Trial: 3 AEs/Gastrointestinal discomfort (3), xeroderma (2) Control: 9 AEs/Xerostomia (2), xeroderma (4), hyperlipidemia (2), gastrointestinal discomfort (1) |
| Peng (2014) [73] | Psoriasis vulgaris | Randomized; Single center; Parallel | Both group 86 (45/41) 51.36 ± 4.22 y Trial: 43 | Both group 86 (45/41) 51.36 ± 4.22 y Control: 43 | Liangxue Jiedu decoction (300 mL, b.i.d) | Compound amino-polypeptide tablets (15 tabs, t.i.d) | 9 ± 3.2 y (Both group) | 9 ± 3.2 y (Both group) | 1. PASI score | 12 w | NR |
| Dou (2014) [74] | Psoriasis vulgaris | Randomized; Single center; Parallel | 33 (21/12) 38.6 ± 11.9 y | 30 (19/11) 36.2 ± 12.5 y | 1. Wutengxiaoyin Decoction 2.10% urea cream (b.i.d) | 1. Compound amino-polypeptide Tablets (15 tabs, t.i.d) 2.10% urea cream (b.i.d) | 13.4 ± 12.5 y | 14.3 ± 8.7 y | 1. PASI 60 response rate 2. PASI score 3. DLQI | 8 w | Trial: 3 AEs/Gastrointestinal discomfort (2), diarrhea (3) Control: 19 AEs/ Xeroderma, xerostomia, scale (19), pruritus (4) |
| Miao (2014) [75] | Psoriasis vulgaris | Randomized; Single center; Parallel | Both group 198 (118/80) 39.6 ± 8 y Trial: 132 | Both group 198 (118/80) 39.6 ± 8 y Control: 66 | Quyin decoction (b.i.d) | Acitretin (10 mg, t.i.d) | Mean 3.9 y (Both group) | Mean 3.9 y (Both group) | 1. PASI 70 response rate | 12 w | Trial: 12 AEs/Headache with dizziness (8), nausea and vomiting (6), liver function abnormality (3) Control: 35 AEs/ Xerostomia (25), xerophtalmia (18), xeroderma (14), pruritus (6), headache and dizziness (6), ALT elevation (8) |
| Xu (2015) [76] | Psoriasis vulgaris | Randomized; Single center; Parallel | 40 (23/17) 58.6 ± 8.8 y | 40 (19/21) 59.8 ± 9.3 y | Liangxue Jiedu Decoction (300 mL, t.i.d) | Acitretin (25 mg, b.i.d) | 12.5 ± 2.6 y | 13.7 ± 2.1 y | 1. PASI 70 response rate 2. PASI score | 8 w | Trial: No AE Control: 15 AEs/Xerostomia, gastrointestinal discomfort (6), pruritus and scale (9) |
| Zhang (2015) [77] | Psoriasis vulgaris | Randomized; Single center; Parallel | 60 (38/25) 31.29 ± 0.04 y | 65 (35/30) Mean 29.22 y | Ziyinqingrexiaofeng san (NR) | 1. Acitretin (20 mg, b.i.d) 2. Compound Flumetasone ointment (b.i.d) | Range 3 m~10 y | Range 1~12 y | 1. PASI 60 response rate | 8 w | Trial: 8 AEs/Burningsensation (5), erythema (2), aggravated pruritus (1) Control: NR |
| Yang (2015) [78] | Psoriasis vulgaris | Randomized; Single center; Parallel | 55 (32/23) 37.6 ± 3.3 y | 55 (30/25) 37.9 ± 3.5 y | Qingre liangxue decoction (b.i.d) | Acitretin (0.5 mg/kg, q.d) | 8.8 ± 0.7 y | 8.6 ± 0.5 y | 1. PASI score | 4 w | Trial: 2 AEs/Gastrointestinal discomfort (2) Control: 3 AEs/ Xerostomia (2), scale (1) |
| Liang (2015) [79] | Psoriasis vulgaris | Randomized; Single center; Parallel | 30 (16/14) 34.28 ± 10.26 y | 30 (18/12) 33.46 ± 10.12 y | 1. Tufuling Qingdai decoction (300 mL, b.i.d) 2.Vaseline (b.i.d) | 1. Compound amino-polypeptide tablets (10 tabs, b.i.d) 2. Vaseline (b.i.d) | Mean 6.8 y | Mean 5.6 y | 1. PASI 60 response rate 2. PASI score 3. TNF-α | 4 w | NR |
| Han (2015) [80] | Psoriatic pustules | Randomized; Single center; Parallel | 30 (17/13) 37.71 ± 12.8 y | 30 (16/14) 36.48 ± 12.34 y | Huayin Jiedu decoction (b.i.d) | Acitretin (20 mg, b.i.d) | 8.64 ± 5.43 y | 8.51 ± 7.89 y | 1. PASI score | 8 w | Trial: No AE Control: 5 AEs/Elevated triacylglycerols |
| Xiang (2016) [81] | Psoriasis vulgaris | Randomized; Single center; Parallel | 30 (16/14) 37.5 ± 7.5 y | 30 (17/13) 37.8 ± 7.2 y | Qinzhu Liangxue decoction (400 mL, b.i.d) | Acitretin (20~30 mg, b.i.d or t.i.d) | 10.23 ± 7.2 y | 11.23 ± 8.2 y | 1. PASI score 2.IL-17 3.IL-23 | 4 w | NR |
| Wang (2016) [82] | Psoriasis vulgaris | Randomized; Single center; Parallel | 50 (28/22) 27.2 ± 5.2 y | 50 (27/23) 27.3 ± 6.2 y | Liangxue Runfu decoction (t.i.d) | Acitretin (10 mg, b.i.d) | NR | NR | 1. PASI 60 response rate 2. TNF-α | NR | NR |
| Zhou (2016) [83] | Psoriasis vulgaris | Randomized; Single center; Parallel | 82 (46/36) 35.7 ± 9.4 y | 82 (48/34) 36.2 ± 9.7 y | Shufeng jiedu capsules (12 caps, t.i.d) | Compound amino-polypeptide tablets (6 tabs, b.i.d) | 4.2 ± 2.1 y | 4.1 ± 2.2 y | 1. PASI 70 response rate | 12 w | NR |
| Du (2016a) [84] | Psoriasis vulgaris | Randomized; Single center; Parallel | 80 (56/24) 47.3 ± 10.3 y | 80 (56/21) 48.7 ± 13.3 y | Shengdi Baimao decoction (300 mL, b.i.d) | 0.025% Tretinoin ointment (b.i.d) | 2.3 ± 1.8 y | 2.5 ± 1.7 y | 1. PASI 60 response rate 2. TNF-α | 20 d | Trial: No AE Control: No AE |
| Du (2016b) [85] | Psoriasis vulgaris | Randomized; Single center; Parallel | 24 (14/10) 41.75 ± 9.03 y | 24 (15/9) 42.11 ± 10.95 y | Heat-clearing and detoxicating oral liquid (60 mL, t.i.d) | Acitretin (30 mg, t.i.d) | 6.7 ± 4.4 y | 7.1 ± 5.2 y | 1. PASI 60 response rate 2. TNF-α | 12 w | NR |
| Mao (2017) [86] | Psoriasis vulgaris | Randomized; Single center; Parallel | 30 (21/9) 48.96 ± 6.88 y | 30 (22/8) 48.02 ± 7.18 y | 1. Xiaoyinfang (200 mL, b.i.d) 2. NB-UVB | 1. Calcipotriol ointment (b.i.d) 2. NB-UVB | 3.77 ± 1.27 y | 3.62 ± 1.07 y | 1. PASI 70 response rate 2. IL-17 3. IL-23 | 8 w | NR |
| Xia (2018) [87] | Psoriasis vulgaris | Randomized; Single center; Parallel | 36 (21/15) 37.25 ± 13.44 y | 34 (23/11) 34.85 ± 12.01 y | Kanli fang (400 mL, b.i.d) | 0.005% Calcipotriol ointment (q.d) | 7.57 ± 7.25 y | 7.57 ± 7.25 y | 1. PASI score | 6 w | Trial: No AE Control: 3 AEs/Burning sense (3) |
| Li (2018) [88] | Psoriasis vulgaris | Randomized; Single center; Parallel | 33 (19/14) 35.52 ± 3.72 y | 33 (18/15) 35.48 ± 3.62 y | Jianpi Jiedu decoction (400 mL, b.i.d) | Acitretin (40 mg, b.i.d) | 6.42 ± 3.65 y | 6.35 ± 3.45 y | 1. PASI 60 response rate 2. DLQI 3. TNF-α | NR | NR |
| Yang (2018) [89] | Psoriasis vulgaris | Randomized; Single center; Parallel | 35 (18/17) 33.89 ± 2.68 y | 35 (17/18) 34.26 ± 2.91 y | Jinji Xiaoyin granule (27 g, t.i.d) | Acitretin (40 mg, b.i.d) | 3.62 ± 3.21 y | 3.26 ± 3.42 y | 1. PASI 70 response rate 2. PASI score 3. IL-17 4. IL-23 5.TNF-α | 8 w | NR |
| Wang (2019) [90] | Psoriasis vulgaris | Randomized; Single center; Parallel | 36 (20/16) 41.4 ± 3.5 y | 36 (18/18) 39.2 ± 2.4 y | Qingying decoction (300 mL, b.i.d) | Acitretin (20 mg, b.i.d) | 15.1 ± 3.5 y | 14.2 ± 27 y | 1. PASI 60 response rate 2. PASI score 3. DLQI 4. IL-17 5. IL-23 | 12 w | NR |
| Zhang (2019) [91] | Psoriasis vulgaris | Randomized; Single center; Parallel | 48 (28/20) 36.84 ± 6.20 y | 48 (28/20) 36.60 ± 6.20 y | Xijiao Dihuang decoction (300 mL, b.i.d) | Acitretin (20 mg, b.i.d) | 56.53 ± 12.30 m | 57.20 ± 12.50 m | 1. PASI 70 response rate 2. PASI score | 12 w | NR |
| Jiang (2019) [92] | Psoriasis vulgaris | Randomized; Single center; Parallel | 30 (18/12) 20.15 ± 2.55 y | 30 (14/16) 15.34 ± 4.71 y | 1. Xijiao Dihuang Jiedu decoction (q.d) 2. Urea ointment for external use (b.i.d.) | 1. Roxithromycin (Adlut: 300 mg, b.i.d; Adolescent: 2.5~5 mg*kg, b.i.d) 2. Urea ointment for external use (b.i.d.) | NR | NR | 1. PASI 70 response rate 2. PASI score | 2 w | Trial: No AE Control: No AE |
| Wen (2019) [93] | Psoriasis vulgaris | Randomized; Single center; Parallel | 38 (26/12) 33.42 ± 8.37 y | 38 (23/15) 31.44 ± 7.42 y | Xiaobi decoction (b.i.d) | Acitretin (40 mg, b.i.d) | 46.87 ± 15.10 m | 43.07 ± 15.98 m | 1. PASI score | 4 w | NR |
| Chen (2020) [94] | Psoriasis vulgaris | Randomized; Single center; Parallel | 20 (11/9) 33.65 ± 5.41 y | 20 (12/8) 33.58 ± 5.26 y | 1. Quyin decoction (200 mL, b.i.d) 2.10% Urea ointment | 1. Compound amino poly-peptide tables (10 tabs, b.i.d) 2. 10% Urea ointment | 10.25 ± 3.35 y | 10.19 ± 3.59 y | 1. PASI 60 response rate | NR | NR |
| First Author (Year) | D1 | D2 | D3 | D4 | D5 | Overall |
|---|---|---|---|---|---|---|
| Zhou (2002) | L | Sc | L | Sc | Sc | Sc |
| Zhao (2003) | L | Sc | L | Sc | Sc | Sc |
| Chen (2004) | L | Sc | L | Sc | Sc | Sc |
| Lu (2005) | L | Sc | L | Sc | Sc | Sc |
| Liu (2005) | L | Sc | L | Sc | Sc | Sc |
| He (2005) | L | Sc | L | Sc | Sc | Sc |
| Qiu (2005) | L | Sc | L | Sc | Sc | Sc |
| Li (2006) | L | Sc | L | Sc | Sc | Sc |
| Li (2008) | L | Sc | L | Sc | Sc | Sc |
| Ye (2008) | L | Sc | L | Sc | Sc | Sc |
| Zhong (2008) | L | Sc | L | Sc | Sc | Sc |
| Hu (2009) | L | Sc | L | Sc | Sc | Sc |
| Wang (2009) | L | Sc | L | Sc | Sc | Sc |
| Feng (2009) | L | Sc | L | Sc | Sc | Sc |
| Xie (2009) | L | Sc | L | Sc | Sc | Sc |
| Hou (2009) | L | Sc | L | Sc | Sc | Sc |
| Ho (2010) | L | L | L | L | Sc | Sc |
| Si (2010) | L | Sc | L | Sc | Sc | Sc |
| Yan (2010) | L | Sc | L | Sc | Sc | Sc |
| Ma (2010) | L | Sc | L | Sc | Sc | Sc |
| Ma (2011) | L | Sc | L | Sc | Sc | Sc |
| Wang (2011) | L | Sc | L | Sc | Sc | Sc |
| Xie (2012) | L | Sc | L | Sc | Sc | Sc |
| Jia (2012) | L | Sc | L | Sc | Sc | Sc |
| Cheng (2012) | L | Sc | L | Sc | Sc | Sc |
| Ma (2012a) | L | Sc | L | Sc | Sc | Sc |
| Ma (2012b) | L | Sc | L | Sc | Sc | Sc |
| Zhang (2013) | L | Sc | L | Sc | Sc | Sc |
| Liu (2013) | L | Sc | L | Sc | Sc | Sc |
| Xu (2013a) | L | Sc | L | Sc | Sc | Sc |
| Xu (2013b) | L | Sc | L | Sc | Sc | Sc |
| Zhu (2014) | L | Sc | L | Sc | Sc | Sc |
| Chen (2014) | L | Sc | L | Sc | Sc | Sc |
| Qian (2014) | L | Sc | L | Sc | Sc | Sc |
| Peng (2014) | L | Sc | L | Sc | Sc | Sc |
| Dou (2014) | L | Sc | L | Sc | Sc | Sc |
| Miao (2014) | L | Sc | L | Sc | Sc | Sc |
| Xu (2015) | L | Sc | L | Sc | Sc | Sc |
| Zhang (2015) | L | Sc | L | Sc | Sc | Sc |
| Yang (2015) | L | Sc | L | Sc | Sc | Sc |
| Liang (2015) | L | Sc | L | Sc | Sc | Sc |
| Han (2015) | L | Sc | L | Sc | Sc | Sc |
| Xiang (2016) | L | Sc | L | Sc | Sc | Sc |
| Wang (2016) | L | Sc | L | Sc | Sc | Sc |
| Zhou (2016) | L | Sc | L | Sc | Sc | Sc |
| Du (2016a) | L | Sc | L | Sc | Sc | Sc |
| Du (2016b) | L | Sc | L | Sc | Sc | Sc |
| Mao (2017) | L | Sc | L | Sc | Sc | Sc |
| Xia (2018) | L | Sc | L | Sc | Sc | Sc |
| Li (2018) | L | Sc | L | Sc | Sc | Sc |
| Yang (2018) | L | Sc | L | Sc | Sc | Sc |
| Wang (2019) | L | Sc | L | Sc | Sc | Sc |
| Zhang (2019) | L | Sc | L | Sc | Sc | Sc |
| Jiang (2019) | L | Sc | L | Sc | Sc | Sc |
| Wen (2019) | L | Sc | L | Sc | Sc | Sc |
| Chen (2020) | L | Sc | L | Sc | Sc | Sc |
| k | Risk Ratio | 95% CI | Heterogeneity (I2) | Psubgroup | |
|---|---|---|---|---|---|
| Type of Comparator | 0.0059 | ||||
| •Acitretin | 14 | 1.1158 | 1.0236 to 1.2163 | 69.5% | |
| •Other conventional medicine | 15 | 1.3114 | 1.2154 to 1.4151 | 13.9% |
| Intervention and Comparator Intervention | Outcomes | Number of Participants (Studies) | Anticipated Absolute Effects (95% CI) | Quality of the Evidence (GRADE) |
|---|---|---|---|---|
| EAHM compared to CM for inflammatory skin manifestation of plaque psoriasis | PASI 70 | 1865 (18 trials) | 161 more per 1000 (from 108 more to 218 more) | ⊕⊕⊕◯ MODERATE a |
| PASI 60 | 2479 (29 trials) | 126 more per 1000 (from 75 more to 182 more) | ⊕⊕◯◯ LOW a,c | |
| Continuous PASI score | 2139 (27 trials) | MD 2.3386 point lower (3.3068 lower to 1.3704 lower) | ⊕⊕◯◯ LOW a,c | |
| IL-17 | 262 (4 trials) | SMD 1.17 SD lower (2.18 lower to 0.16 lower) | ⊕⊕◯◯ LOW a,c | |
| IL-23 | 262 (4 trials) | SMD 1.3204 SD lower (3.0143 lower to 0.3734 higher) | ⊕◯◯◯ VERY LOW a,b,c | |
| TNF-α | 584 (5 trials) | SMD 1.4396 SD lower (2.8303 lower to 0.499 lower) | ⊕⊕◯◯ LOW a,c | |
| DLQI | 259 (4 trials) | MD 3.1161 point lower (4.2796 lower to 1.9526 lower) | ⊕⊕⊕◯ MODERATE a |
| First Author (Year) | EAHM Prescription Name | Source | Ingredients of EAHM Prescription (Latin Name) | Types of Preparation |
|---|---|---|---|---|
| Zhou (2002) | Yuyin capsule | Prepared by Zhou (2002) | Rehmanniae Radix Recens 20 g, Smilacis Rhizoma 20 g, Salviae Miltiorrhizae Radix 15 g, Sophorae Tonkinensis Radix Et Rhizoma 10 g, Paeoniae Radix Alba 10 g, Moutan Radicis Cortex 10 g, Manitis Squama 10 g, Zaocys 10 g, Hedyotidis Herba 10 g, Glycyrrhizae Radix et Rhizoma 5 g | Capsule |
| Zhao (2003) | Xiaoyin decoction | Prepared by Zhao (2003) | Sophorae Tonkinensis Radix Et Rhizoma 15 g, Scutellariae Barbatae Herba 15 g, Sargentodoxa Cuneata 20 g, Rhizoma Paridis 15 g, Smilax china Linn 30 g, Rehmanniae Radix Recens 30 g, Moutan Radicis Cortex 15 g, Sophorae Flos 15 g, Gentianae Macrophyllae Radix 30 g, Smilacis Rhizoma 20 g, Tripterygium wilfordii 20 g, Scorpio 5 g, Scolopendra 2 pieces, Vespae Nidus 20 g, Euonymi Lignum Suberalatum 20 g, Radix Paeoniae Rubra 15 g | Decoction |
| Chen (2004) | Sanlong Sanchong decoction | Shaanxi Yulin Chinese Pharmaceutical Co., Ltd. | Portulacae Herba, Smilacis Rhizoma, Dictamni Radicis Cortex, Angelicae Dahuricae Radix, Indigo Pulverata Levis, Lithospermi Radix, Salviae Miltiorrhizae Radix, Taraxaci Herba, Dryopteridis Crassirhizomatis Rhizoma, Tokoro Rhizoma, Mume Fructus, Schisandrae Fructus, Crataegi Fructus, Massa Medicata Fermentata | Capsule |
| Lu (2005) | Yinxieling capsule | Prepared by Lu (2005) | Bubali Cornu, Rehmanniae Radix Recens, Imperatae Rhizoma, Sophorae Flos, Lithospermi Radix, Angelicae Gigantis Radix, Salviae Miltiorrhizae Radix, Moutan Radicis Cortex, Smilacis Rhizoma, Dictamni Radicis Cortex, Hedyotidis Herba, Deinagkistrodon | Capsule |
| Liu (2005) | Jiedu Liangxue decoction | Prepared by Liu (2005) | Rehmanniae Radix Recens 30 g, Lithospermi Radix 15 g, Sophorae Flos 30 g, Isatidis Radix 15 g, Dictamni Radicis Cortex 15 g, Rhizoma Paridis 15 g, Salviae Miltiorrhizae Radix 15 g, Scrophulariae Radix 15 g, Glycyrrhizae Radix et Rhizoma 6 g | Decoction |
| He (2005) | Antidote decoction | Prepared by He (2005) | Sparganii Rhizoma 10 g, Curcumae Rhizoma 10 g, Scutellariae Radix 10 g, Smilacis Rhizoma 30 g, Hedyotidis Herba15 g, Lithospermi Radix 12 g, Taraxaci Herba 15 g, Mume Fructus 10 g | Decoction |
| Qiu (2005) | Sanyu Xiaoyin decoction | Prepared by Qiu (2005) | Sparganii Rhizoma 10 g, Curcumae Rhizoma 10 g, Persicae Semen 10 g, Carthami Flos 10 g, Spatholobi Caulis 10 g, Euonymi Lignum Suberalatum 30 g, Hedyotidis Herba 30 g, Salviae Miltiorrhizae Radix 30 g, Citri Unshius Pericarpium 30 g | Decoction |
| Li (2006) | EAHM prescription for individual clinical trial | Prepared by Li (2006) | Lithospermi Radix 15 g, Rubiae Radix 15 g, Isatidis Radix 30 g, Imperatae Rhizoma 30 g, Rehmanniae Radix Recens 15 g, Radix Paeoniae Rubra 15 g, Salviae Miltiorrhizae Radix 15 g, Hedyotidis Herba 15 g, Spatholobi Caulis 30 g, Smilacis Rhizoma 15 g, Sophorae Flos 15 g, Gazellae seu Saigae Cornu 0.6 g | Decoction |
| Li (2008) | Qinzhu Liangxue decoction | Prepared by Li (2008) | Magenetitum 30 g, Margaritifera Concha 25 g, Ostreae Testa 30 g, Scutellariae Radix 9 g, Lithospermi Radix 9 g, Cynanchi Paniculati Radix Et Rhizoma 9 g, Coicis Semen 10 g, Saposhnikoviae Radix 9 g, Glycyrrhizae Radix et Rhizoma 6 g | Decoction |
| Ye (2008) | Zhixue Jiedu Xiaoyin decoction | Prepared by Ye (2008) | Rehmanniae Radix Recens 10–20 g, Lithospermi Radix 10 g, Radix Paeoniae Rubra 10 g, Moutan Radicis Cortex 10 g, Angelicae Gigantis Radix 10 g, Cnidii Rhizoma 6–10 g, Carthami Flos 3–6 g, Flos Persicae 6–10 g, Zaocys 10–15 g, Tribuli Fructus 10 g, Lonicerae Flos 10–15 g, Smilacis Rhizoma 10–30 g | Decoction |
| Zhong (2008) | Xiaoyin granule | Epons pharmaceutical Co., Ltd. | Rehmanniae Radix Recens 10 g, Angelicae Gigantis Radix 10 g, Radix Paeoniae Rubra 10 g, Cnidii Rhizoma 6 g, Hedyotidis Herba 15 g, Lithospermi Radix 6 g, Curcumae Rhizoma 10 g, Smilacis Rhizoma 15 g, Mume Fructus 10 g, Scutellariae Barbatae Herba 15 g, Glycyrrhizae Radix et Rhizoma 3 g | Decoction |
| Hu (2009) | Liangxue decoction | Prepared by Hu (2009) | Bubali Cornu 30 g, Notoginseng Radix et Rhizoma 3 g, Lithospermi Radix 10 g, Coptidis Rhizoma 3 g, Scutellariae Radix 10 g, Phellodendri Cortex 15 g, Coicis Semen 15 g, Poria Sclerotium 15 g, Glycyrrhizae Radix et Rhizoma 5 g | Decoction |
| Hou (2009) | Huoxueliangxue decoction | Prepared by Hou (2009) | Sophorae Flos 30 g, Imperatae Rhizoma 30 g, Lithospermi Radix 15 g, Moutan Radicis Cortex 15 g, Rubiae Radix 15 g, Rehmanniae Radix Recens 30 g, Salviae Miltiorrhizae Radix 15 g, Spatholobi Caulis 30 g, Isatidis Radix 30 g, Dictamni Radicis Cortex 15 g | Decoction |
| Wang (2009) | Baiji decoction | Prepared by Wang (2009) | Rehmanniae Radix Recens 30 g, Moutan Radicis Cortex 15 g, Isatidis Folium 30 g, Imperatae Rhizoma 30 g, Hedyotidis Herba 20 g, Salviae Miltiorrhizae Radix 30 g, Cicadidae Periostracum 15 g, Batryticatus Bombyx 15 g, Zaocys 15 g, Astragali Radix 30 g, Glycyrrhizae Radix et Rhizoma 10 g | Decoction |
| Feng (2009) | Wushe decoction | Prepared by Fang (2009) | Zaocys 20 g, Kalopanacis Cortex 15 g, Radix Paeoniae Rubra 10 g, Saposhnikoviae Radix 10 g, Ecliptae Herba 10 g, Rehmanniae Radix Recens 10 g, Dictamni Radicis Cortex 15 g, Junci Medulla 6 g, Smilacis Rhizoma 15 g, Spatholobi Caulis 15 g, Persicae Semen 10 g, Lithospermi Radix 10 g, Glycyrrhizae Radix et Rhizoma 6 g | Decoction |
| Xie (2009) | Kangyin 1 decoction | Prepared by Xie (2009) | Rehmanniae Radix Recens 30 g, Hedyotidis Herba 30 g, Smilacis Rhizoma 30 g, Dictamni Radicis Cortex 20 g, Salviae Miltiorrhizae Radix 15 g, Isatidis Folium 15 g, Sophorae Flos 15 g, Polygoni Multiflori Caulis 15 g, Moutan Radicis Cortex 12 g, Radix Paeoniae Rubra 12 g, Lithospermi Radix 12 g Sophorae Tonkinensis Radix Et Rhizoma 6 g, Glycyrrhizae Radix et Rhizoma 6 g | Decoction |
| Ho (2010) | Wen-tong-hua-yu formulation | Prepared by Ho (2010) | Ephedrae Herba 6 g, Aconiti Lateralis Radix Preparata 10 g, Sinapis Semen 10 g, Cinnamomi Cortex 3 g, Zingiberis Rhizoma 3 g, Cornu Cervi Degelatinatum 15 g, Rehmanniae Radix Preparata 10 g, Smilacis Rhizoma 60 g, Dictamni Radicis Cortex 30 g, Imperatae Rhizoma 30 g, Salviae Miltiorrhizae Radix 15 g, Spatholobi Caulis 30 g, Lithospermi Radix 30 g, Sophorae Flos 30 g, Glycyrrhizae Radix et Rhizoma 6 g, Indigo Pulverata Levis 6 g | Decoction |
| Si (2010) | Jiawei Xiaoyaosan | Prepared by Si (2010) | Moutan Radicis Cortex 10 g, Gardeniae Fructus 10 g, Bupleuri Radix 6 g, Angelicae Gigantis Radix 10 g, Paeoniae Radix Alba 10 g, Poria Sclerotium 12 g, Atractylodis Rhizoma Alba 10 g, Menthae Herba 6 g, Zingiberis Rhizoma Recens 3 g, Glycyrrhizae Radix et Rhizoma 6 g | Decoction |
| Yan (2010) | Quyin decoction | Prepared by Yan (2010) | Rehmanniae Radix Recens, Rehmanniae Radix Preparata, Angelicae Gigantis Radix, Persicae Semen, Lithospermi Radix, Salviae Miltiorrhizae Radix, Carthami Flos, Cremastrae Tuber | Decoction |
| Ma (2010) | Psoriasis prescription | Prepared by Ma (2010) | Eupolyphaga 10 g, Indigo Pulverata Levis 10 g, Glycyrrhizae Radix et Rhizoma 10 g, Salviae Miltiorrhizae Radix 30 g, Hedyotidis Herba 30 g, Rehmanniae Radix Recens 30 g | Decoction |
| Ma (2011) | Keyin I prescription | Prepared by Ma (2011) | Lithospermi Radix 15 g, Radix Paeoniae Rubra 12 g, Rehmanniae Radix Recens 15 g, Carthami Flos 12 g, Angelicae Gigantis Radix 12 g, Scorpio 6 g, Bubali Cornu 20 g, Scolopendra 2 pieces, Sargentodoxa Cuneata 30 g, Scutellariae Radix 12 g, Forsythiae Fructus 12 g | Decoction |
| Wang (2011) | Tufuling Qingdai decoction | Prepared by Wang (2011) | Smilacis Rhizoma 30 g, Indigo Pulverata Levis 6 g, Lonicerae Flos 20 g, Glycyrrhizae Radix et Rhizoma 6 g, Tribuli Fructus 30 g, Sophorae Tonkinensis Radix Et Rhizoma 10 g, Dryopteridis Crassirhizomatis Rhizoma 15 g, Euphorbiae Humifusae Herba 30 g, Scorpio 3 g, Scolopendra 2 pieces, Lycii Radicis Cortex 15 g, Moutan Radicis Cortex 10 g | Decoction |
| Xie (2012) | Liangxie Runfu decoction | Prepared by Xie (2012) | Rehmanniae Radix Recens 30 g, Isatidis Folium 30 g, Smilacis Rhizoma 30 g, Imperatae Rhizoma 12 g, Sophorae Flos 15 g, Moutan Radicis Cortex 15 g, Glycyrrhizae Radix et Rhizoma 6 g | Decoction |
| Jia (2012) | Xiaobi decoction | Prepared by Jia (2012) | Lonicerae Flos 15 g, Forsythiae Fructus 15 g, Violae Herba 15 g, Taraxaci Herba 15 g, Rehmanniae Radix Recens 20 g, Zaocys 15 g, Vespae Nidus 10 g, Scutellariae Radix 15 g, Carthami Flos 15 g, Hirudo 10 g, Pinelliae Tuber 15 g | Decoction |
| Cheng (2012) | EAHM prescription for individual clinical trial | Prepared by Cheng (2012) | Bubali Cornu 10–15 g, Smilacis Rhizoma 6–10 g, Imperatae Rhizoma 10–15 g, Scutellariae Radix 6–10 g, Taraxaci Herba 10–15 g, Moutan Radicis Cortex 6–10 g, Kochiae Fructus 6 g, Spatholobi Caulis 6 g, Isatidis Radix 10 g | Decoction |
| Ma (2012a) | Liangxue jiedu decoction | Prepared by Ma (2012a) | Sophorae Flos 30 g, Imperatae Rhizoma 30 g, Lithospermi Radix 15 g, Radix Paeoniae Rubra 15 g, Rehmanniae Radix Recens 15 g, Moutan Radicis Cortex 15 g, Salviae Miltiorrhizae Radix 15 g, Isatidis Radix 30 g, Isatidis Folium 30 g, Lonicerae Flos 15 g, Forsythiae Fructus 12 g, Dictamni Radicis Cortex 15 g | Decoction |
| Ma (2012b) | Yinxiaobing decoction | Prepared by Ma (2012b) | Eupolyphaga 10 g, Salviae Miltiorrhizae Radix 30 g, Hedyotidis Herba 30 g, Indigo Pulverata Levis 10 g, Rehmanniae Radix Recens 30 g, Glycyrrhizae Radix et Rhizoma 10 g | Decoction |
| Zhang (2013) | Blood-cooling decoction | Prepared by Zhang (2013) | Rehmanniae Radix Recens 20 g, Moutan Radicis Cortex 15 g, Radix Paeoniae Rubra 15 g, Scrophulariae Radix 10 g, Sophorae Flos 10 g, Dictamni Radicis Cortex 10 g, Forsythiae Fructus 10 g, Lonicerae Flos 10 g, Smilacis Rhizoma 10 g, Saposhnikoviae Radix 10 g, Cicadidae Periostracum 10 g, Glycyrrhizae Radix et Rhizoma 10 g | Decoction |
| Liu (2013) | Wanji decoction | Prepared by Liu (2013) | Rehmanniae Radix Recens 20 g, Moutan Radicis Cortex 10 g, Dictamni Radicis Cortex 20 g, Hedyotidis Herba 20 g, Rhizoma Paridis 15 g, Schizonepetae Spica 10 g, Saposhnikoviae Radix 10 g, Mori Radicis Cortex 20 g, Scutellariae Radix 15 g, Lonicerae Flos 20 g, Taraxaci Herba 20 g, Forsythiae Fructus 20 g, Isatidis Radix 20 g, Isatidis Folium 10 g, Glycyrrhizae Radix et Rhizoma 10 g | Decoction |
| Xu (2013a) | Shufeng Yangxue decoction | Prepared by Xu (2013a) | Rehmanniae Radix Recens 18 g, Radix Paeoniae Rubra 15 g, Angelicae Gigantis Radix 15 g, Cnidii Rhizoma 10 g, Isatidis Radix 20 g, Lithospermi Radix 10 g, Cnidi Fructus 18 g, Sophorae Radix 18 g, Dictamni Radicis Cortex 15 g, Glycyrrhizae Radix et Rhizoma 6 g | Decoction |
| Xu (2013b) | Qingre Liangxue decoction | Prepared by Xu (2013b) | Bubali Cornu 15 g, Flos Sophorae Immaturus 12 g, Imperatae Rhizoma 30 g, Scutellariae Radix 30 g, Rehmanniae Radix Recens 30 g, Radix Paeoniae Rubra 12 g, Moutan Radicis Cortex 12 g, Spatholobi Caulis 30 g, Campsitis Flos 12 g, Salviae Miltiorrhizae Radix 30 g, Isatidis Radix 30 g | Decoction |
| Zhu (2014) | Dahuang Zhechong Capsule | Jiangsu Ehai Pharmaceutical Co., Ltd. | Rhei Radix et Rhizoma, Eupolyphaga, Persicae Semen, Lacca Rhois Exsiccata, Hirudo, Tabanus, Holotrichia, Scutellariae Radix, Persicae Semen, Armeniacae Semen, Rehmanniae Radix Preparata, Glycyrrhizae Radix et Rhizoma, Paeoniae Radix Alba | Capsule |
| Chen (2014) | Liangxue No.1 formula | Prepared by Chen (2014) | Bubali Cornu 20 g, Angelicae Gigantis Radix 10 g, Rehmanniae Radix Recens 15 g, Saposhnikoviae Radix 10 g, Cicadidae Periostracum 6 g, Anemarrhenae Rhizoma 6 g, Sophorae Radix 6 g, Sesami Semen Nigra 6 g, Schizonepetae Spica 10 g, Atractylodis Rhizoma 6 g, Arctii Fructus 6 g, Gypsum Fibrosum 10 g, Glycyrrhizae Radix et Rhizoma 3 g, Akebiae Caulis 3 g, Moutan Radicis Cortex 10 g, Radix Paeoniae Rubra 10 g | Decoction |
| Qian (2014) | Liangxue Runfu decoction | Prepared by Qian (2014) | Radix Paeoniae Rubra 12 g, Dictamni Radicis Cortex 12 g, Salviae Miltiorrhizae Radix 12 g, Saposhnikoviae Radix 15 g, Tribuli Fructus 15 g, Smilacis Rhizoma 30 g, Isatidis Folium 30 g, Rehmanniae Radix Recens 30 g, Sophorae Flos 15 g, Solani Nigri Herba 15 g, Moutan Radicis Cortex 15 g, Imperatae Rhizoma 12 g, Glycyrrhizae Radix et Rhizoma 6 g | Decoction |
| Peng (2014) | Liangxue Jiedu decoction | Prepared by Peng (2014) | Sophorae Flos 30 g, Isatidis Radix 30 g, Smilacis Rhizoma 30 g, Isatidis Folium 30 g, Imperatae Rhizoma 15~30 g, Sophorae Tonkinensis Radix Et Rhizoma 15 g, Lithospermi Radix 15 g, Rehmanniae Radix Recens 15 g, Moutan Radicis Cortex 15 g, Salviae Miltiorrhizae Radix 15 g, Lonicerae Flos 15 g, Dictamni Radicis Cortex 15 g, Forsythiae Fructus 12 g | Decoction |
| Dou (2014) | Wuteng Xiaoyin decoction | Prepared by Dou (2014) | Zaocys 15~30 g, Sargentodoxa Cuneata 20 g, Polygoni Multiflori Radix 20 g, Spatholobi Caulis 20 g, Curcumae Radix 15 g, Salviae Miltiorrhizae Radix 30 g, Radix Paeoniae Rubra 15 g, Rehmanniae Radix Recens 20 g, Persicae Semen 10 g, Rhei Radix et Rhizoma 3 g, Smilacis Rhizoma 30 g, Dictamni Radicis Cortex 20 g, Glycyrrhizae Radix et Rhizoma 6 g | Decoction |
| Miao (2014) | Quyin decoction | Prepared by Miao (2014) | Tripterygium wilfordii 50 g, Hedyotidis Herba 50 g, Astragali Radix 30 g, Salviae Miltiorrhizae Radix 30 g | Decoction |
| Xu (2015) | Liangxue Jiedu decoction | Prepared by Xu (2015) | Bubali Cornu 30 g, Rehmanniae Radix Recens 30 g, Moutan Radicis Cortex 10 g, Radix Paeoniae Rubra 10 g, Angelicae Gigantis Radix 12 g, Cnidii Rhizoma 10 g, Schizonepetae Spica 10 g, Saposhnikoviae Radix 10 g, Hedyotidis Herba 15 g, Lonicerae Flos 10 g, Salviae Miltiorrhizae Radix 15 g, Spatholobi Caulis 20 g, Lithospermi Radix 15 g, Dictamni Radicis Cortex 15 g, Glycyrrhizae Radix et Rhizoma 10 g | Decoction |
| Zhang (2015) | Zinyin Qingre Xiaofeng san | Prepared by Zhang (2015) | Rehmanniae Radix Recens 30 g, Moutan Radicis Cortex 10 g, Radix Paeoniae Rubra 10 g, Liriopis seu Ophiopogonis Tuber 10 g, Scrophulariae Radix 10 g, Salviae Miltiorrhizae Radix 10 g, Cannabis Semen 10 g, Isatidis Folium 10 g, Sophorae Tonkinensis Radix Et Rhizoma 10 g, Dictamni Radicis Cortex 10 g | Decoction |
| Yang (2015) | Qingre Liangxue decoction | Prepared by Yang (2015) | Bubali Cornu 15 g, Radix Paeoniae Rubra 12 g, Flos Sophorae Immaturus 12 g, Moutan Radicis Cortex 12 g, Campsitis Flos 12 g, Scutellariae Radix 30 g, Isatidis Radix 30 g, Rehmanniae Radix Recens 30 g, Spatholobi Caulis 30 g, Imperatae Rhizoma 30 g, Salviae Miltiorrhizae Radix 30 g | Decoction |
| Liang (2015) | Tufuling Qingdai decoction | Prepared by Liang (2015) | Smilacis Rhizoma 30 g, Indigo Pulverata Levis 6 g, Lonicerae Flos 20 g, Glycyrrhizae Radix et Rhizoma 6 g, Tribuli Fructus 30 g, Sophorae Tonkinensis Radix Et Rhizoma 10 g, Dryopteridis Crassirhizomatis Rhizoma 15 g, Lithospermi Radix 20 g, Euphorbiae Humifusae Herba 30 g, Scorpio 3 g, Scolopendra 2 pieces, Moutan Radicis Cortex 10 g | Decoction |
| Han (2015) | Huayinjiedu decoction | Prepared by Han (2015) | Rehmanniae Radix Recens, Radix Paeoniae Rubra, Salviae Miltiorrhizae Radix, Lithospermi Radix, Imperatae Rhizoma, Lonicerae Flos, Dioscorea bulbifera Rhizoma, Tribuli Fructus, Smilacis Rhizoma | Decoction |
| Xiang (2016) | Qinzhu Liangxue decoction | Prepared by Xiang (2016) | Scutellariae Radix 12 g, Margaritifera Concha 12 g, Salviae Miltiorrhizae Radix 15 g, Lithospermi Radix 9 g, Fluoritum 30 g, | Decoction |
| Wang (2016) | Liangxue Runfu decoction | Prepared by Wang (2006) | Smilacis Rhizoma 30 g, Isatidis Folium 30 g, Rehmanniae Radix Recens 30 g, Radix Paeoniae Rubra 12 g, Dictamni Radicis Cortex 12 g, Salviae Miltiorrhizae Radix 12 g, Saposhnikoviae Radix 15 g, Tribuli Fructus 15 g, Sophorae Flos 12 g, Solani Nigri Herba 12 g, Moutan Radicis Cortex 12 g, Imperatae Rhizoma 12 g, Glycyrrhizae Radix et Rhizoma 6 g. | Decoction |
| Zhou (2016) | Shufeng jiedu capsules | Anhui Jiren Pharmaceutical Industry Co., Ltd. | Isatidis Radix, Polygoni Cuspidati Rhizoma et Radix, Forsythiae Fructus, Patriniae Radix, Bupleuri Radix, Phragmitis Rhizoma, Verbenae Herba, Glycyrrhizae Radix et Rhizoma | Capsule |
| Du (2016a) | Shengdi Baimao decoction | Prepared by Du (2016a) | Rehmanniae Radix Recens 20 g, Imperatae Rhizoma 20 g, Smilacis Rhizoma 20 g, Poria Sclerotium 20 g, Coicis Semen 20 g, Sophorae Flos 15 g, Lithospermi Radix 10 g, Spatholobi Caulis 10 g, Atractylodis Rhizoma 10 g | Decoction |
| Du (2016b) | Heat-clearing and detoxicating oral liquid | Beijing Tongrentang Technology Development Co., Ltd. | Gypsum Fibrosum, Lonicerae Flos, Scrophulariae Radix, Rehmanniae Radix Recens, Forsythiae Fructus, Gardeniae Fructus, Gueldenstaedtia Verna, Gentianae Scabrae Radix et Rhizoma, Isatidis Radix, Anemarrhenae Rhizoma, Liriopis seu Ophiopogonis Tuber | Liquid |
| Mao (2017) | Xiaoyinfang | Prepared by Mao (2017) | Sophorae Tonkinensis Radix Et Rhizoma, Imperatae Rhizoma, Isatidis Radix, Sophorae Radix, Euphorbiae Helioscopiae Herba, Lithospermi Radix, Rhei Radix et Rhizoma, Rehmanniae Radix Recens, Scutellariae Radix, Salviae Miltiorrhizae Radix, Sargentodoxa Cuneata, Dictamni Radicis Cortex | Deccotion |
| Xia (2018) | Kanli fang | Prepared by Xia (2018) | Anemarrhenae Rhizoma 10 g, Rehmanniae Radix Recens 10 g, Glehniae Radix 10 g, Liriopis seu Ophiopogonis Tuber 10 g, Gardeniae Fructus 10 g, Lilii Bulbus 10 g, Lophatheri Herba 10 g, Zizyphi Semen 10 g, Phellodendri Cortex 10 g, Corni Fructus 9 g, Prunellae Spica 10 g | Decoction |
| Li (2018) | Jianpi Jiedu decoction | Prepared by Li (2018) | Smilacis Rhizoma 30 g, Poria Sclerotium 12 g, Atractylodis Rhizoma Alba 10 g, Tokoro Rhizoma 10 g, Sophorae Radix 10 g, Hedyotidis Herba 30 g, Forsythiae Fructus 15 g, Salviae Miltiorrhizae Radix 10 g, Phellodendri Cortex 10 g, Coicis Semen 30 g | Decoction |
| Yang (2018) | Jinyu Xiaoyin granules | Shaanxi Kanghui Pharmaceutical Co., Ltd. | Curcumae Radix, Tribuli Fructus, Angelicae Gigantis Radix, Curcumae Rhizoma, Sargentodoxa Cuneata, Clematidis Radix, Paeoniae Radix Alba, Dictamni Radicis Cortex, Cnidi Fructus | Granule |
| Wang (2019) | Qingying tang | Prepared by Wang (2019) | Gazellae seu Saigae Cornu 0.6 g, Rehmanniae Radix Recens 15 g, Moutan Radicis Cortex 12 g, Forsythiae Fructus 15 g, Isatidis Radix 15 g, Imperatae Rhizoma 15 g, Radix Paeoniae Rubra 10 g, Plantaginis Semen 15 g, Spatholobi Caulis 10 g, Lonicerae Flos 20 g, Taraxaci Herba 10 g | Decoction |
| Zhang (2019) | Xijiao Dihuang decoction | Prepared by Zhang (2019) | Bubali Cornu 30 g, Rehmanniae Radix Recens 24 g, Forsythiae Fructus 15 g, Lonicerae Flos 15 g, Paeoniae Radix Alba 12 g, Moutan Radicis Cortex 9 g, Platycodonis Radix 6 g, Menthae Herba 6 g, Arctii Fructus 6 g, Glycyrrhizae Radix et Rhizoma 5 g, Schizonepetae Spica 5 g, Glycine Semen Preparata 6 g, Lophatheri Herba 4 g | Decoction |
| Jiang (2019) | Xijiao Dihuang Jiedu decoction | Prepared by Jiang (2019) | Bubali Cornu 30 g, Zingiberis Rhizoma Recens 20 g, Moutan Radicis Cortex 20 g, Radix Paeoniae Rubra 20 g | Decoction |
| Wen (2019) | Xiaobi decoction | Prepared by Wen (2019) | Lonicerae Flos 15 g, Forsythiae Fructus 15 g, Scutellariae Radix 15 g, Rehmanniae Radix Recens 20 g, Zaocys 15 g, Violae Herba 15 g, Pinelliae Tuber 15 g, Persicae Semen 15 g, Carthami Flos 15 g, Taraxaci Herba 15 g, Vespae Nidus 10 g, Hirudo 10 g, Cinnamomi Ramulus 8 g | Decoction |
| Chen (2020) | Quyin decoction | Prepared by Chen (2020) | Glycyrrhizae Radix et Rhizoma 5 g, Smilacis Rhizoma 30 g, Moutan Radicis Cortex 10 g, Bubali Cornu 15 g, Rehmanniae Radix Recens 15 g, Isatidis Radix 15 g, Dictamni Radicis Cortex 10 g, Scrophulariae Radix 15 g, Kochiae Fructus 15 g, Hedyotidis Herba 30 g, Plantaginis Herba 10 g, Salviae Miltiorrhizae Radix 15 g, Alismatis Rhizoma 10 g | Decoction |
| No. | EAHM (Latin Name) | Frequency of Prescription | Relative Frequency (%) | Properties | Flavors | Action Category |
|---|---|---|---|---|---|---|
| 1 | Rehmanniae Radix Recens | 39 | 69.64 | Cold | Sweet | Clearing heat to cool blood |
| 2 | Salviae Miltiorrhizae Radix | 28 | 50.00 | Cold | Bitter | Activating blood and removing blood stasis |
| 3 | Glycyrrhizae Radix et Rhizoma | 27 | 48.21 | Neutral | Sweet | Tonifying qi |
| 4 | Moutan Radicis Cortex | 27 | 48.21 | Cold | Bitter | Clearing heat to cool blood |
| 5 | Lithospermi Radix | 24 | 42.86 | Cold | Sweet | Clearing heat to cool blood |
| 6 | Smilacis Rhizoma | 24 | 42.86 | Neutral | Sweet | Clearing heat and toxic materials |
| 7 | Radix Paeoniae Rubra | 21 | 37.50 | Cold | Bitter | Clearing heat to cool blood |
| 8 | Dictamni Radicis Cortex | 20 | 35.71 | Cold | Bitter | Clearing heat and dampness |
| 9 | Imperatae Rhizoma | 17 | 30.36 | Cold | Sweet | Cooling blood to arrest bleeding |
| 10 | Hedyotidis Herba | 15 | 26.79 | Cold | Bitter | Clearing heat and toxic materials |
| 11 | Isatidis Radix | 15 | 26.79 | Cold | Bitter | Clearing heat and toxic materials |
| 12 | Lonicerae Flos | 14 | 25.00 | Cold | Sweet | Clearing heat and toxic materials |
| 13 | Sophorae Flos | 14 | 25.00 | Cold | Bitter | Cooling blood to arrest bleeding |
| 14 | Scutellariae Radix | 13 | 23.21 | Cold | Bitter | Clearing heat and dampness |
| 15 | Forsythiae Fructus | 12 | 21.43 | Cold | Bitter | Clearing heat and toxic materials |
| 16 | Spatholobi Caulis | 12 | 21.43 | Warm | Bitter | Activating blood and removing blood stasis |
| First Author (Year) | EAHM (Latin Name) | Target Cell Line or Animal Model | Possible Active Ingredients | Possible Mechanisms |
|---|---|---|---|---|
| Sui (2013) [109] | Rehmanniae Radix Recens | -UVB ray treated mice | Radix Rehmanniae polysaccharides | -Enhancing serum IL-2, IL-4, and IL-10 levels -Enhancing skin GSH, SOD, CAT, and GSH-Px activities -Decreasing skin MDA level |
| Ma (2016) [110] | Salviae Miltiorrhizae Radix | -lipopolysaccharide-stimulated THP-1 macrophages | Tanshinone IIB, Danshixinkun B, Danshenol A, Arucadiol, Tanshindiol C, Salviolone, and Sugiol | -Inhibiting the expression of TNF-α, IL-1β, and IL-8 |
| Yu (2017) [111] | Glycyrrhizae Radix et Rhizoma | -human monocyte model THP-1 -AD-like skin lesion model mice | Isoliquiritigenin | -Suppressing the up-regulation of CD86 and CD54 and abolished the DNCB-induced p38-α and ERK activation -Suppressing the DNCB-induced IgE and Th2 cytokines up-regulation -Inhibiting DNCB-induced pro-inflammatory cytokines such as TNF-α, IL-6 as well as IL-4 expressions |
| Yun (2013) [112] | Moutan Radicis Cortex | -stimulated with LPS in cultured HGFs | Paeonol, Paeoniflorin | -Inhibiting a wide variety of activations of inflammation-related genes |
| Kim (2007) [113] | Lithospermi Radix | -rat peritoneal mast cells -PCA rat | Shikonin | -Inhibiting the release of histamine in a dose-dependent manner -Inhibiting the anti-DNP IgE-induced passive cutaneous anaphylaxis reaction and IL-6, IL-8, and TNF-α expression -Inhibiting NF-κB activation and IκB-alpha degradation |
| Ki (2016) [114] | Smilacis Rhizoma | -AD-like skin lesion model mice | Astilbin, Neoastilbin, Isoastilbin, Neoisoastilbin, Engeletin and Isoengeletin | -Decreasing in both Th2 and Th1 serum antibodies -Suppressing expression of IL-4, IL-13, IL-17, IL-18, TSLP, and IFN-γ genes |
| Zhao (2016) [115] | Radix Paeoniae Rubra | -IMQ-induced psoriasis mice | Paeoniflorin | -Inhibiting IMQ-induced psoriasis by regulating Th17 cell response and cytokine secretion via phosphorylation of Stat3. |
| Yang (2017) [116] | Dictamni Radicis Cortex | -DNFB-induced CD mice | Fraxinellone | -Reducing the levels of TNF-α, IFN-γ, and IL-6 in inflamed tissues -Inhibiting enlargement of dorsal skin and prevented epidermal hyperplasia, hyperkeratosis, and spongiotic changes in inflamed tissues -Ameliorating skin lesions such as crust, scales, incrustation and petechiae, and lowered erythema index on skin surface |
| Ruan (2022) [117] | Imperatae Rhizoma | -LPS stimulated RAW 264.7 cells | Imperphenoside A, Imperphenols B and C, Imperphenosides D-F, Imperlignanosides A-D | -Nitric oxide inhibitory effects -Restraining the phosphorylation of nuclear factor kappa-B kinase to down-regulate the protein expression of inflammatory cytokines such as inducible nitric oxide synthase, interleukin-6 and tumor necrosis factor-α |
| Chen (2016) [118] | Hedyotidis Herba | -LPS stimulated RAW 264.7 cells | Total flavonoids | -Inhibiting the LPS-induced activation of NF-κB via the suppression of inhibitor of κB (IκB) phosphorylation -Reducing the phosphorylation of MAPK signaling molecules, which resulted in the inhibition of cytokine expression |
| Fan (2021) [119] | Isatidis Radix | -LPS stimulated RAW 264.7 cells | Acidic fraction | -Inhibiting the secretion of inflammatory cytokines (PGE2, IL-6, IL-1β, and NO, other than TNF-α) in a dose-dependent manner -Downregulating the expression of iNOS and COX-2 -Suppressing the phosphorylation of ERK1/2, JNK, and p38 -Reducing the translocation of NF-κB from the cytoplasm to nucleus |
| Wu (2020) [120] | Lonicerae Flos | -TPA (12-O-tetradecanoylphorbol-13-acetate)-induced ear edema mouse model -LPS-stimulated RAW264.7 cells | Chrysoeriol | -Lowering protein levels of phospho-p65 (Ser536), phospho-STAT3 (Tyr705), iNOS, COX-2, IL-6, IL-1β, and TNF-α -Decreasing the production of NO and PGE2 -Inhibiting the phosphorylation of inhibitor of κB (Ser32), p65 (Ser536), and Janus kinase 2 (Tyr1007/1008) -Decreasing nuclear localization of p50, p65, and STAT3 -Down regulating mRNA levels of pro-inflammatory cytokines IL-6, IL-1β and TNF-α |
| Lee (2013) [121] | Sophorae Flos | -BALB/c mice | Sophoricoside | -Inhibiting the phosphorylation and degradation of IκBα/β and the nuclear translocation of NF-κB p65 in B cells -Ameliorating DNCB-induced acute and chronic contact dermatitis |
| Wang (2022) [122] | Scutellariae Radix | - BALB/c mice treated with DNCB to induce AD-like skin lesions | Baicalin | -Up-regulating the protein expressions of filaggrin, involucrin, and loricrin -Inhibited the inflammatory response and the activation of NF-κB and JAK/STAT pathways -Inhibiting the release of IgE, histamine, TNF-α and IL-4 |
| Sung (2016) [123] | Forsythiae Fructus | -Dermatophagoides farinae-induced atopic dermatitis in NC/Nga mice | Forsythoside A, Phillyrin, Pinoresinol, Phylligenin | -Attenuating serum levels of IgE, TNF-α, and histamine -Inhibiting the expression of chemokines, cytokines, and adhesion molecules -Inhibiting the production of chemokines in TNF-α/IFN-γ-activated human keratinocytes. |
| Tang (2020) [124] | Spatholobi Caulis | -cell model of oxygen-glucose deprivation | Spatholobi Caulis total extract | -Decreasing the protein expression of tissue factor -Enhancing SIRT1 protein expression and reduced Ace-p65 nuclear protein expression -Promotin protein expressions of nuclear Nrf2 and total HO-1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.-G.; Kim, H.; Lee, D. Oral Administration of East Asian Herbal Medicine for Inflammatory Skin Lesions in Plaque Psoriasis: A Systematic Review, Meta-Analysis, and Exploration of Core Herbal Materials. Nutrients 2022, 14, 2434. https://doi.org/10.3390/nu14122434
Jo H-G, Kim H, Lee D. Oral Administration of East Asian Herbal Medicine for Inflammatory Skin Lesions in Plaque Psoriasis: A Systematic Review, Meta-Analysis, and Exploration of Core Herbal Materials. Nutrients. 2022; 14(12):2434. https://doi.org/10.3390/nu14122434
Chicago/Turabian StyleJo, Hee-Geun, Hyehwa Kim, and Donghun Lee. 2022. "Oral Administration of East Asian Herbal Medicine for Inflammatory Skin Lesions in Plaque Psoriasis: A Systematic Review, Meta-Analysis, and Exploration of Core Herbal Materials" Nutrients 14, no. 12: 2434. https://doi.org/10.3390/nu14122434
APA StyleJo, H.-G., Kim, H., & Lee, D. (2022). Oral Administration of East Asian Herbal Medicine for Inflammatory Skin Lesions in Plaque Psoriasis: A Systematic Review, Meta-Analysis, and Exploration of Core Herbal Materials. Nutrients, 14(12), 2434. https://doi.org/10.3390/nu14122434








