Glucose as a Potential Key to Fuel Inflammation in Rheumatoid Arthritis
Abstract
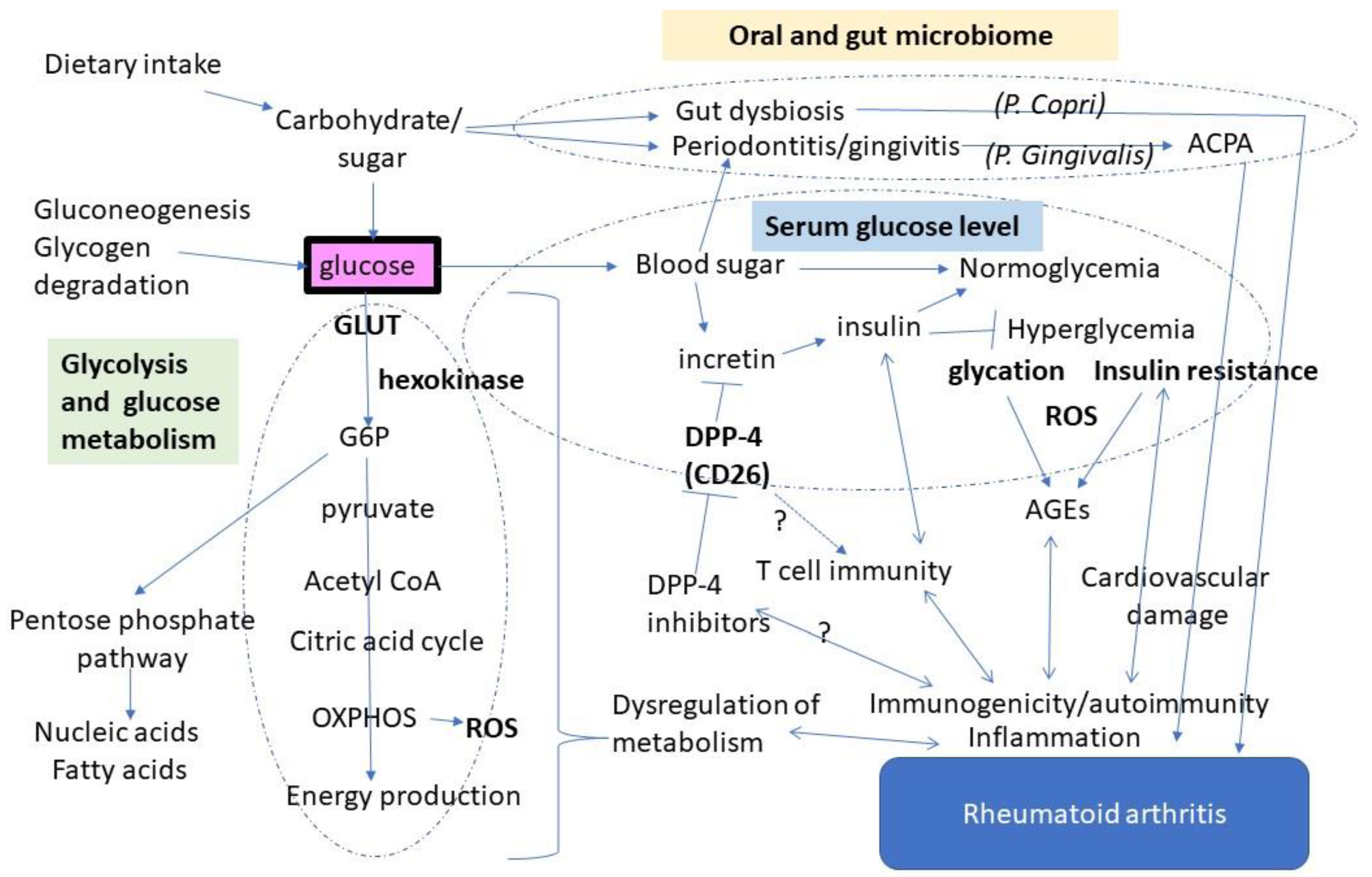
1. Introduction
2. Glucose Metabolism Shifts to Glycolysis in Rheumatoid Synovium
3. Glucose May Enhance Autoimmune Responses via Antigen Modification
4. Diabetes and Autoimmunity: Is There a Link through DPP-4?
5. Modulation of the Gut and Oral Microbiome by Glucose
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scheepers, A.; Joost, H.G.; Schürmann, A. The glucose transporter families SGLT and GLUT: Molecular basis of normal and aberrant function. JPEN J. Parenter. Enter. Nutr. 2004, 28, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heredero, G.; Gómez de Las Heras, M.M.; Gabandé-Rodríguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef] [PubMed]
- Warmoes, M.O.; Locasale, J.W. Heterogeneity of glycolysis in cancers and therapeutic opportunities. Biochem. Pharm. 2014, 92, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A. Drivers of the Warburg phenotype. Cancer J. 2015, 21, 56–61. [Google Scholar] [CrossRef]
- Gill, K.S.; Fernandes, P.; O’Donovan, T.R.; McKenna, S.L.; Doddakula, K.K.; Power, D.G.; Soden, D.M.; Forde, P.F. Glycolysis inhibition as a cancer treatment and its role in an anti-tumour immune response. Biochim. Biophys. Acta. 2016, 1866, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.F.; Garcia-Carbonell, R.; Whisenant, K.D.; Guma, M. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2017, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonell, R.; Divakaruni, A.S.; Lodi, A.; Vicente-Suarez, I.; Saha, A.; Cheroutre, H.; Boss, G.R.; Tiziani, S.; Murphy, A.N.; Guma, M. Critical role of glucose metabolism in rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheumatol. 2016, 68, 1614–1626. [Google Scholar] [CrossRef]
- Bustamante, M.F.; Oliveira, P.G.; Garcia-Carbonell, R.; Croft, A.P.; Smith, J.M.; Serrano, R.L.; Sanchez-Lopez, E.; Liu, X.; Kisseleva, T.; Hay, N.; et al. Hexokinase 2 as a novel selective metabolic target for rheumatoid arthritis. Ann. Rheum. Dis. 2018, 77, 1636–1643. [Google Scholar] [CrossRef]
- Biniecka, M.; Canavan, M.; McGarry, T.; Gao, W.; McCormick, J.; Cregan, S.; Gallagher, L.; Smith, T.; Phelan, J.J.; Ryan, J.; et al. Dysregulated bioenergetics: A key regulator of joint inflammation. Ann. Rheum. Dis. 2016, 75, 2192–2200. [Google Scholar] [CrossRef]
- Koedderitzsch, K.; Zezina, E.; Li, L.; Herrmann, M.; Biesemann, N. TNF induces glycolytic shift in fibroblast like synoviocytes via GLUT1 and HIF1A. Sci. Rep. 2021, 11, 19385. [Google Scholar] [CrossRef]
- Kvacskay, P.; Yao, N.; Schnotz, J.H.; Scarpone, R.; Carvalho, R.A.; Klika, K.D.; Merkt, W.; Tretter, T.; Lorenz, H.M.; Tykocinski, L.O. Increase of aerobic glycolysis mediated by activated T helper cells drives synovial fibroblasts towards an inflammatory phenotype: New targets for therapy? Arthritis Res. 2021, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of glycemic indices (hyperglycemia, glucose variability, and hypoglycemia) with oxodative stress and diabetic compliations. J. Diabetee Res. 2020, 7489795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collier, B.; Dossett, L.A.; May, A.K.; Diaz, J.J. Glucose control and the inflammatory response. Nutr. Clin. Pr. 2008, 23, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharm. 2019, 70, 809–824. [Google Scholar]
- Chu, C.Q. Highlights of strategies targeting fibroblasts for novel therapies for rheumatoid arthritis. Front. Med. 2022, 9, 846300. [Google Scholar] [CrossRef]
- Monu, P.; Agnihotri, P.; Biswas, S. AGE/non-AGE glycation: An important event in rheumatoid arthritis pathophysiology. Inflammation 2022, 45, 477–496. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, L.; Regueiro, C.; Amhaz-Escanlar, S.; Pena, C.; Herbello-Hermelo, P.; Moreda-Pineiro, A.; Rodriguez-Garcia, J.; Mera-Varela, A.; Perez-Pampin, E.; Gonzalez, A. Antibodies against 4 atypical post-translational protein modifications in patients with rheumatoid arthritis. Diagnostics 2022, 12, 352. [Google Scholar] [CrossRef]
- Kwon, E.J.; Ju, J.H. Impact of posttranslational modification in pathogenesis of rheumatoid arthritis: Focusing on citrullination, carbamylation, and acetylation. Int. J. Mol. Sci. 2021, 22, 10576. [Google Scholar] [CrossRef]
- Trouw, L.A.; Rispens, T.; Toes, R.E.M. Beyond citrullination: Other post-translational protein modifications in rheumatoid arthritis. Nat. Rev. Rheumatol. 2017, 13, 331–339. [Google Scholar] [CrossRef]
- Rantapää-Dahlqvist, S.; de Jong, B.A.; Berglin, E.; Hallmans, G.; Wadell, G.; Stenlund, H.; Sundin, U.; van Venrooij, W.J. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003, 48, 2741–2749. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative stress and advanced lipoxidation and glycation end products (ALEs and AGEs) in aging and age-related diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef] [PubMed]
- Egawa, T.; Hayashi, T. Association of glycative stress with motor and muscle function. Front. Physiol. 2022, 13, 855358. [Google Scholar] [CrossRef] [PubMed]
- Vytásek, R.; Sedová, L.; Vilím, V. Increased concentration of two different advanced glycation end-products detected by enzyme immunoassays with new monoclonal antibodies in sera of patients with rheumatoid arthritis. BMC Musculoskelet Disord. 2010, 11, 83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khan, M.W.A.; Al Otaibi, A.; Sherwani, S.; Khan, W.A.; Alshammari, E.M.; Al-Zahrani, S.A.; Saleem, M.; Khan, S.N.; Alouffi, S. Glycation and oxidative stress increase autoantibodies in the elderly. Molecules 2020, 25, 3675. [Google Scholar] [CrossRef]
- Ahmad, S.; Moinuddin, A.A. Immunological studies on glycated human IgG. Life Sci. 2012, 90, 980–987. [Google Scholar] [CrossRef]
- Albano, S.A.; Santana-Sahagun, E.; Weisman, M.H. Cigarette smoking and rheumatoid arthritis. Semin. Arthritis Rheum. 2001, 31, 146–159. [Google Scholar] [CrossRef]
- Sugiyama, D.; Nishimura, K.; Tamaki, K.; Tsuji, G.; Nakazawa, T.; Morinobu, A.; Kumagai, S. Impact of smoking as a risk factor for developing rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2010, 69, 70–81. [Google Scholar] [CrossRef]
- Yoshida, K.; Wang, J.; Malspeis, S.; Marchand, N.; Lu, B.; Prisco, L.C.; Martin, L.W.; Ford, J.A.; Costenbader, K.H.; Karlson, E.W.; et al. Passive smoking throughout the life course and the risk of incident rheumatoid arthritis in adulthood among women. Arthritis Rheumatol. 2021, 73, 2219–2228. [Google Scholar] [CrossRef]
- Sharma, H.; Sahlot, R.; Purwar, N.; Garg, U.; Saran, S.; Sharma, B.; Mathur, S.K. Co-existence of type 1 diabetes and other autoimmune ailments in subjects with autoimmune thyroid disorders. Diabetes Metab. Syndr. 2022, 16, 102405. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Liu, Q.; Tian, Z.; Zhang, Y. Mechanistic and therapeutic links between rheumatoid arthritis and diabetes mellitus. Clin. Exp. Med. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, L.; Cregan, S.; Biniecka, M.; Cunningham, C.; Veale, D.J.; Kane, D.J.; Fearon, U.; Mullan, R.H. Insulin-resistant pathways are associated with disease activity in rheumatoid arthritis and are subject to disease modification through metabolic reprogramming: A potential novel therapeutic approach. Arthritis Rheumatol. 2020, 72, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Tang, X.; Pang, M. Prevalence of metabolic syndrome in patients with rheumatoid arthritis: An updated systematic review and meta-analysis. Front. Med. 2022, 9, 855141. [Google Scholar] [CrossRef] [PubMed]
- Aussel, C.; Desmoulins, D.; Agneray, J.; Ekindjian, O.G. Effect of insulin on aminoisobutyric acid uptake by human non-rheumatoid and rheumatoid synovial cells. FEBS Lett. 1987, 214, 327–330. [Google Scholar] [CrossRef]
- Han, J.M.; Patterson, S.J.; Speck, M.; Ehses, J.A.; Levings, M.K. Insulin inhibits IL-10-mediated regulatory T cell function: Implications for obesity. J. Immunol. 2014, 192, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Y.; Lin, S.H.; Li, N.; Han, Y.; Huang, Q.; Zhao, Y.; Xie, F.; Guo, Y.; Deng, B.; et al. Insulin signaling establishes a developmental trajectory of adipose regulatory T. cells. Nat. Immunol. 2021, 22, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.G.; Farinon, M.; Sanchez-Lopez, E.; Miyamoto, S.; Guma, M. Fibroblast-like synoviocytes glucose metabolism as a therapeutic target in rheumatoid arthritis. Front. Immunol. 2019, 10, 1743. [Google Scholar] [CrossRef]
- Masoumi, M.; Mehrabzadeh, M.; Mahmoudzehi, S.; Mousavi, M.J.; Jamalzehi, S.; Sahebkar, A.; Karami, J. Role of glucose metabolism in aggressive phenotype of fibroblast-like synoviocytes: Latest evidence and therapeutic approaches in rheumatoid arthritis. Int. Immunopharmacol 2020, 89, 107064. [Google Scholar] [CrossRef]
- Tripolino, C.; Ciaffi, J.; Pucino, V.; Ruscitti, P.; van Leeuwen, N.; Borghi, C.; Giacomelli, R.; Meliconi, R.; Ursini, F. Insulin signaling in arthritis. Front. Immunol. 2021, 12, 672519. [Google Scholar] [CrossRef]
- Holst, J.J. The incretin system in healthy humans: The role of GIP and GLP-1. Metabolism 2019, 96, 46–55. [Google Scholar] [CrossRef]
- Deacon, C.F. Physiology and pharmacology of DPP-4 in glucose homeostasis and the treatment of Type 2 diabetes. Front. Endocrinol. 2019, 10, 80. [Google Scholar] [CrossRef]
- Shao, S.; Xu, Q.; Yu, X.; Pan, R.; Chen, Y. Dipeptidyl peptidase 4 inhibitors and their potential immune modulatory functions. Pharm. Ther. 2020, 209, 107503. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, L.; Wang, X.; Zhou, Z. The new insights from DPP-4 inhibitors: Their potential immune modulatory function in autoimmune diabetes. Diabetes Metab. Res. Rev. 2014, 30, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Cordero, O.J.; Varela-Calvino, R.; Lopez-Gonzalez, T.; Calvino-Sampedro, C.; Vinuela, J.E.; Mourino, C.; Hernandez-Rodriguez, I.; Rodriguez-Lopez, M.; Aspe de la Iglesia, B.; Pego-Reigosa, J.M. CD26 expression on T helper populations and sCD26 serum levels in patients with rheumatoid arthritis. PLoS ONE 2015, 1, e0131992. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, F.; Mousavi, S.M.J.; Hosseinzadeh-Sarband, S.; Ahmadzadeh, A.; Bahrami-Motlagh, H.; Hoseini, M.H.M.; Sattari, M.; Sohrabi, M.R.; Pouriran, R.; Dehghan, P. Association of CD26/dipeptidyl peptidase IV mRNA level in peripheral blood mononuclear cells with disease activity and bone erosion in rheumatoid arthritis. Clin. Rheumatol. Assoc. CD26 2018, 37, 3183–3190. [Google Scholar] [CrossRef]
- Yamauchi, K.; Sato, Y.; Yamashita, K.; Funase, Y.; Kaneko, T.; Hashimoto, T.; Aizawa, T. RS3PE in association with dipeptidyl peptidase-4 inhibitor: Report of two cases. Diabetes Care 2012, 35, e7. [Google Scholar] [CrossRef][Green Version]
- Yokota, K.; Igaki, N. Sitagliptin (DPP-4 inhibitor)-induced rheumatoid arthritis in type 2 diabetes mellitus: A case report. Intern. Med. 2012, 51, 2041–2044. [Google Scholar] [CrossRef]
- Padron, S.; Rogers, E.; Demory Beckler, M.; Kesselman, M. DPP-4 inhibitor (sitagliptin)-induced seronegative rheumatoid arthritis. BMJ Case Rep. 2019, 12, e228981. [Google Scholar] [CrossRef]
- Douros, A.; Abrahami, D.; Yin, H.; Yu, O.H.Y.; Renoux, C.; Hudson, M.; Azoulay, L. Use of dipeptidyl peptidase-4 inhibitors and new-onset rheumatoid arthritis in patients with Type 2 diabetes. Epidemiology 2018, 29, 904–912. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, T.H.; Sun, C.C.; Chen, J.Y.; Chang, S.S.; Yeung, L.; Tsai, Y.W. Dipeptidyl peptidase-4 inhibitors and the risks of autoimmune diseases in type 2 diabetes mellitus patients in Taiwan: A nationwide population-based cohort study. Acta. Diabetol. 2020, 57, 1181–1192. [Google Scholar] [CrossRef]
- Charoenngam, N.; Rittiphairoj, T.; Ponvilawan, B.; Ungprasert, P. Use of dipeptidyl peptidase-4 inhibitors is associated with a lower risk of rheumatoid arthritis in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of cohort studies. Diabetes Metab. Syndr. 2021, 15, 249–255. [Google Scholar] [CrossRef]
- Suezawa, M.; Dainichi, T.; Kaku, Y.; Izumi, M.; Kataoka, K.; Ishii, N.; Koga, H.; Izumi, K.; Nishie, W. Dipeptidyl peptidase 4 inhibitor-associated mucous membrane pemphigoid. J. Dermatol. 2021, 48, 1584–1587. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Sahoo, J.; Narayanan, N.; Merugu, C.; Kamalanathan, S.; Naik, D. Dipeptidyl peptidase-4 inhibitor-induced autoimmune diseases: Current evidence. World J. Diabetes 2021, 12, 1426–1441. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, X.; Wei, Y.; Li, X.; Gao, S.; Dong, L.; Rao, X.; Zhong, J. Emerging role of dipeptidyl peptidase-4 in autoimmune disease. Front. Immunol. 2022, 13, 830863. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Takeda, K. Role of gut microbiota in rheumatoid arthritis. J. Clin. Med. 2017, 6, 60. [Google Scholar] [CrossRef]
- Rosser, E.C.; Mauri, C. A clinical update on the significance of the gut microbiota in systemic autoimmunity. J. Autoimmun. 2016, 74, 85–93. [Google Scholar] [CrossRef]
- Bergot, A.S.; Giri, R.; Thomas, R. The microbiome and rheumatoid arthritis. Best Pr. Res. Clin. Rheumatol. 2019, 33, 101497. [Google Scholar] [CrossRef]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumatol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Alpizar-Rodriguez, D.; Lesker, T.R.; Gronow, A.; Gilbert, B.; Raemy, E.; Lamacchia, C.; Gabay, C.; Finckh, A.; Strowig, T. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 590–593. [Google Scholar] [CrossRef]
- Leite, A.Z.; Rodrigues, N.C.; Gonzaga, M.I.; Paiolo, J.C.C.; de Souza, C.A.; Stefanutto, N.A.V.; Omori, W.P.; Pinheiro, D.G.; Brisotti, J.L.; Matheucci Junior, E.; et al. Detection of increased plasma interleukin-6 levels and prevalence of Prevotella copri and Bacteroides vulgatus in the feces of Type 2 diabetes patients. Front. Immunol. 2017, 8, 1107. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Nguyen, L.H.; Li, Y.; Yan, Y.; Ma, W.; Rinott, E.; Ivey, K.L.; Shai, I.; Willett, W.C.; Hu, F.B.; et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat. Med. 2021, 27, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Picchianti Diamanti, A.; Panebianco, C.; Salerno, G.; Di Rosa, R.; Salemi, S.; Sorgi, M.L.; Meneguzzi, G.; Mariani, M.B.; Rai, A.; Iacono, D.; et al. Impact of Mediterranean diet on disease activity and gut microbiota composition of rheumatoid arthritis patients. Microorganisms 2020, 8, 1989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ju, Z.; Zuo, T. Time for food: The impact of diet on gut microbiota and human health. Nutrition 2018, 51–52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Liu, Q. Factors affecting gut microbiome in daily diet. Front. Nutr. 2021, 8, 644138. [Google Scholar] [CrossRef]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Khatib, H.A.; et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef]
- Moszak, M.; Szulińska, M.; Bogdański, P. You are what you eat-the relationship between diet, microbiota, and metabolic disorders-A review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef]
- Fajstova, A.; Galanova, N.; Coufal, S.; Malkova, J.; Kostovcik, M.; Cermakova, M.; Pelantova, H.; Kuzma, M.; Sediva, B.; Hudcovic, T.; et al. Diet rich in simple sugars promotes pro-inflammatory response via gut microbiota alteration and TLR4 signaling. Cells 2020, 9, 2701. [Google Scholar] [CrossRef]
- Correa-Rodríguez, M.; Pocovi-Gerardino, G.; Callejas-Rubio, J.L.; Ríos Fernández, R.; Martín-Amada, M.; Cruz-Caparros, M.G.; Medina-Martínez, I.; Ortego-Centeno, N.; Rueda-Medina, B. Dietary intake of free sugars is associated with disease activity and dyslipidemia in systemic lupus erythematosus patients. Nutrients 2020, 12, 1094. [Google Scholar] [CrossRef]
- Anhê, F.F.; Barra, N.G.; Schertzer, J.D. Glucose alters the symbiotic relationships between gut microbiota and host physiology. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E111–E116. [Google Scholar] [CrossRef]
- Silva, D.N.A.; Casarin, M.; Monajemzadeh, S.; Bezerra, B.B.; Lux, R.; Pirih, F.Q. The Microbiome in Periodontitis and Diabetes. Front Oral Health. 2022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hung, S.-L.; Lee, N.G.; Chang, L.Y.; Chen, Y.T.; Lai, Y.L. Stimulatory effects of glucose and Porphyromonas gingivalis lipopolysaccharide on the secretion of inflammatory mediators from human macrophages. J. Periodontol. 2014, 85, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moles, M.A.; Ramos-Garcia, P. State of evidence on oral health problems in diabetic patients: A critical review of the literature. J. Clin. Med. 2021, 10, 5383. [Google Scholar] [CrossRef] [PubMed]
- Bingham, C.O., III; Moni, M. Periodontal disease and rheumatoid arthritis: The evidence accumulates for complex pathobiologic interactions. Curr. Opin. Rheumatol. 2013, 25, 345–353. [Google Scholar] [CrossRef]
- Liu, J.; Gao, J.; Wu, Z.; Mi, L.; Li, N.; Wang, Y.; Peng, X.; Xu, K.; Wu, F.; Zhang, L. Anti-citrullinated Protein Antibody Generation, Pathogenesis, Clinical Application, and Prospects. Front Med 2022, 8, 802934. [Google Scholar]
- González-Febles, J.; Sanz, M. Periodontitis and rheumatoid arthritis: What have we learned about their connection and their treatment? Periodontol 2000 2021, 87, 181–203. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masuko, K. Glucose as a Potential Key to Fuel Inflammation in Rheumatoid Arthritis. Nutrients 2022, 14, 2349. https://doi.org/10.3390/nu14112349
Masuko K. Glucose as a Potential Key to Fuel Inflammation in Rheumatoid Arthritis. Nutrients. 2022; 14(11):2349. https://doi.org/10.3390/nu14112349
Chicago/Turabian StyleMasuko, Kayo. 2022. "Glucose as a Potential Key to Fuel Inflammation in Rheumatoid Arthritis" Nutrients 14, no. 11: 2349. https://doi.org/10.3390/nu14112349
APA StyleMasuko, K. (2022). Glucose as a Potential Key to Fuel Inflammation in Rheumatoid Arthritis. Nutrients, 14(11), 2349. https://doi.org/10.3390/nu14112349





