Piceatannol, a Dietary Polyphenol, Alleviates Adipose Tissue Loss in Pre-Clinical Model of Cancer-Associated Cachexia via Lipolysis Inhibition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. In Vitro Model of CAC-Induced Lipolysis
2.3. Western Blot
2.4. Isolation of Total RNA and PCR
2.5. Cell Viability
2.6. Animal Experiment
2.7. Statistical Analysis
3. Results
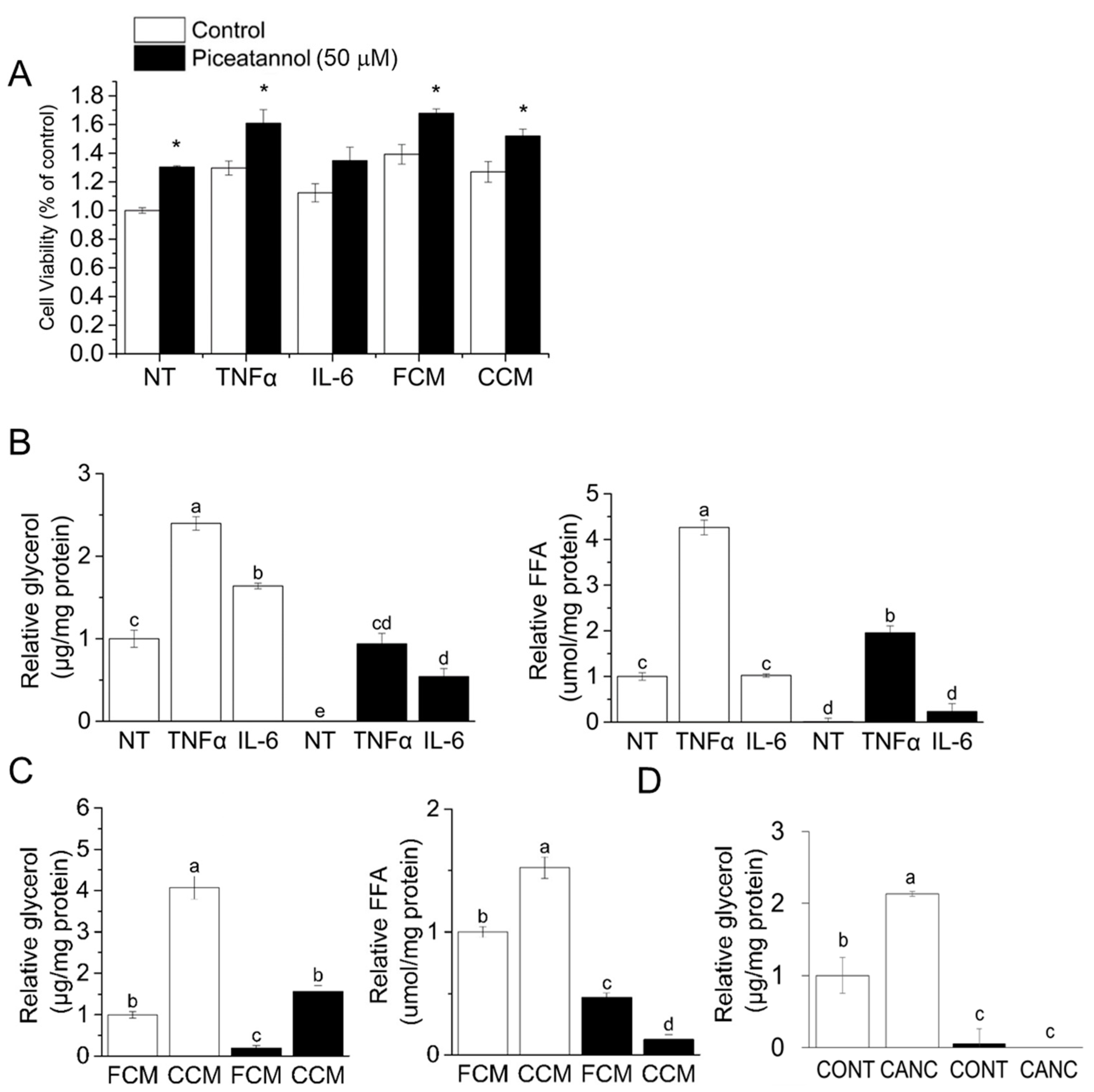
3.1. Piceatannol Blocks Cancer-Associated Lipolysis In Vitro
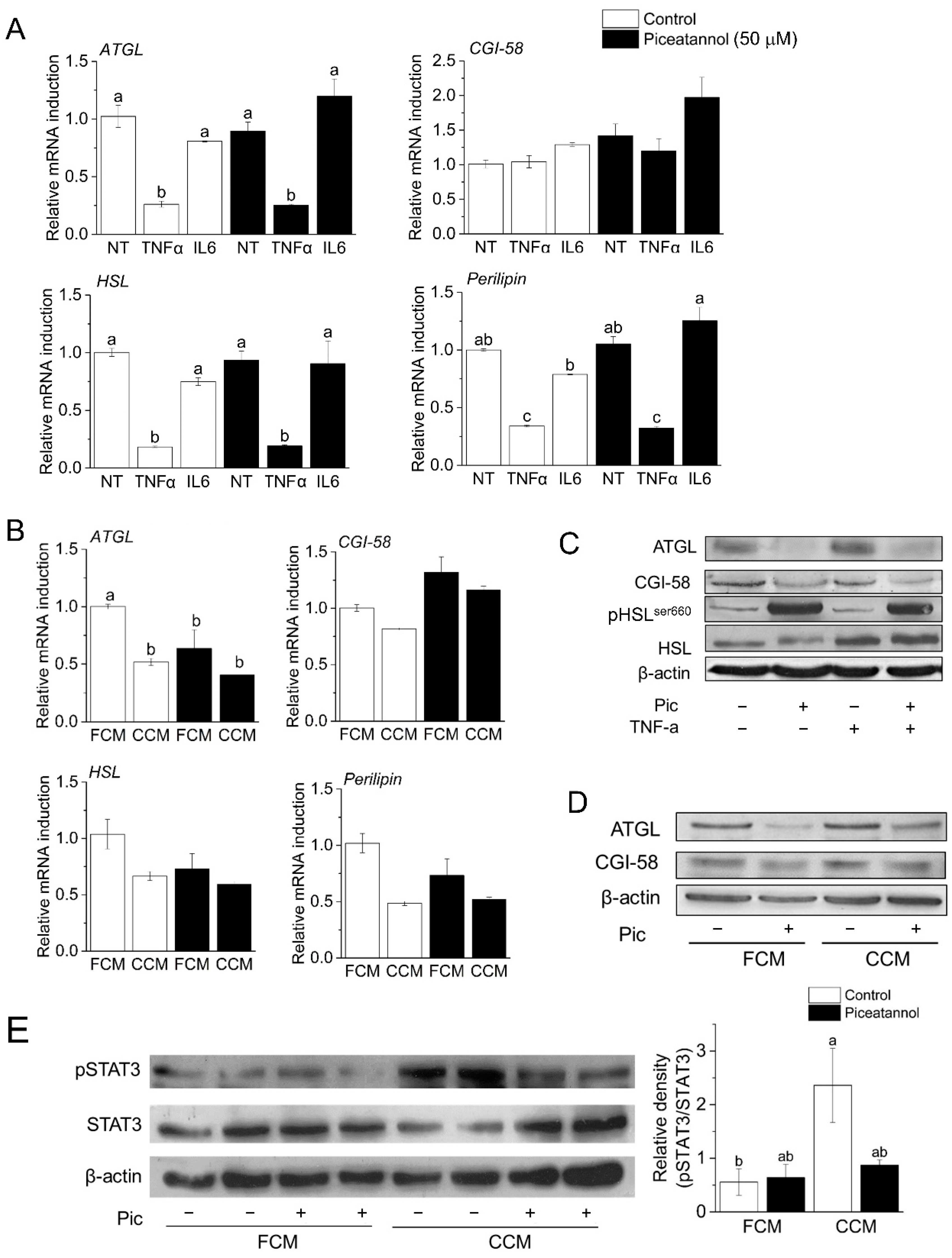
3.2. Piceatannol Induces Post-Transcriptional Degradation of ATGL and CGI-58
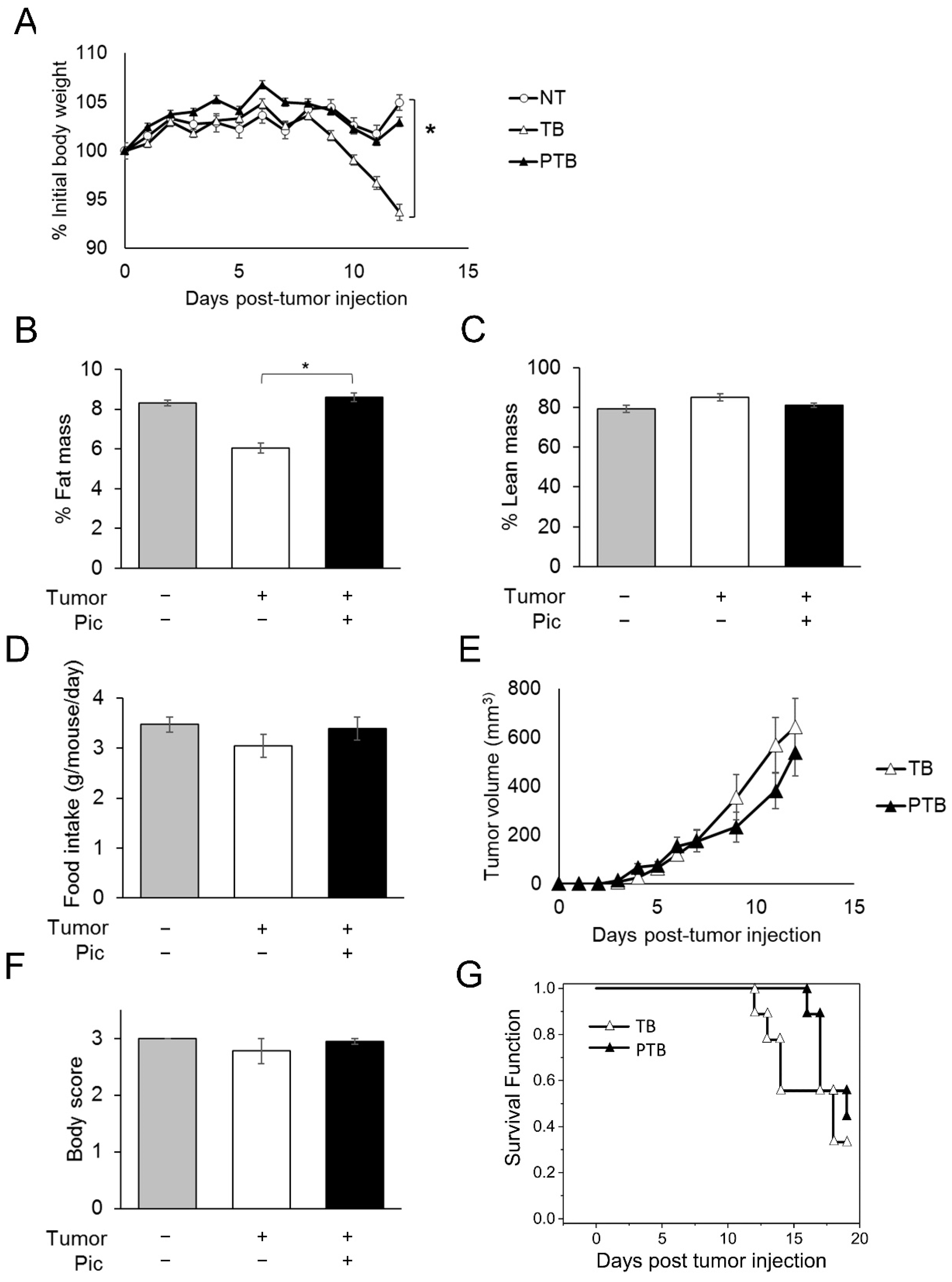
3.3. Piceatannol Slows Weight Loss and Wasting in Tumor-Bearing Mice by Preserving Adipose Tissue Mass
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tisdale, M.J. Cachexia in cancer patients. Nat. Rev. Cancer 2002, 2, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Dewys, W.D.; Begg, C.; Lavin, P.T.; Band, P.R.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O., Jr.; Engstrom, P.F.; Ezdinli, E.Z.; et al. Prognostic effect of weight loss prior tochemotherapy in cancer patients. Am. J. Med. 1980, 69, 491–497. [Google Scholar] [CrossRef]
- Fearon, K.C. The Sir David Cuthbertson Medal Lecture 1991. The mechanisms and treatment of weight loss in cancer. Proc. Nutr. Soc. 1992, 51, 251–265. [Google Scholar] [CrossRef] [Green Version]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Ni, J.; Zhang, L. Cancer Cachexia: Definition, Staging, and Emerging Treatments. Cancer Manag. Res. 2020, 12, 5597–5605. [Google Scholar] [CrossRef]
- Rohm, M.; Schafer, M.; Laurent, V.; Ustunel, B.E.; Niopek, K.; Algire, C.; Hautzinger, O.; Sijmonsma, T.P.; Zota, A.; Medrikova, D.; et al. An AMP-activated protein kinase-stabilizing peptide ameliorates adipose tissue wasting in cancer cachexia in mice. Nat. Med. 2016, 22, 1120–1130. [Google Scholar] [CrossRef]
- Petruzzelli, M.; Schweiger, M.; Schreiber, R.; Campos-Olivas, R.; Tsoli, M.; Allen, J.; Swarbrick, M.; Rose-John, S.; Rincon, M.; Robertson, G.; et al. A Switch from White to Brown Fat Increases Energy Expenditure in Cancer-Associated Cachexia. Cell Metab. 2014, 20, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Kir, S.; White, J.P.; Kleiner, S.; Kazak, L.; Cohen, P.; Baracos, V.E.; Spiegelman, B.M. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 2014, 513, 100–104. [Google Scholar] [CrossRef]
- Fearon, K.C. Cancer cachexia and fat-muscle physiology. N. Engl. J. Med. 2011, 365, 565–567. [Google Scholar] [CrossRef]
- Davidson, W.; Ash, S.; Capra, S.; Bauer, J. Weight stabilisation is associated with improved survival duration and quality of life in unresectable pancreatic cancer. Clin. Nutr. 2004, 23, 239–247. [Google Scholar] [CrossRef]
- Bing, C. Lipid mobilization in cachexia: Mechanisms and mediators. Curr. Opin. Support. Palliat. Care 2011, 5, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.A.; Wilke, M.S.; Perrine, M.; Pawlowicz, M.; Mourtzakis, M.; Lieffers, J.R.; Maneshgar, M.; Bruera, E.; Clandinin, M.T.; Baracos, V.E.; et al. Loss of adipose tissue and plasma phospholipids: Relationship to survival in advanced cancer patients. Clin. Nutr. 2010, 29, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Fouladiun, M.; Korner, U.; Bosaeus, I.; Daneryd, P.; Hyltander, A.; Lundholm, K.G. Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care—Correlations with food intake, metabolism, exercise capacity, and hormones. Cancer 2005, 103, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- Ryden, M.; Agustsson, T.; Laurencikiene, J.; Britton, T.; Sjolin, E.; Isaksson, B.; Permert, J.; Arner, P. Lipolysis--not inflammation, cell death, or lipogenesis—Is involved in adipose tissue loss in cancer cachexia. Cancer 2008, 113, 1695–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryden, M.; Arner, P. Fat loss in cachexia—Is there a role for adipocyte lipolysis? Clin. Nutr. 2007, 26, 1–6. [Google Scholar] [CrossRef]
- Dalal, S. Lipid metabolism in cancer cachexia. Ann. Palliat. Med. 2019, 8, 13–23. [Google Scholar] [CrossRef]
- Das, S.K. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 2011, 333, 1576. [Google Scholar] [CrossRef] [Green Version]
- Bezaire, V.; Mairal, A.; Ribet, C.; Lefort, C.; Girousse, A.; Jocken, J.; Laurencikiene, J.; Anesia, R.; Rodriguez, A.M.; Ryden, M.; et al. Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in hMADS adipocytes. J. Biol. Chem. 2009, 284, 18282–18291. [Google Scholar] [CrossRef] [Green Version]
- Schweiger, M.; Schreiber, R.; Haemmerle, G.; Lass, A.; Fledelius, C.; Jacobsen, P.; Tornqvist, H.; Zechner, R.; Zimmermann, R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J. Biol. Chem. 2006, 281, 40236–40241. [Google Scholar] [CrossRef] [Green Version]
- Schweiger, M.; Romauch, M.; Schreiber, R.; Grabner, G.F.; Hutter, S.; Kotzbeck, P.; Benedikt, P.; Eichmann, T.O.; Yamada, S.; Knittelfelder, O.; et al. Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. Nat. Commun. 2017, 8, 14859. [Google Scholar] [CrossRef]
- Tisdale, M.J. Mechanisms of cancer cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karayiannakis, A.J.; Syrigos, K.N.; Polychronidis, A.; Pitiakoudis, M.; Bounovas, A.; Simopoulos, K. Serum levels of tumor necrosis factor-alpha and nutritional status in pancreatic cancer patients. Anticancer Res. 2001, 21, 1355–1358. [Google Scholar] [PubMed]
- Chung, T.H.; Kuo, M.Y.P.; Chen, J.K.; Huang, D.M. YC-1 rescues cancer cachexia by affecting lipolysis and adipogenesis. Int. J. Cancer 2011, 129, 2274–2283. [Google Scholar] [CrossRef] [PubMed]
- Nara-Ashizawa, N.; Akiyama, Y.; Maruyama, K.; Tsukada, T.; Yamaguchi, K. Lipolytic and lipoprotein lipase (LPL)-inhibiting activities produced by a human lung cancer cell line responsible for cachexia induction. Anticancer Res. 2001, 21, 3381–3387. [Google Scholar] [PubMed]
- Chitti, S.V.; Marzan, A.L.; Shahi, S.; Kang, T.; Fonseka, P.; Mathivanan, S. Repurposing of Antibiotic Sulfisoxazole Inhibits Lipolysis in Pre-Clinical Model of Cancer-Associated Cachexia. Biology 2021, 10, 700. [Google Scholar] [CrossRef]
- Kershaw, J.; Kim, K.H. The Therapeutic Potential of Piceatannol, a Natural Stilbene, in Metabolic Diseases: A Review. J. Med. Food 2017, 20, 427–438. [Google Scholar] [CrossRef] [Green Version]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res.-Rev. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Seo, S.G.; Heo, Y.S.; Yue, S.H.; Cheng, J.X.; Lee, K.W.; Kim, K.H. Piceatannol, Natural Polyphenolic Stilbene, Inhibits Adipogenesis via Modulation of Mitotic Clonal Expansion and Insulin Receptor-dependent Insulin Signaling in Early Phase of Differentiation. J. Biol. Chem. 2012, 287, 11566–11578. [Google Scholar] [CrossRef] [Green Version]
- Les Parellada, F.; Deleruyelle, S.; Cassagnes, L.E.; Boutin, J.A.; Balogh, B.; Arbones-Mainar, J.M.; Biron, S.; Marceau, P.; Richard, D.; Nepveu, F.; et al. Piceatannol and resveratrol share inhibitory effects on hydrogen peroxide release, monoamine oxidase and lipogenic activities in adipose tissue, but differ in their antilipolytic properties. Chem.-Biol. Interact. 2016, 258, 115–125. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Kershaw, J.; Chen, C.Y.; Komanetsky, S.M.; Zhu, Y.; Guo, X.; Myer, P.R.; Applegate, B.; Kim, K.H. Piceatannol antagonizes lipolysis by promoting autophagy-lysosome-dependent degradation of lipolytic protein clusters in adipocytes. J. Nutr. Biochem. 2022, 105, 108998. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Heckmann, B.L.; Lu, X.; Liu, J. Relative contribution of adipose triglyceride lipase and hormone-sensitive lipase to tumor necrosis factor-alpha (TNF-alpha)-induced lipolysis in adipocytes. J. Biol. Chem. 2011, 286, 40477–40485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonetto, A.; Rupert, J.E.; Barreto, R.; Zimmers, T.A. The Colon-26 Carcinoma Tumor-bearing Mouse as a Model for the Study of Cancer Cachexia. J. Vis. Exp. JoVE 2016, e54893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullman-Cullere, M.H.; Foltz, C.J. Body condition scoring: A rapid and accurate method for assessing health status in mice. Comp. Med. 1999, 49, 319–323. [Google Scholar]
- Anthonsen, M.W.; Ronnstrand, L.; Wernstedt, C.; Degerman, E.; Holm, C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J. Biol. Chem. 1998, 273, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Tsoli, M.; Schweiger, M.; Vanniasinghe, A.S.; Painter, A.; Zechner, R.; Clarke, S.; Robertson, G. Depletion of white adipose tissue in cancer cachexia syndrome is associated with inflammatory signaling and disrupted circadian regulation. PLoS ONE 2014, 9, e92966. [Google Scholar] [CrossRef]
- Jeong, S.O.; Son, Y.; Lee, J.H.; Cheong, Y.K.; Park, S.H.; Chung, H.T.; Pae, H.O. Resveratrol analog piceatannol restores the palmitic acid-induced impairment of insulin signaling and production of endothelial nitric oxide via activation of anti-inflammatory and antioxidative heme oxygenase-1 in human endothelial cells. Mol. Med. Rep. 2015, 12, 937–944. [Google Scholar] [CrossRef] [Green Version]
- Kwon, G.T.; Jung, J.I.; Song, H.R.; Woo, E.Y.; Jun, J.G.; Kim, J.K.; Her, S.; Park, J.H. Piceatannol inhibits migration and invasion of prostate cancer cells: Possible mediation by decreased interleukin-6 signaling. J. Nutr. Biochem. 2012, 23, 228–238. [Google Scholar] [CrossRef]
- Aulino, P.; Berardi, E.; Cardillo, V.M.; Rizzuto, E.; Perniconi, B.; Ramina, C.; Padula, F.; Spugnini, E.P.; Baldi, A.; Faiola, F.; et al. Molecular, cellular and physiological characterization of the cancer cachexia-inducing C26 colon carcinoma in mouse. BMC Cancer 2010, 10, 363. [Google Scholar] [CrossRef]
- Galgani, J.E.; Smith, S.R.; Ravussin, E. Assessment of EchoMRI-AH versus dual-energy X-ray absorptiometry to measure human body composition. Int. J. Obes. 2011, 35, 1241–1246. [Google Scholar] [CrossRef] [Green Version]
- Shen, Q.; Kuang, J.X.; Miao, C.X.; Zhang, W.L.; Li, Y.W.; Zhang, X.W.; Liu, X. Alantolactone ameliorates cancer cachexia-associated muscle atrophy mainly by inhibiting the STAT3 signaling pathway. Phytomedicine Int. J. Phytother. Phytopharm. 2022, 95, 153858. [Google Scholar] [CrossRef]
- Park, J.M.; Han, Y.M.; Lee, H.J.; Park, Y.J.; Hahm, K.B. Nicotinamide Riboside Vitamin B3 Mitigated C26 Adenocarcinoma-Induced Cancer Cachexia. Front. Pharmacol. 2021, 12, 665493. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, L.; Zou, J.; Zhou, T.; Wang, B.; Sun, H.; Yu, S. Coix seed oil ameliorates cancer cachexia by counteracting muscle loss and fat lipolysis. BMC Complement. Altern. Med. 2019, 19, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.; Li, Y.; Shen, Q.; Zhang, W.; Gu, X.; Ma, M.; Li, Y.; Zhang, L.; Liu, X.; Zhang, X. Carnosol and its analogues attenuate muscle atrophy and fat lipolysis induced by cancer cachexia. J. Cachexia Sarcopenia Muscle 2021, 12, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Arner, P. Medicine. Lipases in cachexia. Science 2011, 333, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Huang, S.; Wang, L.; Leng, Y.; Lu, W. Design and synthesis of Atglistatin derivatives as adipose triglyceride lipase inhibitors. Chem. Biol. Drug Des. 2017, 90, 1122–1133. [Google Scholar] [CrossRef]
- Mayer, N.; Schweiger, M.; Romauch, M.; Grabner, G.F.; Eichmann, T.O.; Fuchs, E.; Ivkovic, J.; Heier, C.; Mrak, I.; Lass, A.; et al. Development of small-molecule inhibitors targeting adipose triglyceride lipase. Nat. Chem. Biol. 2013, 9, 785–787. [Google Scholar] [CrossRef] [Green Version]
- MacPherson, R.E.; Dragos, S.M.; Ramos, S.; Sutton, C.; Frendo-Cumbo, S.; Castellani, L.; Watt, M.J.; Perry, C.G.; Mutch, D.M.; Wright, D.C. Reduced ATGL-mediated lipolysis attenuates beta-adrenergic-induced AMPK signaling, but not the induction of PKA-targeted genes, in adipocytes and adipose tissue. Am. J. Physiol. Cell Physiol. 2016, 311, C269–C276. [Google Scholar] [CrossRef] [Green Version]
- Kliewer, K.L.; Ke, J.Y.; Tian, M.; Cole, R.M.; Andridge, R.R.; Belury, M.A. Adipose tissue lipolysis and energy metabolism in early cancer cachexia in mice. Cancer Biol. Ther. 2015, 16, 886–897. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Jung, J.I.; Cho, H.J.; Her, S.; Kwon, S.H.; Yu, R.; Kang, Y.H.; Lee, K.W.; Park, J.H. Inhibition of tumor progression by oral piceatannol in mouse 4T1 mammary cancer is associated with decreased angiogenesis and macrophage infiltration. J. Nutr. Biochem. 2015, 26, 1368–1378. [Google Scholar] [CrossRef]
- Farrand, L.; Byun, S.; Kim, J.Y.; Im-Aram, A.; Lee, J.; Lim, S.; Lee, K.W.; Suh, J.Y.; Lee, H.J.; Tsang, B.K. Piceatannol enhances cisplatin sensitivity in ovarian cancer via modulation of p53, X-linked inhibitor of apoptosis protein (XIAP), and mitochondrial fission. J. Biol. Chem. 2013, 288, 23740–23750. [Google Scholar] [CrossRef] [Green Version]
- Dias, S.J.; Li, K.; Rimando, A.M.; Dhar, S.; Mizuno, C.S.; Penman, A.D.; Levenson, A.S. Trimethoxy-resveratrol and piceatannol administered orally suppress and inhibit tumor formation and growth in prostate cancer xenografts. Prostate 2013, 73, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Arends, J.; Baracos, V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 2013, 10, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Blum, D.; Omlin, A.; Baracos, V.E.; Solheim, T.S.; Tan, B.H.; Stone, P.; Kaasa, S.; Fearon, K.; Strasser, F. Cancer cachexia: A systematic literature review of items and domains associated with involuntary weight loss in cancer. Crit. Rev. Oncol./Hematol. 2011, 80, 114–144. [Google Scholar] [CrossRef] [PubMed]
- Tsoli, M.; Robertson, G. Cancer cachexia: Malignant inflammation, tumorkines, and metabolic mayhem. Trends Endocrinol. Metab. 2013, 24, 174–183. [Google Scholar] [CrossRef]
- Arner, P.; Langin, D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol. Metab. 2014, 25, 255–262. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kershaw, J.C.; Elzey, B.D.; Guo, X.-X.; Kim, K.-H. Piceatannol, a Dietary Polyphenol, Alleviates Adipose Tissue Loss in Pre-Clinical Model of Cancer-Associated Cachexia via Lipolysis Inhibition. Nutrients 2022, 14, 2306. https://doi.org/10.3390/nu14112306
Kershaw JC, Elzey BD, Guo X-X, Kim K-H. Piceatannol, a Dietary Polyphenol, Alleviates Adipose Tissue Loss in Pre-Clinical Model of Cancer-Associated Cachexia via Lipolysis Inhibition. Nutrients. 2022; 14(11):2306. https://doi.org/10.3390/nu14112306
Chicago/Turabian StyleKershaw, Jonathan C., Bennett D. Elzey, Xiao-Xuan Guo, and Kee-Hong Kim. 2022. "Piceatannol, a Dietary Polyphenol, Alleviates Adipose Tissue Loss in Pre-Clinical Model of Cancer-Associated Cachexia via Lipolysis Inhibition" Nutrients 14, no. 11: 2306. https://doi.org/10.3390/nu14112306
APA StyleKershaw, J. C., Elzey, B. D., Guo, X.-X., & Kim, K.-H. (2022). Piceatannol, a Dietary Polyphenol, Alleviates Adipose Tissue Loss in Pre-Clinical Model of Cancer-Associated Cachexia via Lipolysis Inhibition. Nutrients, 14(11), 2306. https://doi.org/10.3390/nu14112306





