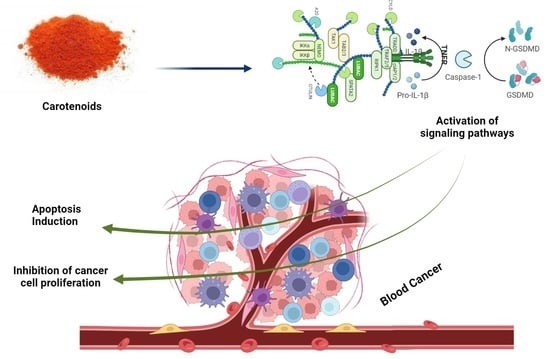
Therapeutic Role of Carotenoids in Blood Cancer: Mechanistic Insights and Therapeutic Potential
Abstract
:1. Introduction
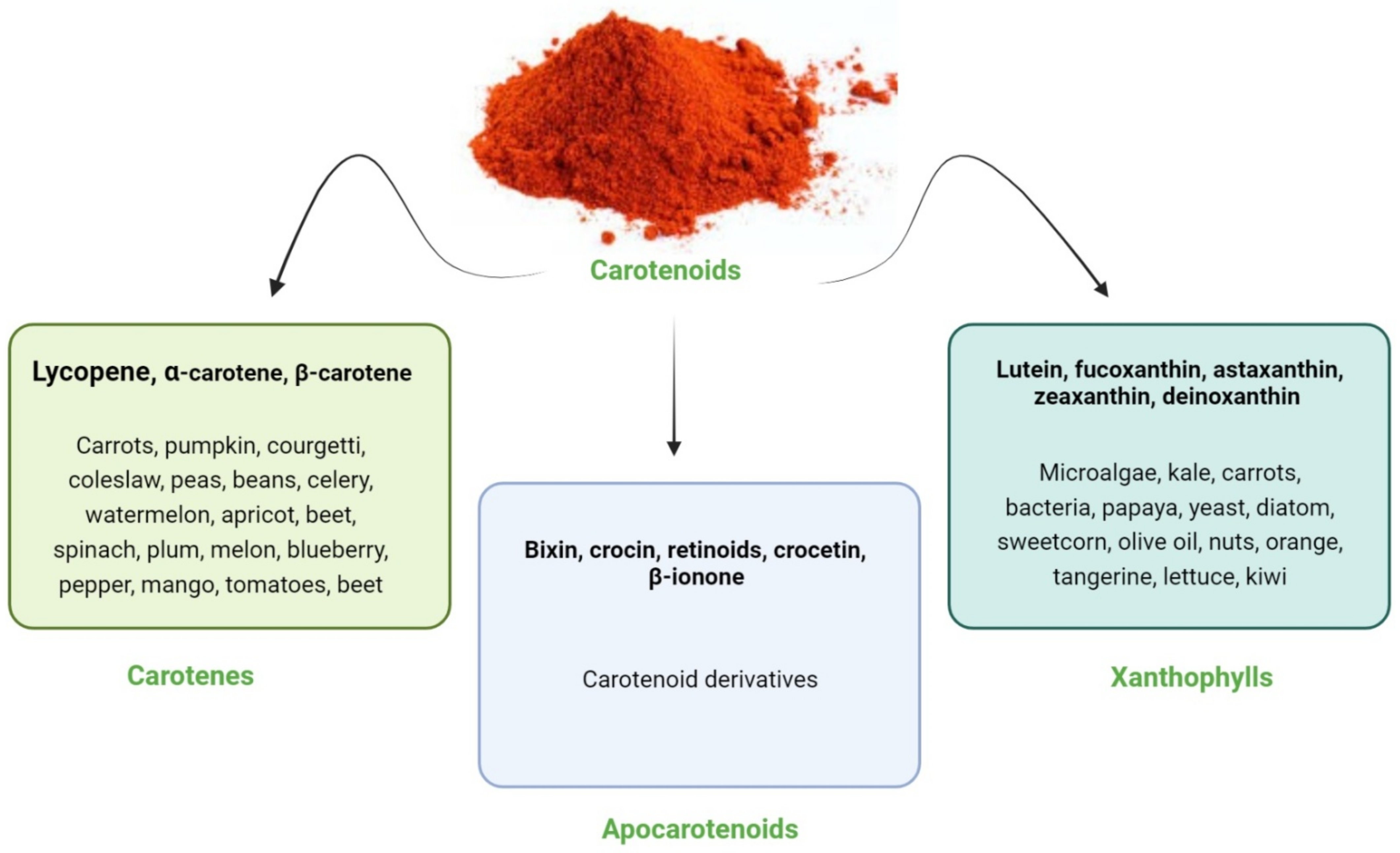
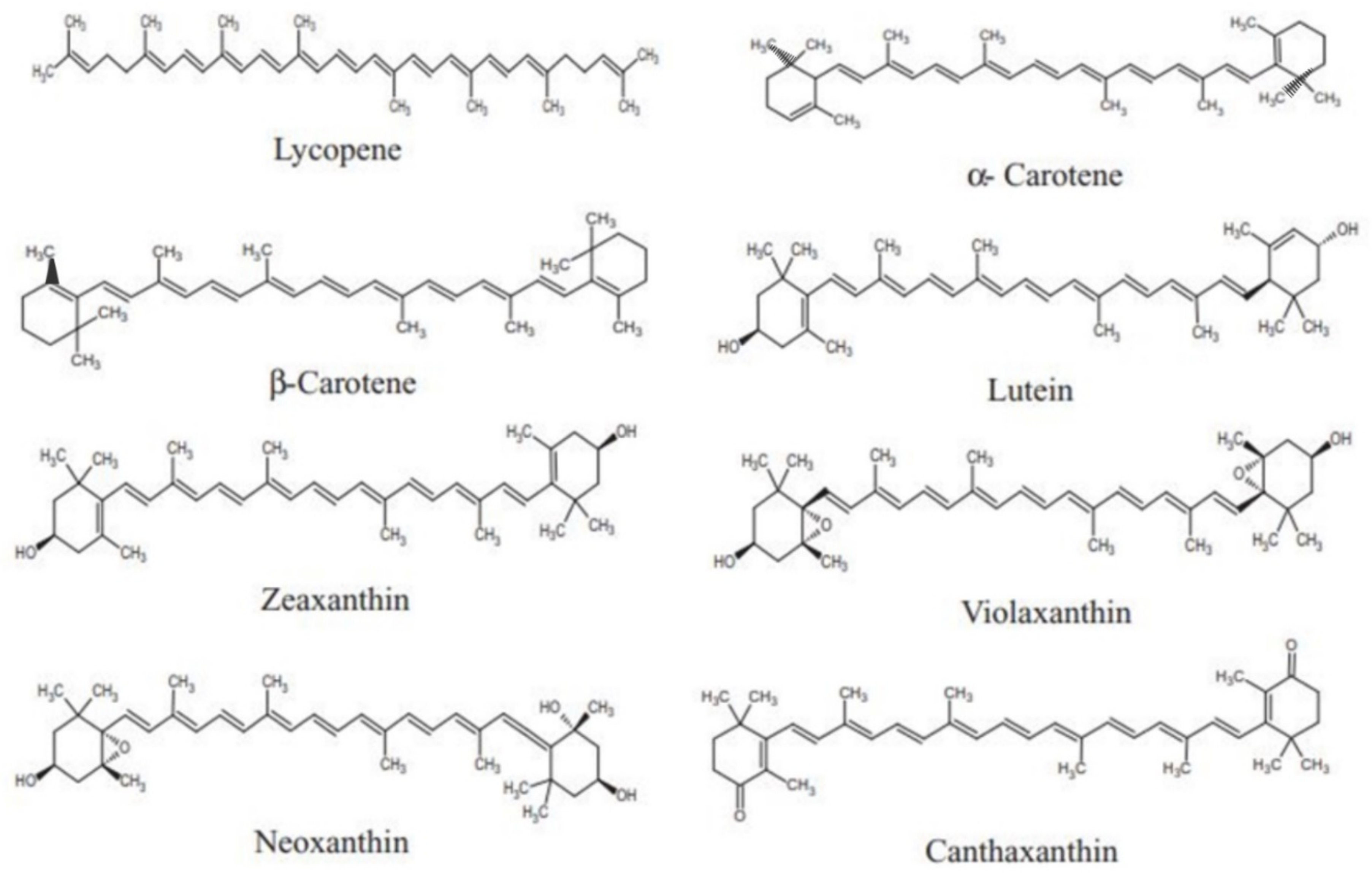
2. Carotenoids: An Overview
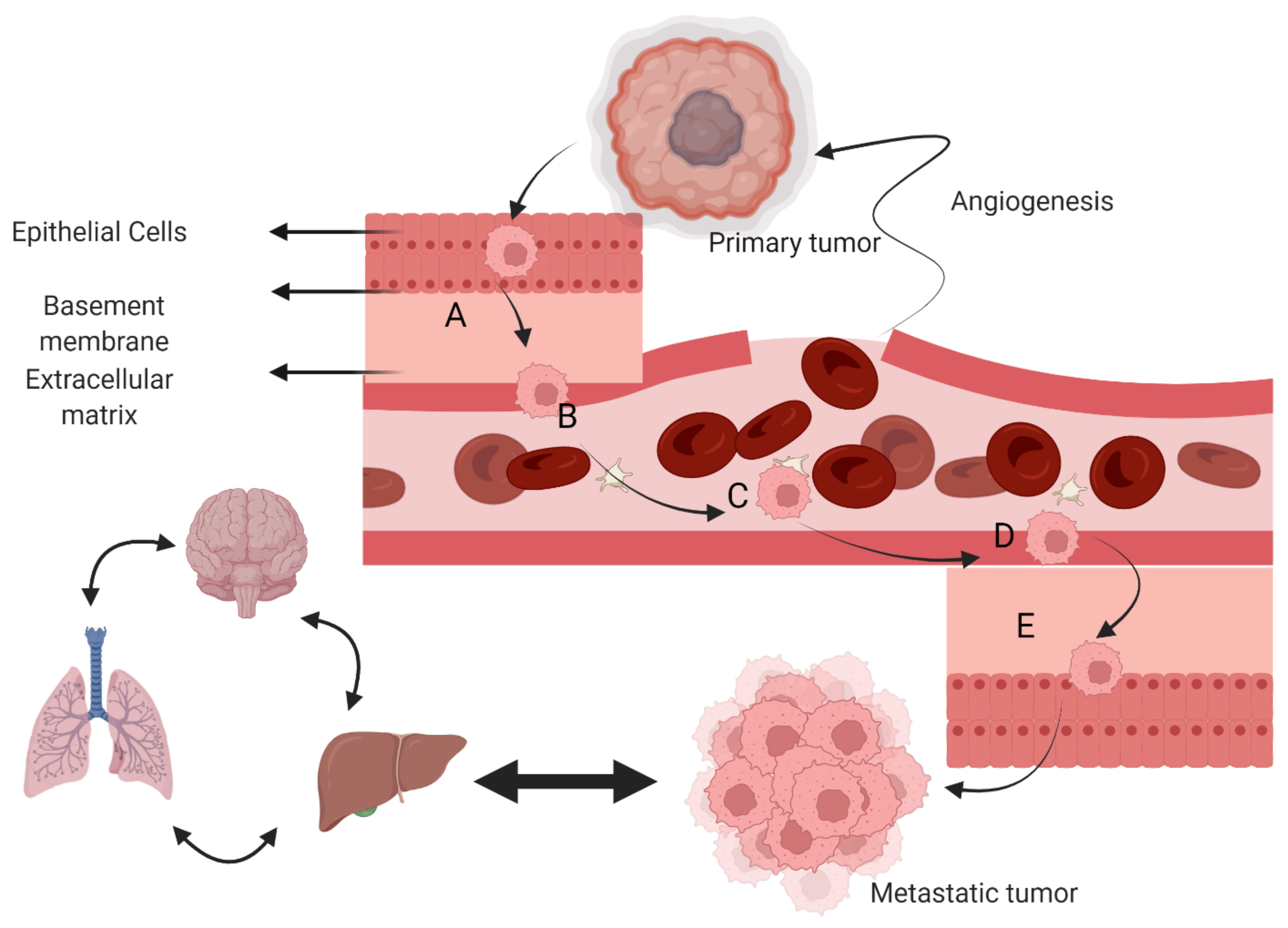
3. Cancer and Metastasis
4. Challenges in Blood Cancer Treatment
5. Carotenoids in Cancer
6. Carotenoids in Blood Cancer
6.1. Carotenoids in Leukemia
6.2. Carotenoids in Lymphoma and Myeloma
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bogeska, R. Focus on blood cancers and liquid biopsy research. Mol. Oncol. 2021, 15, 2251. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tebbi, C. Etiology of acute leukemia: A review. Cancer 2021, 13, 2256. [Google Scholar] [CrossRef] [PubMed]
- Umeno, D.; Tobias, A.V.; Arnold, F. Evolution of the C30 carotenoid synthase CrtM for function in a C40 pathway. J. Bacteriol. 2002, 184, 6690–6699. [Google Scholar] [CrossRef] [Green Version]
- Giuffrida, D.; Sutthiwong, N.; Dugo, P.; Donato, P.; Cacciola, F.; Girard-Valenciennes, E.; Le Mao, Y.; Monnet, C.; Fouillaud, M.; Caro, Y. Characterisation of the C50 carotenoids produced by strains of the cheese-ripening bacterium Arthrobacter arilaitensis. Int. Dairy J. 2016, 55, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Najib, M.Z.B.M. Biogranule Containing Photosynthetic Bacteria for Carbon Dioxide Reduction in Palm Oil Mill Effluent Treatment. Ph.D. Thesis, Universiti Teknologi Malaysia, Skudai, Malaysia, June 2017. [Google Scholar]
- Amarowicz, R. Lycopene as a Natural Antioxidant; Wiley Online Library: Weinheim, Germany, 2011; Volume 113, pp. 675–677. [Google Scholar]
- Stahl, W.; Sies, H.J. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2005, 1740, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Koklesova, L.; Liskova, A.; Samec, M.; Zhai, K.; Abotaleb, M.; Ashrafizadeh, M.; Brockmueller, A.; Shakibaei, M.; Biringer, K.; Bugos, O.J.B. Carotenoids in cancer metastasis—Status quo and outlook. Eur. J. Med. Chem. 2020, 10, 1653. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M.J. Health protective effects of carotenoids and their interactions with other biological antioxidants. FASEB J. 2013, 70, 102–110. [Google Scholar] [CrossRef]
- Parker, R. Absorption, metabolism, and transport of carotenoids. J. Nutr. Biochem. 1996, 10, 542–551. [Google Scholar] [CrossRef]
- Furr, H.C.; Clark, R.M. Intestinal absorption and tissue distribution of carotenoids. J. Nutr. Biochem. 1997, 8, 364–377. [Google Scholar] [CrossRef]
- Olson, J.A.J.P.; Chemistry, A. Absorption, transport and metabolism of carotenoids in humans. Pure Appl. Chem. 1994, 66, 1011–1016. [Google Scholar] [CrossRef] [Green Version]
- Van Bennekum, A.; Werder, M.; Thuahnai, S.T.; Han, C.-H.; Duong, P.; Williams, D.L.; Wettstein, P.; Schulthess, G.; Phillips, M.C.; Hauser, H.J.B. Class B scavenger receptor-mediated intestinal absorption of dietary β-carotene and cholesterol. Biochemistry 2005, 44, 4517–4525. [Google Scholar] [CrossRef]
- SENECA Investigators Buijsse Brian Brian. Plasma carotene and α-tocopherol in relation to 10-y all-cause and cause-specific mortality in European elderly: The Survey in Europe on Nutrition and the Elderly, a Concerted Action (SENECA). Am. J. Clin. Nutr. 2005, 82, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T.J. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef]
- Donaldson, M.S. A carotenoid health index based on plasma carotenoids and health outcomes. Nutrients 2011, 3, 1003–1022. [Google Scholar] [CrossRef] [Green Version]
- Koklesova, L.; Liskova, A.; Samec, M.; Buhrmann, C.; Samuel, S.M.; Varghese, E.; Ashrafizadeh, M.; Najafi, M.; Shakibaei, M.; Büsselberg, D. Carotenoids in cancer apoptosis—The road from bench to bedside and back. Cancers 2020, 12, 2425. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K.J.N. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [Green Version]
- Rowles, J.L., III; Erdman, J.W., Jr. Carotenoids and their role in cancer prevention. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158613. [Google Scholar]
- Athreya, K.; Xavier, M.F.J.N. Antioxidants in the treatment of cancer. Nutr. Cancer 2017, 69, 1099–1104. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S.; Daglia, M.; Rengasamy, K.R. Dietary carotenoids in cancer chemoprevention and chemotherapy: A review of emerging evidence. Pharmacol. Res. 2020, 157, 104830. [Google Scholar] [CrossRef]
- Qian, C.-N.; Mei, Y.; Zhang, J. Cancer metastasis: Issues and challenges. Chin. J. Cancer 2017, 36, 38. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y.J. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Liskova, A.; Koklesova, L.; Samec, M.; Smejkal, K.; Samuel, S.M.; Varghese, E.; Abotaleb, M.; Biringer, K.; Kudela, E.; Danko, J.; et al. Flavonoids in cancer metastasis. Cancers 2020, 12, 1498. [Google Scholar] [CrossRef]
- Loud, J.T.; Murphy, J. Cancer screening and early detection in the 21st century. In Seminars in Oncology Nursing; Elsevier: Amsterdam, The Netherlands, 2017; pp. 121–128. [Google Scholar]
- Haun, M.W.; Estel, S.; Ruecker, G.; Friederich, H.C.; Villalobos, M.; Thomas, M.; Hartmann, M.J. Early palliative care for adults with advanced cancer. Cochrane Database Syst. Rev. 2017, 6. [Google Scholar] [CrossRef]
- Menezes, M.; Das, S.; Minn, I.; Emdad, L.; Wang, X.-Y.; Sarkar, D.; Pomper, M.; Fisher, P.J. Detecting tumor metastases: The road to therapy starts here. Adv. Cancer Res. 2016, 132, 1–44. [Google Scholar]
- Hanahan, D.; Weinberg, R. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef]
- Miles, L.A.; Bowman, R.L.; Merlinsky, T.R.; Csete, I.S.; Ooi, A.T.; Durruthy-Durruthy, R.; Bowman, M.; Famulare, C.; Patel, M.A.; Mendez, P.; et al. Single-cell mutation analysis of clonal evolution in myeloid malignancies. Nature 2020, 587, 477–482. [Google Scholar] [CrossRef]
- Vetrie, D.; Helgason, G.V.; Copland, M. The leukaemia stem cell: Similarities, differences and clinical prospects in CML and AML. Nat. Rev. Cancer 2020, 20, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.S.; Lazarides, K.; Lewis, J.B.; von Andrian, U.H.; Van Etten, R. Selectins and their ligands are required for homing and engraftment of BCR-ABL1+ leukemic stem cells in the bone marrow niche. J. Am. Soc. Hematol. 2014, 123, 1361–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafouresse, F.; Bellard, E.; Laurent, C.; Moussion, C.; Fournié, J.-J.; Ysebaert, L.; Girard, J.-P. L-selectin controls trafficking of chronic lymphocytic leukemia cells in lymph node high endothelial venules in vivo. J. Am. Soc. Hematol. 2015, 126, 1336–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavor, S.; Petit, I.; Porozov, S.; Avigdor, A.; Dar, A.; Leider-Trejo, L.; Shemtov, N.; Deutsch, V.; Naparstek, E.; Nagler, A.; et al. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res. 2004, 64, 2817–2824. [Google Scholar] [CrossRef] [Green Version]
- Viadana, E.; Bross, I.; Pickren, J.W. An autopsy study of the metastatic patterns of human leukemias. Oncology 1978, 35, 87–96. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. J. Am. Soc. Hematol. 2017, 129, 424–447. [Google Scholar] [CrossRef] [Green Version]
- Inaba, H.; Greaves, M.; Mullighan, C. Acute lymphoblastic leukaemia. Lancet 2013, 381, 1943–1955. [Google Scholar] [CrossRef] [Green Version]
- Naidu, M.U.R.; Ramana, G.V.; Rani, P.U.; Suman, A.; Roy, P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis-complicating the treatment of cancer. Neoplasia 2004, 6, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Vagace, J.M.; Gervasini, G. Chemotherapy Toxicity in Patients with Acute Leukemia; Intechopen: London, UK, 2011. [Google Scholar]
- Bae, Y.H. Drug targeting and tumor heterogeneity. J. Control. Release 2009, 133, 2. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.; Ma, K.; Kim, T.H.; Lee, E.S.; Oh, K.T.; Park, E.-S.; Lee, K.C.; Youn, Y.S. Doxorubicin-loaded human serum albumin nanoparticles surface-modified with TNF-related apoptosis-inducing ligand and transferrin for targeting multiple tumor types. Biomaterials 2012, 33, 1536–1546. [Google Scholar] [CrossRef]
- De Palma, M.; Venneri, M.A.; Roca, C.; Naldini, L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat. Med. 2003, 9, 789–795. [Google Scholar] [CrossRef]
- Navya, P.; Kaphle, A.; Daima, H. Nanomedicine in sensing, delivery, imaging and tissue engineering: Advances, opportunities and challenges. In Nanoscience; The Royal Society of Chemistry: Cambridge, UK, 2018; pp. 30–56. [Google Scholar]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Hu, W.; Wang, G.; Huang, D.; Sui, M.; Xu, Y. Cancer immunotherapy based on natural killer cells: Current progress and new opportunities. Front. Immunol. 2019, 10, 1205. [Google Scholar] [CrossRef]
- Sha, H.-H.; Wang, D.-D.; Yan, D.-L.; Hu, Y.; Yang, S.-J.; Liu, S.-W.; Feng, J.-F. Chimaeric antigen receptor T-cell therapy for tumour immunotherapy. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wu, Z.; Liu, Y.; Han, W.J. New development in CAR-T cell therapy. J. Hematol. Oncol. 2017, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Dana, H.; Chalbatani, G.M.; Jalali, S.A.; Mirzaei, H.R.; Grupp, S.A.; Suarez, E.R.; Rapôso, C.; Webster, T.J. CAR-T cells: Early successes in blood cancer and challenges in solid tumors. Acta Pharm. Sin. B 2021, 11, 1129–1147. [Google Scholar] [CrossRef]
- Zhang, C.; Oberoi, P.; Oelsner, S.; Waldmann, A.; Lindner, A.; Tonn, T.; Wels, W.S. Chimeric antigen receptor-engineered NK-92 cells: An off-the-shelf cellular therapeutic for targeted elimination of cancer cells and induction of protective antitumor immunity. Front. Immunol. 2017, 8, 533. [Google Scholar] [CrossRef]
- Huong, P.T.; Nguyen, L.T.; Nguyen, X.-B.; Lee, S.K.; Bach, D.-H. The role of platelets in the tumor-microenvironment and the drug resistance of cancer cells. Cancers 2019, 11, 240. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Charles Lin, P.; Zhou, B.P. Inflammation fuels tumor progress and metastasis. Curr. Pharm. Des. 2015, 21, 3032–3040. [Google Scholar] [CrossRef] [Green Version]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef] [Green Version]
- Rachidi, S.; Metelli, A.; Riesenberg, B.; Wu, B.X.; Nelson, M.H.; Wallace, C.; Paulos, C.M.; Rubinstein, M.P.; Garrett-Mayer, E.; Hennig, M.; et al. Platelets subvert T cell immunity against cancer via GARP-TGFβ axis. Sci. Immunol. 2017, 2, eaai7911. [Google Scholar] [CrossRef] [Green Version]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Chapuis, A.G.; Egan, D.N.; Bar, M.; Schmitt, T.M.; McAfee, M.S.; Paulson, K.G.; Voillet, V.; Gottardo, R.; Ragnarsson, G.B.; Bleakley, M.; et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat. Med. 2019, 25, 1064–1072. [Google Scholar] [CrossRef]
- Sonneveld, P.; Avet-Loiseau, H.; Lonial, S.; Usmani, S.; Siegel, D.; Anderson, K.C.; Chng, W.-J.; Moreau, P.; Attal, M.; Kyle, R.A.; et al. Treatment of multiple myeloma with high-risk cytogenetics: A consensus of the International Myeloma Working Group. J. Am. Soc. Hematol. 2016, 127, 2955–2962. [Google Scholar] [CrossRef]
- Hunger, S.P.; Lu, X.; Devidas, M.; Camitta, B.M.; Gaynon, P.S.; Winick, N.J.; Reaman, G.H.; Carroll, W.L. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children’s oncology group. J. Clin. Oncol. 2012, 30, 1663. [Google Scholar] [CrossRef]
- Thomas, X.; Boiron, J.-M.; Huguet, F.; Dombret, H.; Bradstock, K.; Vey, N.; Kovacsovics, T.; Delannoy, A.; Fegueux, N.; Fenaux, P.J.; et al. Outcome of treatment in adults with acute lymphoblastic leukemia: Analysis of the LALA-94 trial. J. Clin. Oncol. 2004, 22, 4075–4086. [Google Scholar] [CrossRef]
- Levine, S.R.; McNeer, J.; Isakoff, M.S. Chalenges faced in the treatment of acute lymphoblastic leukemia in adolescents and young adults. Clin. Oncol. Adolesc Young Adults 2016, 6, 11–20. [Google Scholar]
- Bar, M.; Wood, B.L.; Radich, J.P.; Doney, K.C.; Woolfrey, A.E.; Delaney, C.; Appelbaum, F.R.; Gooley, T. Impact of minimal residual disease, detected by flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia. Leuk. Res. Treat. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Holtick, U.; Albrecht, M.; Chemnitz, J.M.; Theurich, S.; Skoetz, N.; Scheid, C.; von Bergwelt-Baildon, M. Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults. Cochrane Database Syst. Rev. 2014, 4. [Google Scholar] [CrossRef]
- Pulsipher, M.A.; Langholz, B.; Wall, D.A.; Schultz, K.R.; Bunin, N.; Carroll, W.; Raetz, E.; Gardner, S.; Goyal, R.K.; Gastier-Foster, J.; et al. Risk factors and timing of relapse after allogeneic transplantation in pediatric ALL: For whom and when should interventions be tested? Bone Marrow Transplant. 2015, 50, 1173–1179. [Google Scholar] [CrossRef]
- Pulsipher, M.A.; Langholz, B.; Wall, D.A.; Schultz, K.R.; Bunin, N.; Carroll, W.L.; Raetz, E.; Gardner, S.; Gastier-Foster, J.M.; Howrie, D.; et al. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: A phase 3 Children’s Oncology Group/Pediatric Blood and Marrow Transplant Consortium trial. J. Am. Soc. Hematol. 2014, 123, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Sivanesan, I.; Keum, Y.-S. Emerging roles of carotenoids in the survival and adaptations of microbes. Indian J. Microbiol. 2019, 59, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Zia-Ul-Haq, M. Historical and introductory aspects of carotenoids. In Carotenoids: Structure and Function in the Human Body; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–42. [Google Scholar]
- Shahidi, F.; Pan, Y. Influence of food matrix and food processing on the chemical interaction and bioaccessibility of dietary phytochemicals: A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Das, A.K.; Ghosh, S.; Sil, P.C. Carotenoids as Coloring Agents. In Carotenoids: Structure and Function in the Human Body; Springer: Berlin/Heidelberg, Germany, 2021; pp. 189–207. [Google Scholar]
- Zhang, Y.; Zhang, T.; Liang, Y.; Jiang, L.; Sui, X. Dietary bioactive lipids: A review on absorption, metabolism, and health properties. J. Agric. Food Chem. 2021, 69, 8929–8943. [Google Scholar] [CrossRef]
- Singh, A.; Mukherjee, T. Application of carotenoids in sustainable energy and green electronics. Mater. Adv. 2022, 3, 1341–1358. [Google Scholar] [CrossRef]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From plants to food and feed industries. Microb. Carotenoids 2018, 1852, 57–71. [Google Scholar]
- Diep, T.T.; Pook, C.; Rush, E.C.; Yoo, M.J.Y. Quantification of carotenoids, α-tocopherol, and ascorbic acid in amber, mulligan, and laird’s large cultivars of New Zealand tamarillos (Solanum betaceum Cav.). Foods 2020, 9, 769. [Google Scholar] [CrossRef]
- Jiao, Y.; Reuss, L.; Wang, Y. β-Cryptoxanthin: Chemistry, occurrence, and potential health benefits. Curr. Pharmacol. Rep. 2019, 5, 20–34. [Google Scholar] [CrossRef]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food sources, biological activities, and human health benefits. Oxidative Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef]
- Arballo, J.; Amengual, J.; Erdman, J.W. Lycopene: A critical review of digestion, absorption, metabolism, and excretion. Antioxidants 2021, 10, 342. [Google Scholar] [CrossRef]
- Xavier, A.A.O.; Pérez-Gálvez, A. Carotenoids as a Source of Antioxidants in the Diet. In Carotenoids in Nature; Springer: Cham, Germany, 2016; pp. 359–375. [Google Scholar]
- Piyarach, K.; Nipawan, K.; Chadapon, C.; Daluwan, S.; Kunjana, R. Effect of drying on β-carotene, α carotene, lutein and zeaxanthin content in vegetables and its application for vegetable seasoning. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2020; p. 02007. [Google Scholar]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Hornero-Méndez, D.; Cerrillo, I.; Ortega, Á.; Rodríguez-Griñolo, M.-R.; Escudero-López, B.; Martín, F.; Fernández-Pachón, M.-S. β-Cryptoxanthin is more bioavailable in humans from fermented orange juice than from orange juice. Food Chem. 2018, 262, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Talvas, J.; Caris-Veyrat, C.; Guy, L.; Rambeau, M.; Lyan, B.; Minet-Quinard, R.; Lobaccaro, J.-M.A.; Vasson, M.-P.; George, S.; Mazur, A.; et al. Differential effects of lycopene consumed in tomato paste and lycopene in the form of a purified extract on target genes of cancer prostatic cells. Am. J. Clin. Nutr. 2010, 91, 1716–1724. [Google Scholar] [CrossRef] [Green Version]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharmacother. 2020, 129, 110459. [Google Scholar] [CrossRef]
- Van Breemen, R.B.; Sharifi, R.; Viana, M.; Pajkovic, N.; Zhu, D.; Yuan, L.; Yang, Y.; Bowen, P.E.; Stacewicz-Sapuntzakis, M. Antioxidant effects of lycopene in African American men with prostate cancer or benign prostate hyperplasia: A randomized, controlled trial. Cancer Prev. Res. 2011, 4, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Udayawara Rudresh, D.; Maradagi, T.; Stephen, N.M.; Niraikulam, A.; Nambi Ramudu, K.; Ponesakki, G. Neoxanthin prevents H2O2-induced cytotoxicity in HepG2 cells by activating endogenous antioxidant signals and suppressing apoptosis signals. Mol. Biol. Rep. 2021, 48, 6923–6934. [Google Scholar] [CrossRef]
- Kavalappa, Y.P.; Gopal, S.S.; Ponesakki, G. Lutein inhibits breast cancer cell growth by suppressing antioxidant and cell survival signals and induces apoptosis. J. Cell. Physiol. 2021, 236, 1798–1809. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, J.; Jiang, W.; Ye, X.; Zhang, S. Long non-coding RNA CASC9/microRNA-590-3p axis participates in lutein-mediated suppression of breast cancer cell proliferation. Oncol. Lett. 2021, 22, 544. [Google Scholar] [CrossRef]
- Gong, X.; Smith, J.R.; Swanson, H.M.; Rubin, L. Carotenoid lutein selectively inhibits breast cancer cell growth and potentiates the effect of chemotherapeutic agents through ROS-mediated mechanisms. Molecules 2018, 23, 905. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Zhang, Y.; Li, Y.; Lu, K.; Shen, Y.; Guo, Y.; Qi, Q.; Wang, M.; Zhang, S. NrF2/ARE and NF-κB pathway regulation may be the mechanism for lutein inhibition of human breast cancer cell. Future Oncol. 2018, 14, 719–726. [Google Scholar] [CrossRef]
- Vijay, K.; Sowmya, P.R.-R.; Arathi, B.P.; Shilpa, S.; Shwetha, H.J.; Raju, M.; Baskaran, V.; Lakshminarayana, R. Low-dose doxorubicin with carotenoids selectively alters redox status and upregulates oxidative stress-mediated apoptosis in breast cancer cells. Food Chem. Toxicol. 2018, 118, 675–690. [Google Scholar] [CrossRef]
- Shin, J.; Song, M.-H.; Oh, J.-W.; Keum, Y.-S.; Saini, R.K. Pro-oxidant actions of carotenoids in triggering apoptosis of cancer cells: A review of emerging evidence. Antioxidants 2020, 9, 532. [Google Scholar] [CrossRef]
- Cheng, J.; Miller, B.; Eroglu, A. The Efficacy of Carotenoids in DNA Repair in Lung Cancer. Curr. Dev. Nutr. 2020, 4, 99. [Google Scholar] [CrossRef]
- Ming, J.X.; Wang, Z.C.; Huang, Y.; Ohishi, H.; Wu, R.J.; Shao, Y.; Wang, H.; Qin, M.Y.; Wu, Z.L.; Li, Y.Y.; et al. Fucoxanthin extracted from Laminaria Japonica inhibits metastasis and enhances the sensitivity of lung cancer to Gefitinib. J. Ethnopharmacol. 2021, 265, 113302. [Google Scholar] [CrossRef]
- Arzi, L.; Hoshyar, R.; Jafarzadeh, N.; Riazi, G.; Sadeghizadeh, M. Anti-metastatic properties of a potent herbal combination in cell and mice models of triple negative breast cancer. Life Sci. 2020, 243, 117245. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Zheng, X.; Wu, X.; Wang, S.; Wang, Y.; Xing, F. All-trans retinoic acid reverses epithelial-mesenchymal transition in paclitaxel-resistant cells by inhibiting nuclear factor kappa B and upregulating gap junctions. Cancer Sci. 2019, 110, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Bi, M.-C.; Hose, N.; Xu, C.-L.; Zhang, C.; Sassoon, J.; Song, E. Nonlethal levels of zeaxanthin inhibit cell migration, invasion, and secretion of MMP-2 via NF-κB pathway in cultured human uveal melanoma cells. J. Ophthalmol. 2016, 2016, 8734309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, S.; Afzal, S.; Elwakeel, A.; Sharma, D.; Radhakrishnan, N.; Dhanjal, J.K.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Marine carotenoid fucoxanthin possesses anti-metastasis activity: Molecular evidence. Mar. Drugs 2019, 17, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Lu, X.; Yu, R. Lycopene inhibits epithelial–mesenchymal transition and promotes apoptosis in oral cancer via PI3K/AKT/m-TOR signal pathway. Drug Des. Dev. Ther. 2020, 14, 2461. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, K.; Radha, K.; Madhyastha, H. Cell cycle regulation and induction of apoptosis by β-carotene in U937 and HL-60 leukemia cells. BMB Rep. 2007, 40, 1009–1015. [Google Scholar] [CrossRef] [Green Version]
- Kikushige, Y. Pathophysiology of chronic lymphocytic leukemia and human B1 cell development. Int. J. Hematol. 2020, 111, 634–641. [Google Scholar] [CrossRef]
- Gomes, L.C.; Ferrão, A.L.M.; Evangelista, F.C.G.; de Almeida, T.D.; Barbosa, R.C.; das Graças Carvalho, M.; de Paula Sabino, A. Advances in chronic lymphocytic leukemia pharmacotherapy. Biomed. Pharmacother. 2018, 97, 349–358. [Google Scholar] [CrossRef]
- Smith, L.D.; Minton, A.R.; Blunt, M.D.; Karydis, L.I.; Dutton, D.A.; Rogers-Broadway, K.-R.; Dobson, R.; Liu, R.; Norster, F.; Hogg, E.; et al. BCR signaling contributes to autophagy regulation in chronic lymphocytic leukemia. Leukemia 2020, 34, 640–644. [Google Scholar] [CrossRef]
- Moccia, S.; Russo, M.; Durante, M.; Lenucci, M.S.; Mita, G.; Russo, G.L. A carotenoid-enriched extract from pumpkin delays cell proliferation in a human chronic lymphocytic leukemia cell line through the modulation of autophagic flux. Curr. Res. Biotechnol. 2020, 2, 74–82. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.-E.; Hu, L.; Zhao, L.; Huang, J. Carotenoids inhibit proliferation and regulate expression of peroxisome proliferators-activated receptor gamma (PPARγ) in K562 cancer cells. Arch. Biochem. Biophys. 2011, 512, 96–106. [Google Scholar] [CrossRef]
- Almeida, T.P.; Ramos, A.A.; Ferreira, J.; Azqueta, A.; Rocha, E. Bioactive compounds from seaweed with anti-leukemic activity: A mini-review on carotenoids and phlorotannins. Mini Rev. Med. Chem. 2020, 20, 39–53. [Google Scholar] [CrossRef]
- Gharib, A.; Faezizadeh, Z.; Godarzee, M. Preparation and characterization of nanoliposomal beta-cryptoxanthin and its effect on proliferation and apoptosis in human leukemia cell line K562. Trop. J. Pharm. Res. 2015, 14, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Saikia, M.; Retnakumari, A.P.; Anwar, S.; Anto, N.P.; Mittal, R.; Shah, S.; Pillai, K.S.; Balachandran, V.S.; Peter, V.; Thomas, R.; et al. Heteronemin, a marine natural product, sensitizes acute myeloid leukemia cells towards cytarabine chemotherapy by regulating farnesylation of Ras. Oncotarget 2018, 9, 18115. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.; Kim, M.; Park, H.; Jeong, M.I.; Jung, W.; Kim, B. Natural products and acute myeloid leukemia: A review highlighting mechanisms of action. Nutrients 2019, 11, 1010. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, T.; Ganesan, P.; Li, Z.; Manabe, Y.; Hirata, T. Siphonaxanthin, a green algal carotenoid, as a novel functional compound. Mar. Drugs 2014, 12, 3660–3668. [Google Scholar] [CrossRef] [Green Version]
- Kenzik, K.M.; Bhatia, R.; Williams, G.R.; Bhatia, S. Medicare and patient spending among beneficiaries diagnosed with chronic myelogenous leukemia. Cancer 2019, 125, 2570–2578. [Google Scholar] [CrossRef]
- Hernandez, F.Y.F.; Khandual, S.; López, I.G.R. Cytotoxic effect of Spirulina platensis extracts on human acute leukemia Kasumi-1 and chronic myelogenous leukemia K-562 cell lines. Asian Pac. J. Trop. Biomed. 2017, 7, 14–19. [Google Scholar] [CrossRef]
- Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-inflammatory and anticancer effects of microalgal carotenoids. Mar. Drugs 2021, 19, 531. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Wang, L.; Wang, L.; Zang, C.; Sun, L. The effect and mechanisms of proliferative inhibition of crocin on human leukaemia jurkat cells. West Indian Med. J. 2015, 64, 473. [Google Scholar]
- Veisi, A.; Akbari, G.; Mard, S.A.; Badfar, G.; Zarezade, V.; Mirshekar, M.A. Role of crocin in several cancer cell lines: An updated review. Iran. J. Basic Med. Sci. 2020, 23, 3. [Google Scholar]
- Colapietro, A.; Mancini, A.; D’Alessandro, A.M.; Festuccia, C. Crocetin and crocin from saffron in cancer chemotherapy and chemoprevention. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti Cancer Agents) 2019, 19, 38–47. [Google Scholar] [CrossRef]
- Kaushik, N.; Kumar, N.; Kim, C.H.; Kaushik, N.K.; Choi, E.H. Dielectric barrier discharge plasma efficiently delivers an apoptotic response in human monocytic lymphoma. Plasma Processes Polym. 2014, 11, 1175–1187. [Google Scholar] [CrossRef]
- Chen, F.; Hu, J.; Liu, P.; Li, J.; Wei, Z.; Liu, P. Carotenoid intake and risk of non-Hodgkin lymphoma: A systematic review and dose-response meta-analysis of observational studies. Ann. Hematol. 2017, 96, 957–965. [Google Scholar] [CrossRef]
- Li, S.; Zhu, X.; Zhu, L.; Hu, X.; Wen, S. Associations between serum carotenoid levels and the risk of non-Hodgkin lymphoma: A case–control study. Br. J. Nutr. 2020, 124, 1311–1319. [Google Scholar] [CrossRef]
- Bozorgpour, A.; Azad, R.; Showkatian, E.; Sulaiman, A. Multi-scale regional attention deeplab3+: Multiple myeloma plasma cells segmentation in microscopic images. arXiv 2021, arXiv:210506238. [Google Scholar]
- Hoshyar, R.; Mollaei, H. A comprehensive review on anticancer mechanisms of the main carotenoid of saffron, crocin. J. Pharm. Pharmacol. 2017, 69, 1419–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, T.; Cui, L.; Liu, Y.; Cheng, Z.; Quan, L.; Zeng, T.; Huang, W.; Dai, Y.; Chen, J.; Liu, L. High expression of chaperonin-containing TCP1 subunit 3 may induce dismal prognosis in multiple myeloma. Pharm. J. 2020, 20, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.H.A.; Naqvi, S.R.Z.; Abdelnour, S.A.; Schreurs, N.; Mohammedsaleh, Z.M.; Khan, I.; Shater, A.F.; Abd El-Hack, M.E.; Khafaga, A.F.; Quan, G.; et al. Beneficial effects and health benefits of Astaxanthin molecules on animal production: A review. Res. Vet. Sci. 2021, 138, 69–78. [Google Scholar] [CrossRef]

| Carotenoid | Diet Source | Reference |
|---|---|---|
| β-Cryptoxanthin | Pepper, papaya, oranges, tangerine | [75] |
| Lycopene | Water melon, tomatoes, pumpkin | [76,77] |
| α-Carotene | Carrots, green leafy vegetables, coleslaw, pumpkin | [78,79] |
| Lutein/zeaxanthin | Cucumber, pumpkin, celery, broccoli, spinach, egg, beans, pepper, grapes, melon, carrots, beans | [80,81] |
| Carotenoids | Cancer Type | Study Design | Mechanism | References |
|---|---|---|---|---|
| Crocin | Breast cancer | 4T1 mammary carcinoma cells injected to BALB/c mice | Inhibition of Wnt/β-catenin target genes | [94] |
| Retinoic acid | Colon cancer | CT26 murine colon cancer cells | Inhibition of nuclear factor-κB, vimentin, β-catenin and increased level of E-cadherin, gap junctions | [95] |
| Zeaxanthin | Uveal melanoma | C918 cultured uveal melanoma cells | Decreased matrix metalloproteinase, invasion and migration | [96] |
| β-cryptoxanthin | Gastric cancer | SGC-7901 gastric cancer cells | Apoptosis induction, reduction in AMP-activated protein kinases, and matrix metalloproteinase | [11] |
| Fucoxanthin | Lung cancer | murine PC9 xenograft, A549 lung cancer cells | Inhibition of Snail family of zinc-finger transcription factors 1, fibronectin and increased level of tissue inhibitors of metalloproteinase | [93] |
| Ovarian cancer | SKOV3 ovarian cancer cells | Decreased β-catenin, vimentin and vascular endothelial growth factor | [97] | |
| Lycopene | Oral cancer | Murine CAL-27 oral cancer xenograft | Inhibition of migration and N-cadherin with elevation in E-cadherin | [98] |
| Lutein | Breast cancer | Human breast cancer cells (MCF-7, MDA-MB-468) | increased ROS generation, activation of p53 signaling, and increased HSP60 expression | [89] |
| β-carotene | Leukemia | U 937, HL-60 cell line | Antioxidant, apoptosis, Cell cycle arrest | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, Y.; Abdullah; Alsharif, K.F.; Aschner, M.; Theyab, A.; Khan, F.; Saso, L.; Khan, H. Therapeutic Role of Carotenoids in Blood Cancer: Mechanistic Insights and Therapeutic Potential. Nutrients 2022, 14, 1949. https://doi.org/10.3390/nu14091949
Hussain Y, Abdullah, Alsharif KF, Aschner M, Theyab A, Khan F, Saso L, Khan H. Therapeutic Role of Carotenoids in Blood Cancer: Mechanistic Insights and Therapeutic Potential. Nutrients. 2022; 14(9):1949. https://doi.org/10.3390/nu14091949
Chicago/Turabian StyleHussain, Yaseen, Abdullah, Khalaf F. Alsharif, Michael Aschner, Abdulrahman Theyab, Fazlullah Khan, Luciano Saso, and Haroon Khan. 2022. "Therapeutic Role of Carotenoids in Blood Cancer: Mechanistic Insights and Therapeutic Potential" Nutrients 14, no. 9: 1949. https://doi.org/10.3390/nu14091949
APA StyleHussain, Y., Abdullah, Alsharif, K. F., Aschner, M., Theyab, A., Khan, F., Saso, L., & Khan, H. (2022). Therapeutic Role of Carotenoids in Blood Cancer: Mechanistic Insights and Therapeutic Potential. Nutrients, 14(9), 1949. https://doi.org/10.3390/nu14091949