Is Time-Restricted Eating Safe in the Treatment of Type 2 Diabetes?—A Review of Intervention Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy for Identifying Studies Investigating TRE in People with Type 2 Diabetes
2.2. Ongoing Studies of TRE in People with Type 2 Diabetes
2.3. Selection and Description of Antidiabetic Drugs
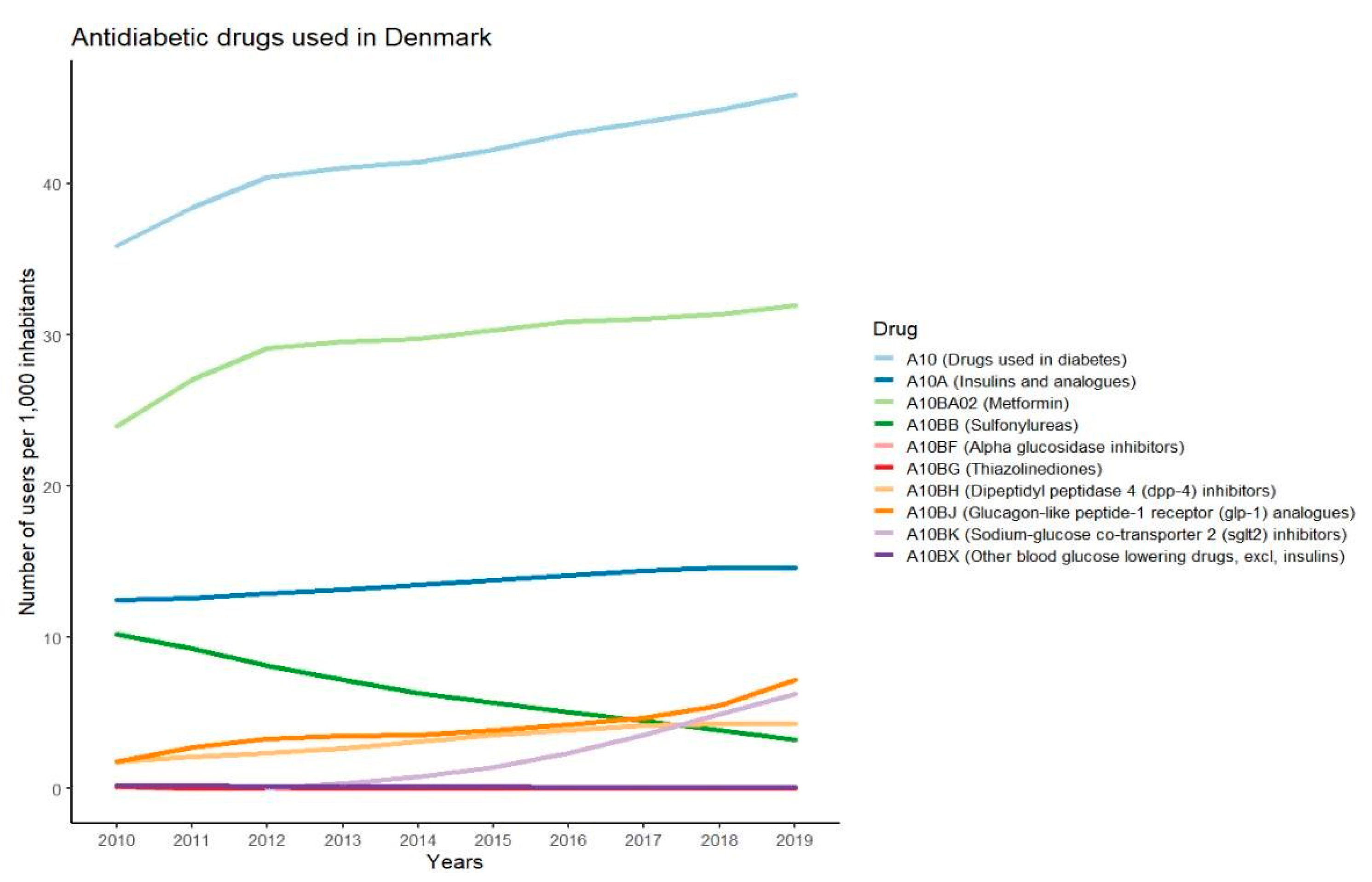
2.4. Statistics on Antidiabetic Drugs
3. Results
3.1. Time-Restricted Eating Interventions in People with Type 2 Diabetes
3.2. Medication Used for Treatment of Type 2 Diabetes
3.3. Most Used Antidiabetic Drugs
3.3.1. Metformin
3.3.2. GLP-1 Receptor Agonists
3.3.3. DPP-4 Inhibitors
3.3.4. SGLT-2 Inhibitors
3.3.5. Sulfonylureas
3.3.6. Basal Insulin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and Its Complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycaemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018, 61, 2461–2498. [Google Scholar] [CrossRef] [Green Version]
- Van Gaal, L.; Scheen, A. Weight Management in Type 2 Diabetes: Current and Emerging Approaches to Treatment. Diabetes Care 2015, 38, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Rajpal, A.; Ismail-Beigi, F. Intermittent Fasting and ‘Metabolic Switch’: Effects on Metabolic Syndrome, Prediabetes and Type 2 Diabetes. Diabetes Obes. Metab. 2020, 22, 1496–1510. [Google Scholar] [CrossRef] [PubMed]
- Rynders, C.A.; Thomas, E.A.; Zaman, A.; Pan, Z.; Catenacci, V.A.; Melanson, E.L. Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients 2019, 11, 2442. [Google Scholar] [CrossRef] [Green Version]
- Manoogian, E.N.C.; Chow, L.S.; Taub, P.R.; Laferrère, B.; Panda, S. Time-Restricted Eating for the Prevention and Management of Metabolic Diseases. Endocr. Rev. 2022, 43, 405–436. [Google Scholar] [CrossRef]
- Queiroz, J.D.N.; Macedo, R.C.O.; Tinsley, G.M.; Reischak-Oliveira, A. Time-Restricted Eating and Circadian Rhythms: The Biological Clock Is Ticking. Crit. Rev. Food Sci. Nutr. 2021, 61, 2863–2875. [Google Scholar] [CrossRef]
- Hawley, J.A.; Sassone-Corsi, P.; Zierath, J.R. Chrono-Nutrition for the Prevention and Treatment of Obesity and Type 2 Diabetes: From Mice to Men. Diabetologia 2020, 63, 2253–2259. [Google Scholar] [CrossRef]
- Moon, S.; Kang, J.; Kim, S.H.; Chung, H.S.; Kim, Y.J.; Yu, J.M.; Cho, S.T.; Oh, C.M.; Kim, T. Beneficial Effects of Time-Restricted Eating on Metabolic Diseases: A Systemic Review and Meta- Analysis. Nutrients 2020, 12, 1267. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Scheer, F.A.J.L.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian Clocks and Insulin Resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; Manoogian, E.N.C.; Melkani, G.C.; Panda, S. Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu. Rev. Nutr. 2019, 39, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; McStay, M.; Gabel, K.; Varady, K.A. Time Restricted Eating for the Prevention of Type 2 Diabetes. J. Physiol. 2022, 600, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Horne, B.D.; Grajower, M.M.; Anderson, J.L. Limited Evidence for the Health Effects and Safety of Intermittent Fasting Among Patients With Type 2 Diabetes. JAMA 2020, 324, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Sundhedsdatastyrelsen. Available online: https://sundhedsdatastyrelsen.dk/da/tal-og-analyser (accessed on 21 December 2021).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Parr, E.B.; Devlin, B.L.; Lim, K.H.C.; Moresi, L.N.Z.; Geils, C.; Brennan, L.; Hawley, J.A. Time-Restricted Eating as a Nutrition Strategy for Individuals with Type 2 Diabetes: A Feasibility Study. Nutrients 2020, 12, 3228. [Google Scholar] [CrossRef]
- Kahleova, H.; Belinova, L.; Malinska, H.; Oliyarnyk, O.; Trnovska, J.; Skop, V.; Kazdova, L.; Dezortova, M.; Hajek, M.; Tura, A.; et al. Eating Two Larger Meals a Day (Breakfast and Lunch) Is More Effective than Six Smaller Meals in a Reduced-Energy Regimen for Patients with Type 2 Diabetes: A Randomised Crossover Study. Diabetologia 2014, 57, 1552–1560. [Google Scholar] [CrossRef] [Green Version]
- Arnason, T.G.; Bowen, M.W.; Mansell, K.D. Effects of Intermittent Fasting on Health Markers in Those with Type 2 Diabetes: A Pilot Study. World J. Diabetes 2017, 8, 154. [Google Scholar] [CrossRef]
- Che, T.; Yan, C.; Tian, D.; Zhang, X.; Liu, X.; Wu, Z. Time-Restricted Feeding Improves Blood Glucose and Insulin Sensitivity in Overweight Patients with Type 2 Diabetes: A Randomised Controlled Trial. Nutr. Metab. 2021, 18, 88. [Google Scholar] [CrossRef]
- ADA. 5. Facilitating Behavior Change and Well-Being to Improve Health Outcomes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S60–S82. [Google Scholar] [CrossRef]
- ADA. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S125–S143. [Google Scholar] [CrossRef]
- Snorgaard, O.; Kristensen, J.K.; Balasubramaniam, K.; Breum, L.; Charles, M.; Højlund, K.; Madsen, G.K.; Bruun, J.M.; Navntoft, D.; Rungby, J.; et al. 2018 Revision Farmakologisk Behandling af Type 2-Diabetes: En Fælles Behandlingsvejledning med Enslydende Kliniske Behandlingsmål. Dansk Endokrinologisk Selskab og Dansk Selskab for Almen Medicin; Aalborg University: Aalborg, Denmark, 2018. [Google Scholar]
- Greiver, M.; Havard, A.; Bowles, J.K.F.; Kalia, S.; Chen, T.; Aliarzadeh, B.; Moineddin, R.; Sherlock, J.; Hinton, W.; Sullivan, F.; et al. Trends in Diabetes Medication Use in Australia, Canada, England, and Scotland: A Repeated Cross-Sectional Analysis in Primary Care. Br. J. Gen. Pract. 2021, 71, e209–e218. [Google Scholar] [CrossRef] [PubMed]
- Persson, F.; Bodegard, J.; Lahtela, J.T.; Nyström, T.; Jørgensen, M.E.; Jensen, M.L.; Gulseth, H.L.; Thuresson, M.; Hoti, F.; Nathanson, D.; et al. Different Patterns of Second-Line Treatment in Type 2 Diabetes after Metformin Monotherapy in Denmark, Finland, Norway and Sweden (D360 Nordic): A Multinational Observational Study. Endocrinol. Diabetes Metab. 2018, 1, e00036. [Google Scholar] [CrossRef] [PubMed]
- Thein, D.; Christiansen, M.N.; Mogensen, U.M.; Bundgaard, J.S.; Rørth, R.; Madelaire, C.; Fosbøl, E.L.; Schou, M.; Torp-Pedersen, C.; Gislason, G.; et al. Add-on Therapy in Metformin-Treated Patients with Type 2 Diabetes at Moderate Cardiovascular Risk: A Nationwide Study. Cardiovasc. Diabetol. 2020, 19, 107. [Google Scholar] [CrossRef]
- Yang, C.T.; Yang, C.Y.; Ou, H.T.; Kuo, S. Comparative Cardiovascular Safety of GLP-1 Receptor Agonists versus Other Glucose-Lowering Agents in Real-World Patients with Type 2 Diabetes: A Nationwide Population-Based Cohort Study. Cardiovasc. Diabetol. 2020, 19, 83. [Google Scholar] [CrossRef]
- Pernicova, I.; Korbonits, M. Metformin-Mode of Action and Clinical Implications for Diabetes and Cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the Glucoregulatory Mechanisms of Metformin in Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef] [Green Version]
- Flory, J.; Lipska, K. Metformin in 2019. JAMA 2019, 321, 1926–1927. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Barnett, A.H.; Bailey, C.J. Pharmacology and Therapeutic Implications of Current Drugs for Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2016, 12, 566–592. [Google Scholar] [CrossRef] [Green Version]
- Cornell, S. A Review of GLP-1 Receptor Agonists in Type 2 Diabetes: A Focus on the Mechanism of Action of Once-Weekly Agents. J. Clin. Pharm. Ther. 2020, 45, 17–27. [Google Scholar] [CrossRef]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like Peptide 1 in Health and Disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Gabery, S.; Salinas, C.G.; Paulsen, S.J.; Ahnfelt-Rønne, J.; Alanentalo, T.; Baquero, A.F.; Buckley, S.T.; Farkas, E.; Fekete, C.; Frederiksen, K.S.; et al. Semaglutide Lowers Body Weight in Rodents via Distributed Neural Pathways. JCI Insight 2020, 5, e133429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deacon, C.F. Diabetes: A Comparative Review. Diabetes Obes. Metab. 2011, 13, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F. Dipeptidyl Peptidase 4 Inhibitors in the Treatment of Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2020, 16, 642–653. [Google Scholar] [CrossRef]
- Chen, X.-W.; He, Z.-X.; Zhou, Z.-W.; Yang, T.; Zhang, X.; Yang, Y.-X.; Duan, W.; Zhou, S.-F. Clinical Pharmacology of Dipeptidyl Peptidase 4 Inhibitors Indicated for the Treatment of Type 2 Diabetes Mellitus. Clin. Exp. Pharmacol. Physiol. 2015, 42, 999–1024. [Google Scholar] [CrossRef]
- Perry, R.J.; Shulman, G.I. Sodium Glucose Cotransporter-2 Inhibitors: Understanding the Mechanisms for Therapeutic Promise and Persisting Risks. J. Biol. Chem. 2020, 295, 14379–14390. [Google Scholar] [CrossRef]
- Scheen, A.J. Sodium–Glucose Cotransporter Type 2 Inhibitors for the Treatment of Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2020, 16, 556–577. [Google Scholar] [CrossRef]
- Lupsa, B.C.; Inzucchi, S.E. Use of SGLT2 Inhibitors in Type 2 Diabetes: Weighing the Risks and Benefits. Diabetologia 2018, 61, 2118–2125. [Google Scholar] [CrossRef] [Green Version]
- Proks, P.; Reimann, F.; Green, N.; Gribble, F.; Ashcroft, F. Sulfonylurea Stimulation of Insulin Secretion. Diabetes 2002, 51, S368–S376. [Google Scholar] [CrossRef] [Green Version]
- Rossi, D.L.; Sola, D.; Rossi, L.; Piero, G.; Schianca, C.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Fra, G.P.; et al. State of the Art Paper Sulfonylureas and Their Use in Clinical Practice. Arch. Med. Sci. 2015, 11, 840–848. [Google Scholar] [CrossRef]
- Del Prato, S.; Pulizzi, N. The Place of Sulfonylureas in the Therapy for Type 2 Diabetes Mellitus. Metabolism 2006, 55, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.; Kapitza, C.; Pettus, J.; Heise, T. Understanding How Pharmacokinetic and Pharmacodynamic Differences of Basal Analog Insulins Influence Clinical Practice. Curr. Med. Res. Opin. 2017, 33, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.C.; Kruger, D.F. Considerations for Insulin-Treated Type 2 Diabetes Patients During Hospitalization: A Narrative Review of What We Need to Know in the Age of Second-Generation Basal Insulin Analogs. Diabetes Ther. 2020, 11, 2775–2790. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Achieving Glycaemic Control with Concentrated Insulin in Patients with Type 2 Diabetes. Drugs 2019, 79, 173–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frier, B.M. Hypoglycaemia in Diabetes Mellitus: Epidemiology and Clinical Implications. Nat. Rev. Endocrinol. 2014, 10, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Diabetes i Danmark | Forskning Og Viden—Diabetes. Available online: https://diabetes.dk/forskning/viden-om-diabetes/diabetes-i-danmark (accessed on 8 April 2022).
- Corley, B.T.; Carroll, R.W.; Hall, R.M.; Weatherall, M.; Parry-Strong, A.; Krebs, J.D. Intermittent Fasting in Type 2 Diabetes Mellitus and the Risk of Hypoglycaemia: A Randomized Controlled Trial. Diabet. Med. 2018, 35, 588–594. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | Study-Design | Intervention Eating Window | Duration of Intervention | Number of Participants | Male M, Female F | Mean Age, Years | Participants Treated with Antidiabetic Drugs | Incidences of Hypoglycaemia or Adverse Events |
|---|---|---|---|---|---|---|---|---|---|
| Arnason et al. (2017) [19] | Canada | Non-randomised Study; 2-week baseline, 2-week intervention, and 2-week follow up. | Daily eating window of 4–6 h | 2 weeks | 10 | M 1 F 9 | 53.8 | Metformin (10) Sulfonylureas (1) Other diabetic medications (1) | No hypoglycaemic events Does not report on other adverse events |
| Kahleova et al. (2014) [18] | Czech Republic | Randomised crossover study; 2 meals a day for 12 weeks, 6 meals a day for 12 weeks. | Both study arms: (resting energy expenditure × 1.5)−500 kcal/day 2 meals a day (1st meal 6–10 a.m. and 2nd meal 12–4 p.m.) OR 6 meals a day Breakfast, lunch and dinner + 3 smaller snacks in between. | 2 × 12 weeks | 54 | M 29 F 25 | 59.4 | Metformin (41) Sulfonylureas (16) Thiazolidinedione (3) Glinides (2) Acarbose (1) DPP-4 inhibitors (19) | Does not report on hypoglycaemic events No other adverse events |
| Parr et al. (2020) [17] | Australia | Pre-post, non-randomised. 2-week baseline, 4-week intervention. | Daily eating window between 10 a.m. and 7 p.m. | 4 weeks | 19 | M 9 F 10 | 50.2 | Metformin (10) SGLT-2 inhibitors (3) DPP-4 inhibitors (2) | No hypoglycaemic or other adverse events |
| Che et al. (2021) [20] | China | Randomised controlled trial | TRE: ad libitum intake: 8 a.m.–6 p.m. CON: Habitual diet | 12 weeks | 120 | TRE: M 31 F 29 CON: M 34 F 26 | TRE: 48.2 CON: 48.8 | OHA (not specified) TRE: 42 (70%) CON: 46 (77%) Insulin TRE: 19 (32%) CON: 15 (25%) | No adverse events including hypoglycaemic events in TRE group. One hypoglycaemic event in CON. |
| Name of Study | Country | Number of Participants | Intervention | Glucose Levels and Antidiabetic Medication | Estimated Timeline | Outcomes |
|---|---|---|---|---|---|---|
| Effect of Time-restricted Eating on Blood Glucose and Behavior in Patients With Type 2 Diabetes Mellitus (TREAT BB) | China | 35 | 12 weeks intervention with daily 10 h eating window | HbA1c 7.0–8.5% No use of insulin, long-acting insulin secretagogues, GLP-1 receptor agonist, DPP-4 inhibitor, SGLT-2 inhibitor | Start: 15 April 2021 Primary completion date: April 2022 Study completion Date: April 2022 | Adverse events (not specified) HbA1c and mean glucose and time in range measured using CGM |
| Application of Time Restriction Feeding in Patients With Type 2 Diabetes Mellitus | USA | 30 | 1 week with daily 12 h eating window | HbA1c ≥ 8% Stable anti-diabetic medication | Start: October 2021 Primary completion date: July 2023 Study completion Date: July 2024 | No outcomes regarding drugs, hypoglycaemia, or adverse events Mean glucose (unspecified method) HbA1c |
| Effect of Eating Within a Limited Time on Sugar Sensitivity and Liver Sugar Stores of People With Type 2 Diabetes. | The Netherlands | 21 | 3 weeks intervention with daily 10 h eating window | Non-insulin-treated type 2 diabetes No use of SGLT-2 inhibitors or insulin | Start: 31 January 2019 Primary completion date: 3 February 2021 Study completion Date: 3 February 2021 | No outcomes regarding drugs, hypoglycaemia, or adverse events Insulin sensitivity measured by hyperinsulinemic clamp |
| A Comparison Between the Effects of Conventional Diets vs Intermittent Fasting Diabetic and Pre-diabetic Patients | Pakistan | 128 | 12 weeks intervention: (1) calorie restriction, (2) TRE with daily 8 h eating window, (3) calorie restriction and TRE with daily 8 h eating window | Glycaemic values belonging to diabetes or prediabetes category (not specified) No use of insulin or sulfonylureas | Start: 1 September 2020 Primary completion date: 15 May 2021 Study completion Date: 15 May 2021 | No outcomes regarding drugs, hypoglycaemia, or adverse events Fasting glucose, HbA1c, OGTT |
| TREAT to Improve Cardiometabolic Health (NY-TREAT) | USA | 52 | 12 months intervention with daily ≤10 h eating window | Prediabetes and/or fasting glucose ≥ 100 mg/dL and/or HbA1c 5.7% or type 2 diabetes diet-controlled and/or treated with metformin and meeting 2 or more of the metabolic syndrome criteria | Start: 26 May 2021 Primary completion date: 31 January 2025 Study completion Date: 30 June 2025 | No outcomes regarding drugs, hypoglycaemia, or adverse events Matsuda index Insulinogenic index |
| Time Restricted Eating As Treatment (TREAT) for Diabetes Mellitus: A Pre-Post 12 Week Study on the Effectiveness of Intermittent Fasting in Asians With Type 2 Diabetes Mellitus | Singapore | 50 | 12 week intervention with daily 8 h eating window | Newly diagnosed T2D Solely dietary control | Start date: 14 January 2019 Primary completion date: 30 June 2020 Study completion Date: 30 June 2020 | No outcomes regarding drugs, hypoglycaemia, or adverse events |
| TREAT (Time Restricted EATing) to Improve Cardiometabolic Health | USA | 52 | Followed up to 12 months. 10 h eating window a day | Prediabetic (ADA criteria 2019) OR T2D solely diet-controlled, and/or treated with metformin and HbA1c ≤ 7% | Start date: November 2020 Primary completion date: December 2024 Study completion date: September 2025 | No outcomes regarding drugs, hypoglycaemia, or adverse events |
| Time Limited Eating in Adolescents With Type 2 Diabetes | USA | 40 | 12 weeks intervention with daily 8 h eating window for 5 days per week | T2D and HbA1c < 9% Monotherapy with metformin | Start date: 1 January 2021 Primary completion date: 1 December 2024 Study completion date: 1 December 2026 | No outcomes regarding drugs, hypoglycaemia, or adverse events |
| Effect of Time Restricted Feeding on Hepatic Glycogen Depletion and Insulin Sensitivity in Adults With Type 2 Diabetes | The Netherlands | 34 | Randomised controlled cross-over design with two 3 weeks arms and a 4 weeks wash-out period. Daily 10 h eating window. | T2D No use of SGLT-2 inhibitors or insulin | Study start date: 31 January 2019 Primary completion date: October 2019 Study completion date: December 2019 | No outcomes regarding drugs, hypoglycaemia, or adverse events |
| Time-Restricted Feeding on Glucose Homeostasis and Quality of Life | Kuwait | 50 | 12 weeks intervention Daily 6 h eating window | T2D with HbA1c 6.5–12% Any diabetes medication | Study start date: 10 July 2019 Primary completion date: 1 March 2020 Study completion date: 1 March 2020 | Secondary outcome: Change in diabetes medications between the intervention and control arms |
| Using Early Time Restricted Feeding and Timed Light Therapy to Improve Glycaemic Control in Adults With Type 2 Diabetes | USA | 344 | 16 weeks intervention 8 h daily eating window | HbA1c 7.0–10.0% and a change of less than 0.7% 6 months prior to study Treatment with metformin, sulfonylureas, DPP-4 inhibitors, and/or GLP-1-RAs on a stable dose for a minimum of 6 months or no use of antidiabetics. | Study start date: October 2020 Primary completion date: August 2023 Completion date: August 2023 | 24 h glucose levels (Time Frame: 16 weeks) Time-weighted mean, fasting, peak, standard deviation, and excursion (maximum–minimum) values (mg/dL) |
| The Impact of Time Restricted Feeding (TRF) in Improving the Health of Patients With Metabolic Syndrome | USA | 35 | 12 weeks intervention 10 h eating window a day | Elevated fasting glucose ≥ 100 mg/dL or drug treatment of elevated blood glucose No use of medication with known effect on appetite | Study start date: 28 July 2017 Primary completion date: 31 January 2019 Study completion date: June 2020 | Mean blood glucose (Time Frame: 12 weeks) Measured using CGM |
| Feasibility Study of a Low-Carb/Time-restricted Feeding Protocol in Insulin-Using Type 2 Diabetics | USA | 20 | 6 months intervention Low carbohydrate diet (30–60 g) 8 h eating window with 2 meals daily | T2D Use of basal insulin glargine or detemir. Stable T2D regimen for > 3 months and HbA1c 7–10% | Study start date: October 2020 Primary completion date: October 2021 Study completion date: October 2021 | Effectiveness of intervention: Changes in insulin dosage |
| Therapeutic Effects of Time Restricted Feeding and Calorie Restriction in Patients With Prediabetes and Diabetes | Pakistan | 250 | 12 weeks intervention Intervention groups of: TRE (8 h eating window a day), CR (deficit of 500 calories), and combined TRE and CR | TDM or prediabetic No use of insulin or sulfonylureas | Study Start Date: September 2020 Primary completion date: January 2021 Study completion date: February 2021 | No outcomes regarding drugs, hypoglycaemia, or adverse events |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uldal, S.; Clemmensen, K.K.B.; Persson, F.; Færch, K.; Quist, J.S. Is Time-Restricted Eating Safe in the Treatment of Type 2 Diabetes?—A Review of Intervention Studies. Nutrients 2022, 14, 2299. https://doi.org/10.3390/nu14112299
Uldal S, Clemmensen KKB, Persson F, Færch K, Quist JS. Is Time-Restricted Eating Safe in the Treatment of Type 2 Diabetes?—A Review of Intervention Studies. Nutrients. 2022; 14(11):2299. https://doi.org/10.3390/nu14112299
Chicago/Turabian StyleUldal, Sarah, Kim Katrine Bjerring Clemmensen, Frederik Persson, Kristine Færch, and Jonas Salling Quist. 2022. "Is Time-Restricted Eating Safe in the Treatment of Type 2 Diabetes?—A Review of Intervention Studies" Nutrients 14, no. 11: 2299. https://doi.org/10.3390/nu14112299
APA StyleUldal, S., Clemmensen, K. K. B., Persson, F., Færch, K., & Quist, J. S. (2022). Is Time-Restricted Eating Safe in the Treatment of Type 2 Diabetes?—A Review of Intervention Studies. Nutrients, 14(11), 2299. https://doi.org/10.3390/nu14112299






