Potentially Bio-Accessible Metabolites from an Extract of Cornus mas Fruit after Gastrointestinal Digestion In Vitro and Gut Microbiota Ex Vivo Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Material Collection
2.3. Extract Preparation
2.4. Digestion Procedures In Vitro
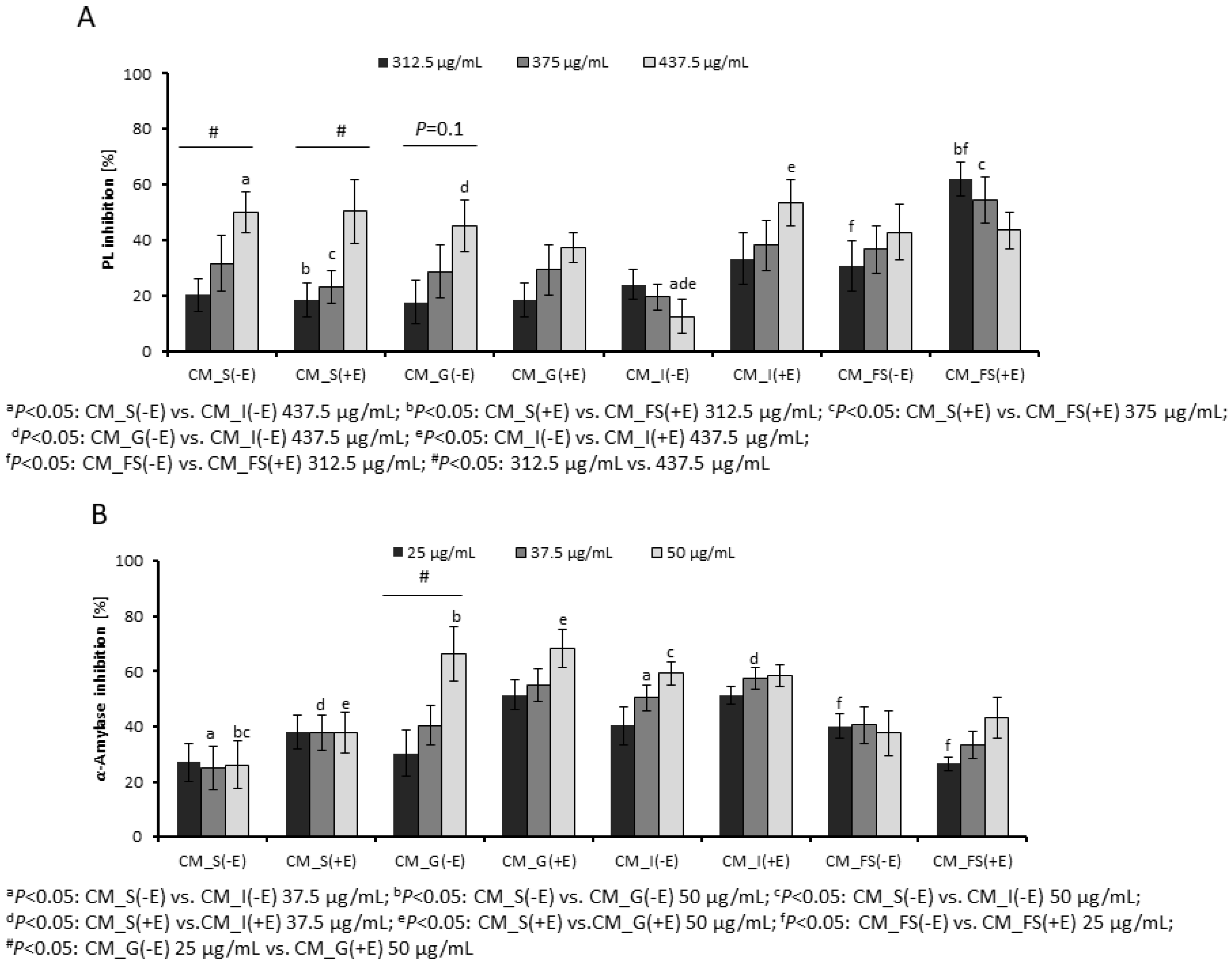
2.5. Inhibition of Pancreatic Lipase and α-Amylase by Digested Fractions
2.5.1. Pancreatic Lipase Inhibitory Assay
2.5.2. α-Amylase Inhibitory Assay
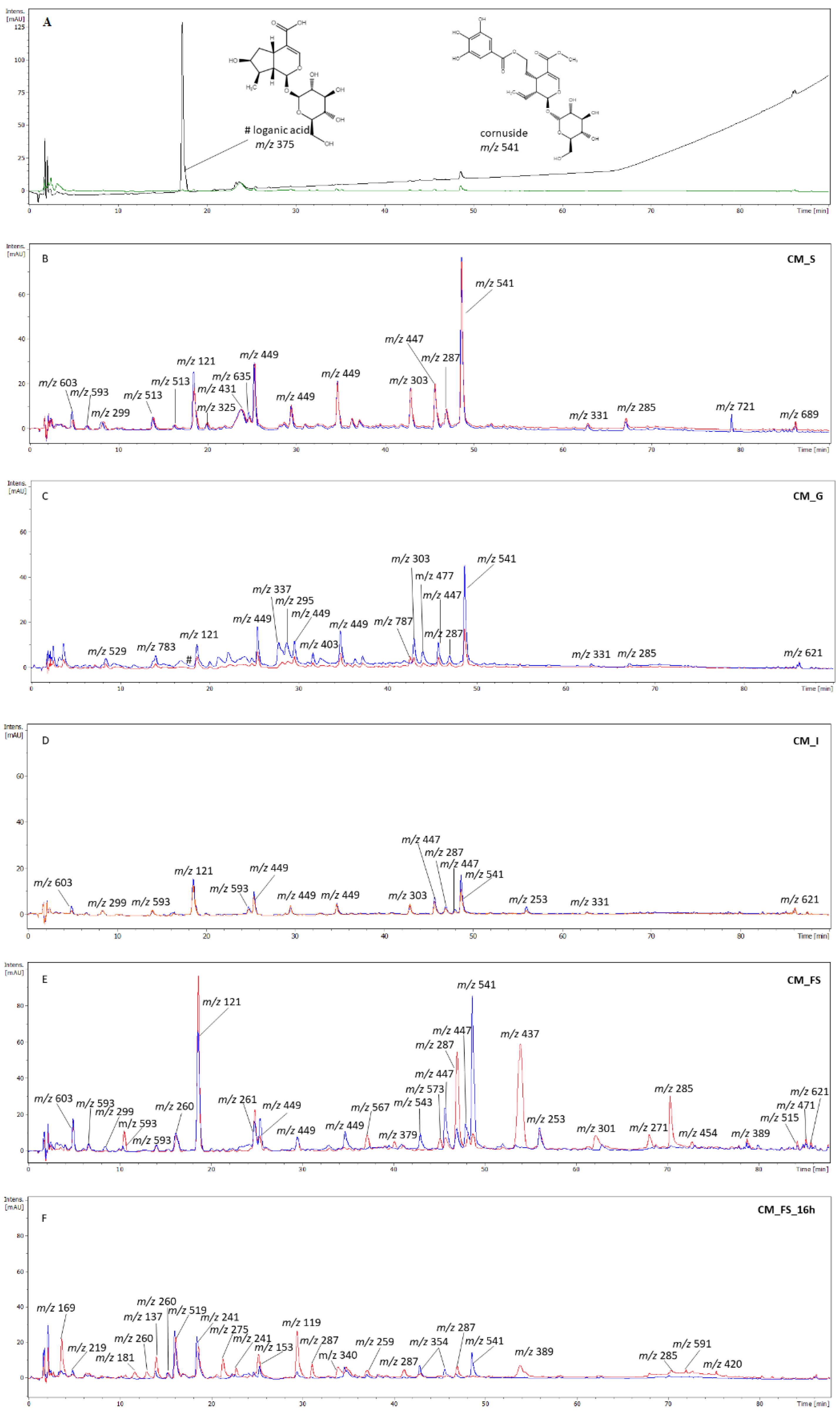
2.6. HPLC Analysis of Digested Fractions
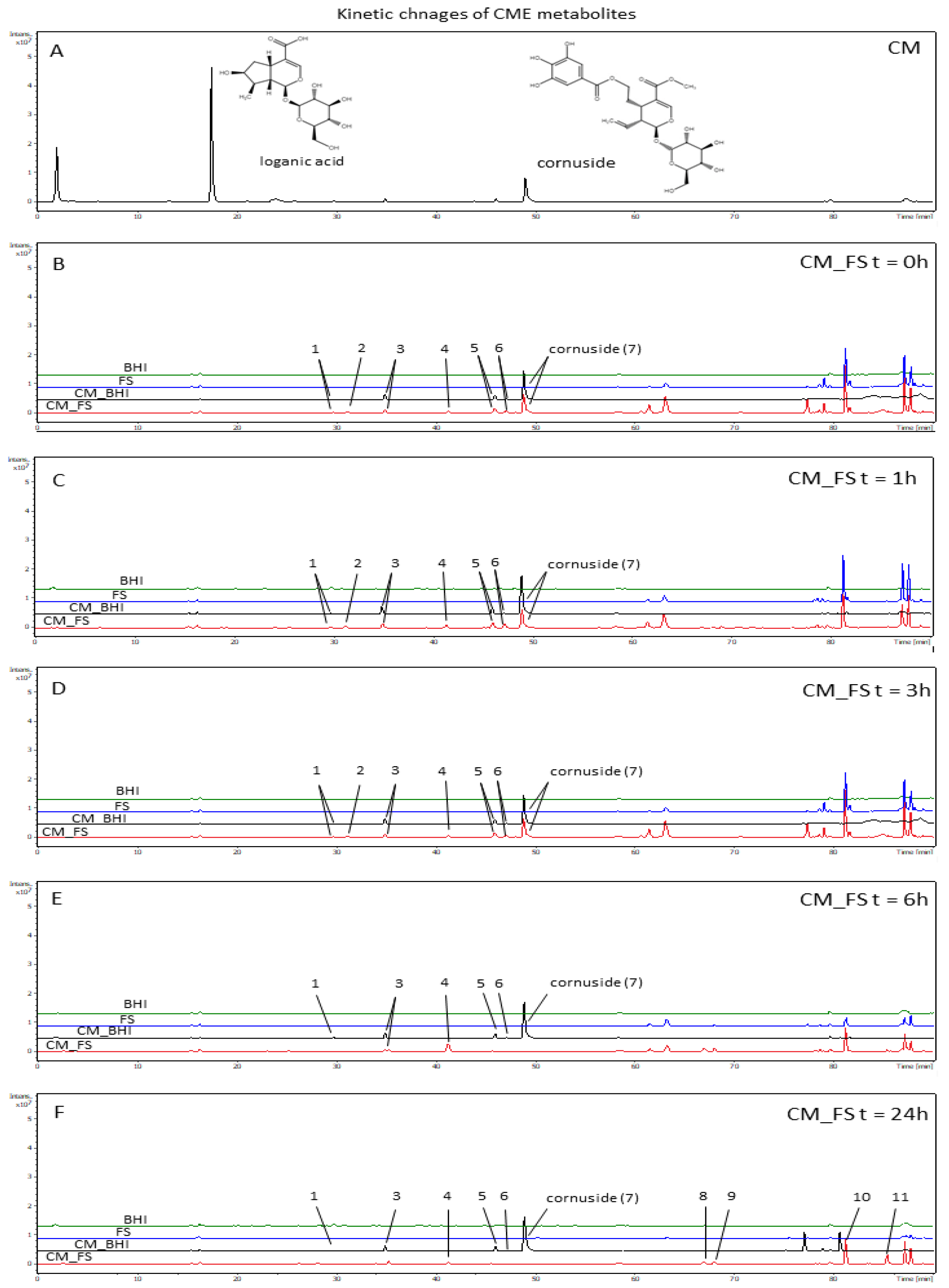
2.7. Kinetic Investigation of Extract Treated with Gut Microbiota
2.8. Statistical Analysis
3. Results
3.1. Inhibition of Digestive Enzymes by Gastrointestinal Fractions
3.2. The Phytochemical Analysis of Gastrointestinal Fractions
3.3. The Analysis of Time-Dependent Changes of Metabolites after Gut Microbiota Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 11 December 2021).
- Kinlen, D.; Cody, D.; O’Shea, D. Complications of obesity. QJM Int. J. Med. 2018, 111, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Schreck, K.; Melzig, M.F. Traditionally Used Plants in the Treatment of Diabetes Mellitus: Screening for Uptake Inhibition of Glucose and Fructose in the Caco2-Cell Model. Front. Pharmacol. 2021, 12, 692566. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.; Nassra, M.; Atgie, C.; Plaisancie, P.; Geloen, A. Oleic acid uptake reveals the rescued enterocyte phenotype of colon cancer Caco-2 by HT29-MTX cells in co-culture mode. Int. J. Mol. Sci. 2017, 18, 1573. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour-Jaghdani, F.; Shomali, T.; Gholipour-Shahraki, S.; Rahimi-Madiseh, M.; Rafieian-Kopaei, M. Cornus mas: A review on traditional uses and pharmacological properties. J. Complement. Integr. Med. 2017, 14, 137. [Google Scholar] [CrossRef]
- Świerczewska, A.; Buchholz, T.; Melzig, M.F.; Czerwińska, M.E. In vitro α-amylase and pancreatic lipase inhibitory activity of Cornus mas L. and Cornus alba L. fruit extracts. J. Food Drug Anal. 2019, 27, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Jayaprakasam, B.; Olson, L.K.; Schutzki, R.E.; Tai, M.H.; Nair, M.G. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas). J. Agric. Food Chem. 2006, 54, 243–248. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Vareed, S.K.; Olson, L.K.; Nair, M.G. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J. Agric. Food Chem. 2005, 53, 28–31. [Google Scholar] [CrossRef]
- Asgary, S.; Rafieian-Kopaei, M.; Shamsi, F.; Najafi, S.; Sahebkar, A. Biochemical and histopathological study of the anti-hyperglycemic and anti-hyperlipidemic effects of cornelian cherry (Cornus mas L.) in alloxan-induced diabetic rats. J. Complement. Integr. Med. 2014, 11, 63–69. [Google Scholar] [CrossRef]
- Soltani, R.; Gorji, A.; Asgary, S.; Sarrafzadegan, N.; Siavash, M. Evaluation of the effects of Cornus mas L. fruit extract on glycemic control and insulin level in type 2 diabetic adult patients: A randomized double-blind placebo-controlled clinical trial. Evid. Based Complement. Alternat. Med. 2015, 2015, 740954. [Google Scholar] [CrossRef] [Green Version]
- Danielewski, M.; Kucharska, A.Z.; Matuszewska, A.; Rapak, A.; Gomulkiewicz, A.; Dzimira, S.; Dziegiel, P.; Nowak, B.; Trocha, M.; Magdalan, J.; et al. Cornelian cherry (Cornus mas L.) iridoid and anthocyanin extract enhances PPAR-α, PPAR-γ expression and reduces I/M ratio in aorta, increases LXR-α expression and alters adipokines and triglycerides levels in cholesterol-rich diet rabbit model. Nutrients 2021, 13, 3621. [Google Scholar] [CrossRef]
- Dzydzan, O.; Bila, I.; Kucharska, A.Z.; Brodyak, I.; Sybirna, N. Antidiabetic effects of extracts of red and yellow fruits of cornelian cherries (Cornus mas L.) on rats with streptozotocin-induced diabetes mellitus. Food Funct. 2019, 10, 6459–6472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moldovan, B.; Filip, A.; Clichici, S.; Suharoschi, R.; Bolfa, P.; David, L. Antioxidant activity of Cornelian cherry (Cornus mas L.) fruits extract and the in vivo evaluation of its anti-inflammatory effects. J. Funct. Food 2016, 26, 77–87. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.Z.; Szumny, A.; Magdalan, J.; Bielska, K.; Merwid-Ląd, A.; Woźniak, A.; Dzimira, S.; Piórecki, N.; Trocha, M. The protective effect of the Cornus mas fruits (Cornelian cherry) on hypertriglyceridemia and atherosclerosis through PPARalpha activation in hypercholesterolemic rabbits. Phytomed. Int. J. Phytother. Phytopharm. 2014, 21, 1774–1784. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.Z.; Rapak, A.; Szumny, D.; Trocha, M.; Merwid-Lad, A.; Dzimira, S.; Piasecki, T.; Piórecki, N.; Magdalan, J.; et al. Iridoid-loganic acid versus anthocyanins from the Cornus mas fruits (Cornelian cherry): Common and different effects on diet-induced atherosclerosis, PPARs expression and inflammation. Atherosclerosis 2016, 254, 151–160. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jin, G.Y.; Li, G.Z.; Yan, G.H. Cornuside suppresses lipopolysaccharide-induced inflammatory mediators by inhibiting nuclear factor-kappa B activation in RAW 264.7 macrophages. Biol. Pharm. Bull. 2011, 34, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Czerwińska, M.E.; Bobińska, A.; Cichocka, K.; Buchholz, T.; Woliński, K.; Melzig, M.F. Cornus mas and Cornus officinalis—A comparison of antioxidant and immunomodulatory activities of standardized fruit extracts in human neutrophils and Caco-2 models. Plants 2021, 10, 2347. [Google Scholar] [CrossRef]
- Brennan, K.; Zheng, J. Interelukin 8. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Aryaeian, N.; Amiri, F.; Rahideh, S.T.; Abolghasemi, J.; Jazayeri, S.; Gholamrezayi, A.; Motevalian, M.; Solaymani-Dodaran, M.; Taghizadeh, M.; Heshmati, E.; et al. The effect of Cornus mas extract consumption on bone biomarkers and inflammation in postmenopausal women: A randomized clinical trial. Phytother. Res. 2021, 35, 4425–4432. [Google Scholar] [CrossRef]
- Rutkowski, L. Klucz do Oznaczania Roślin Naczyniowych Polski Niżowej, 2nd ed.; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2011. [Google Scholar]
- Siegień, J.; Buchholz, T.; Popowski, D.; Granica, S.; Osińska, E.; Melzig, M.F.; Czerwińska, M.E. Pancreatic lipase and α-amylase inhibitory activity of extracts from selected plant materials after gastrointestinal digestion in vitro. Food Chem. 2021, 355, 129414. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Granica, S.; Zwierzyńska, M.; Stefańska, J.; Schopohl, P.; Melzig, M.F.; Kiss, A.K. Role of human gut microbiota metabolism in the anti-inflammatory effect of traditionally used ellagitannin-rich plant materials. J. Ethnopharmacol. 2014, 155, 801–809. [Google Scholar] [CrossRef]
- Buchholz, T.; Melzig, M.F. Medicinal plants traditionally used for treatment of obesity and diabetes mellitus—Screening for pancreatic lipase and α-amylase inhibition. Phytother. Res. 2016, 30, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A.Z.; Szumny, A.; Sokół-Łętowska, A.; Piórecki, N.; Klymenko, S.V. Iridoids and anthocyanins in cornelian cherry (Cornus mas L.) cultivars. J. Food Comp. Anal. 2015, 40, 95–102. [Google Scholar] [CrossRef]
- Szandruk-Bender, M.; Rutkowska, M.; Merwid-Lad, A.; Wiatrak, B.; Szelag, A.; Dzimira, S.; Sobieszczanska, B.; Krzystek-Korpacka, M.; Kucharska, A.Z.; Matuszewska, A.; et al. Cornelian cherry iridoid-polyphenolic extract improves mucosal epithelial barrier integrity in rat experimental colitis and exerts antimicrobial and antiadhesive activities in vitro. Oxidative Med. Cell. Longev. 2020, 2020, 7697851. [Google Scholar] [CrossRef] [PubMed]
- Pawlowska, A.M.; Camangi, F.; Braca, A. Quali-quantitative analysis of flavonoids of Cornus mas L. (Cornaceae) fruits. Food Chem. 2010, 119, 1257–1261. [Google Scholar] [CrossRef] [Green Version]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abad-García, B.; Garmón-Lobato, S.; Berrueta, L.A.; Gallo, B.; Vicente, F. A fragmentation study of dihydroquercetin using triple quadrupole mass spectrometry and its application for identification of dihydroflavonols in Citrus juices. Rapid Commun. Mass Spectrom. 2009, 23, 2785–2792. [Google Scholar] [CrossRef]
- Przybylska, D.; Kucharska, A.Z.; Cybulska, I.; Sozanski, T.; Piorecki, N.; Fecka, I. Cornus mas L. stones: A valuable by-product as an ellagitannin source with high antioxidant potential. Molecules 2020, 25, 4646. [Google Scholar] [CrossRef]
- Wang, D.; Wei, X.; Yan, X.; Jin, T.; Ling, W. Protocatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein E-deficient mice. J. Agric. Food Chem. 2010, 58, 12722–12728. [Google Scholar] [CrossRef]
- Stuby, J.; Gravestock, I.; Wolfram, E.; Pichierri, G.; Steurer, J.; Burgstaller, J.M. Appetite-suppressing and satiety-increasing bioactive phytochemicals: A systematic review. Nutrients 2019, 11, 2238. [Google Scholar] [CrossRef] [Green Version]
- Allegra, M. Antioxidant and anti-inflammatory properties of plants extract. Antioxidants 2019, 8, 549. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.-T.; Liu, X.-T.; Chen, Q.-X.; Shi, Y. Lipase inhibitors for obesity: A review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kumar, V.; Nayak, S.K.; Wadhwa, P.; Kaur, P.; Sahu, S.K. Alpha-amylase as molecular target for treatment of diabetes mellitus: A comprehensive review. Chem. Biol. Drug Des. 2021, 98, 539–560. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; Rosa, L.A.d.l.; Vargas-Requena, C.L.; Bustos-Jaimes, I.; Alvarez-Parrilla, A.E. Polyphenolic compounds and digestive Enzymes: In vitro non-covalent interactions. Molecules 2017, 22, 669. [Google Scholar] [CrossRef] [Green Version]
- Pereira, F.C.; Berry, D. Microbial nutrient niches in the gut. Environ. Microbiol. 2017, 19, 1366–1378. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Jin, G.; Jiang, J.; Zheng, M.; Jin, Y.; Lin, Z.; Li, G.; Choi, Y.; Yan, G. Cornuside inhibits mast cell-mediated allergic response by down-regulating MAPK and NF-kappaB signaling pathways. Biochem. Biophys. Res. Commun. 2016, 473, 408–414. [Google Scholar] [CrossRef]
- Li, L.; Jin, G.; Jin, Y.; Jiang, J.; Yang, J.; Choi, Y.; Jin, Z.; Zheng, M.; Yan, G. Cornuside ameliorates airway inflammation via toll-like receptor 4 and Notch1 in asthmatic mice induced by lipopolysaccharide and ovalbumin. Int. J. Clin. Exp. Med. 2016, 9, 15274–15284. [Google Scholar]
- Bhakta, H.K.; Park, C.H.; Yokozawa, T.; Tanaka, T.; Jung, H.A.; Choi, J.S. Potential anti-cholinesterase and beta-site amyloid precursor protein cleaving enzyme 1 inhibitory activities of cornuside and gallotannins from Cornus officinalis fruits. Arch. Pharm. Res. 2017, 40, 836–853. [Google Scholar] [CrossRef]
- Jiang, W.L.; Zhang, S.M.; Tang, X.X.; Liu, H.Z. Protective roles of cornuside in acute myocardial ischemia and reperfusion injury in rats. Phytomed. Int. J. Phytother. Phytopharm. 2011, 18, 266–271. [Google Scholar] [CrossRef]
- Liu, L.; Shu, A.; Zhu, Y.; Chen, Y. Cornuside alleviates diabetes mellitus-induced testicular damage by modulating the gut microbiota. Evid.-Based Complement. Alternat. Med. 2021, 2021, 5301942. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Asp. Med. 2018, 61, 41–49. [Google Scholar] [CrossRef]
- Kucharska, A.Z. Związki Aktywne Owoców Derenia (Cornus mas L.); Wrocław University of Environmental and Life Sciences: Wrocław, Poland, 2012. [Google Scholar]
- Sharma, S.; Tripathi, P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; DuPont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.; Morgan, M.R.; Williamson, G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Day, A.J.; Canada, F.J.; Diaz, J.C.; Kroon, P.A.; McLauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, T.; Horio, F.; Osawa, T. Absorption and metabolism of cyanidin 3-O-beta-D-glucoside in rats. FEBS Lett. 1999, 449, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Karamać, M.; Amarowicz, R. Inhibition of pancreatic lipase by phenolic acids—Examination in vitro. Z. Nat. C J. Biosci. 1996, 51, 903–905. [Google Scholar] [CrossRef]
- Adamenko, K.; Kawa-Rygielska, J.; Kucharska, A.; Piórecki, N. Fruit low-alcoholic beverages with high contents of iridoids and phenolics from apple and cornelian cherry (Cornus mas L.) fermented with Saccharomyces bayanus. Pol. J. Food Nutr. Sci. 2019, 69, 307–317. [Google Scholar] [CrossRef]
- Savaş, E.; Tavşanlı, H.; Catalkaya, G.; Capanoglu, E.; Tamer, C.E. The antimicrobial and antioxidant properties of garagurt: Traditional Cornelian cherry (Cornus mas) marmalade. Qual. Assur. Saf. Crops Foods 2012, 12, 12–23. [Google Scholar] [CrossRef]
- Salejda, A.M.; Kucharska, A.Z.; Krasnowska, G. Effect of Cornelian cherry (Cornus mas L.) juice on selected quality properties of beef burgers. J. Food Qual. 2018, 2018, 1563651. [Google Scholar] [CrossRef] [Green Version]
- Czyżowska, A.; Kucharska, A.Z.; Nowak, A.; Sokół-Łętowska, A.; Motyl, I.; Piórecki, N. Suitability of the probiotic lactic acid bacteria strains as the starter cultures in unripe cornelian cherry (Cornus mas L.) fermentation. J. Food Sci. Technol. 2017, 54, 2936–2946. [Google Scholar] [CrossRef] [PubMed]
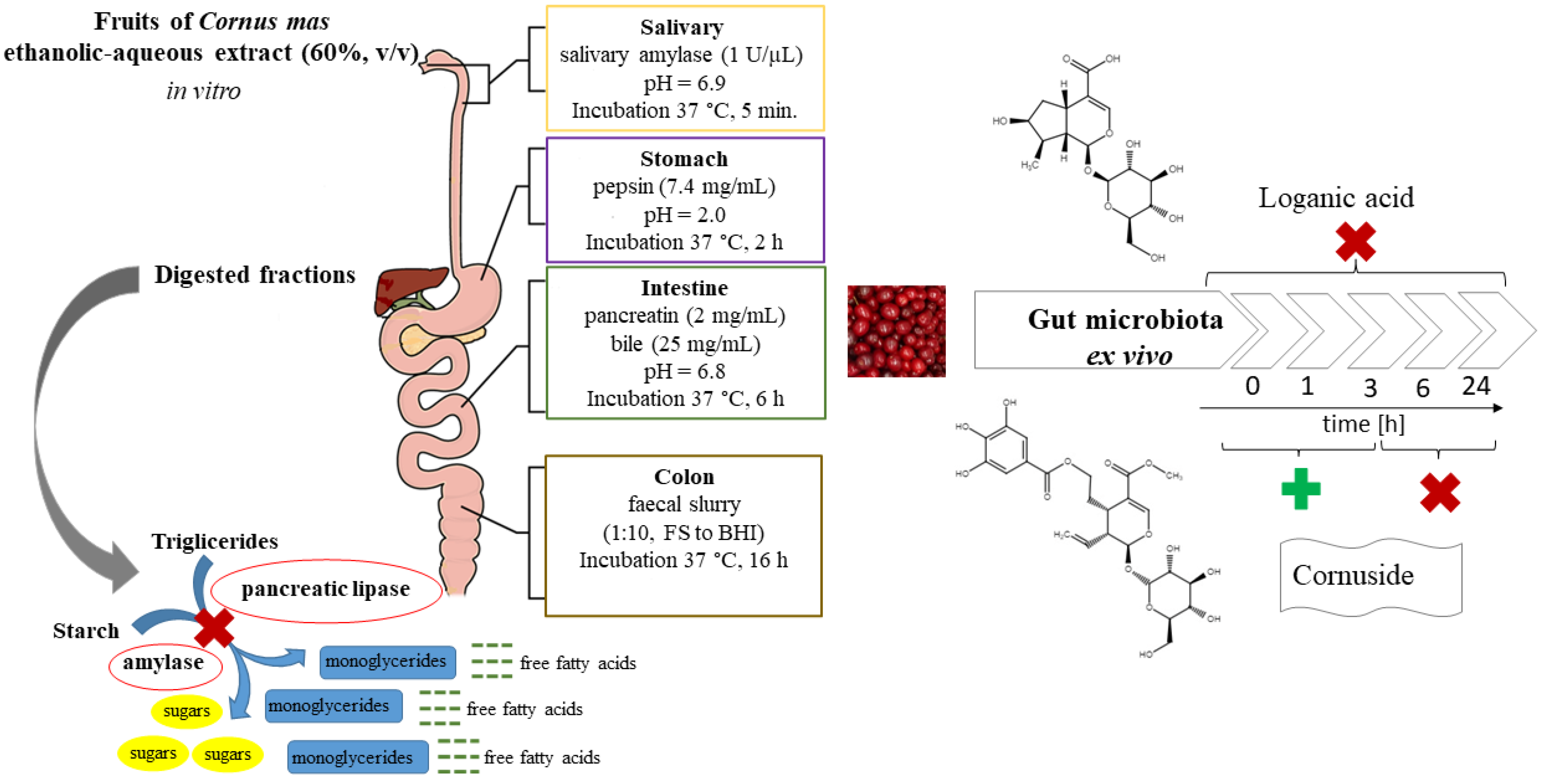
| Medium | Salivary Fraction | Gastric Fraction | Intestinal Fraction | Colon Fraction |
|---|---|---|---|---|
| Enzymes | α-amylase (1 U/μL) | pepsin (7.4 mg/mL) | pancreatin (2 mg/mL) | fecal slurry (1:10, m/v) |
| pH | 6.8 | 2.0 | 6.9 | - |
| Additives | KCl (110 g/L) | |||
| KSCN (25 g/L) | ||||
| NaH2PO4 (110 g/L) | bile salts (25 mg/mL) | |||
| Na2SO4 (70 g/L) | HCl (150 mM) | NaHCO3 (0.5 M) | BHI | |
| NaCl (220 g/L) | ||||
| NaHCO3 (105 g/L) | ||||
| urea (30 g/L) |
| No. | Retention Time [min] | λmax [nm] | [M − H]− | MS2− | Assigned Compound |
|---|---|---|---|---|---|
| 1 | 29.7 | 350 | 449 | 431, 355, 329, 287, 269 | aromadendrin hexoside |
| 2 | 31.2 | 265, 310, 364 | 287 | 259 | aromadendrin isomer |
| 3 | 34.9 | 260, 300, 350 | 449 | 431, 287, 269 | pelargonidin hexuronide |
| 4 | 41.3 | 265, 310, 364 | 287 | 269, 243, 165 | aromadendrin isomer |
| 5 | 45.7 | 345 | 447 | 327, 284 | kaempferol hexoside |
| 6 | 47.2 | 270, 310, 365 | 433 | 287 | aromadendrin |
| 7 | 49.0 | 280 | 541 | 379 | cornuside |
| 8 | 67.0 | 265, 310, 350 | 745 | 630, 388. 257 | unidentified |
| 9 | 68.1 | 270, 320, 360 | 595 | 549, 505, 462 | unidentified |
| 10 | 81.2 | 270, 320, 355 | 391 | 345 | unidentified |
| 11 | 85.4 | 260, 310, 350 | 297 | 279, 185 | unidentified |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olędzka, A.; Cichocka, K.; Woliński, K.; Melzig, M.F.; Czerwińska, M.E. Potentially Bio-Accessible Metabolites from an Extract of Cornus mas Fruit after Gastrointestinal Digestion In Vitro and Gut Microbiota Ex Vivo Treatment. Nutrients 2022, 14, 2287. https://doi.org/10.3390/nu14112287
Olędzka A, Cichocka K, Woliński K, Melzig MF, Czerwińska ME. Potentially Bio-Accessible Metabolites from an Extract of Cornus mas Fruit after Gastrointestinal Digestion In Vitro and Gut Microbiota Ex Vivo Treatment. Nutrients. 2022; 14(11):2287. https://doi.org/10.3390/nu14112287
Chicago/Turabian StyleOlędzka, Agata, Katarzyna Cichocka, Konrad Woliński, Matthias F. Melzig, and Monika E. Czerwińska. 2022. "Potentially Bio-Accessible Metabolites from an Extract of Cornus mas Fruit after Gastrointestinal Digestion In Vitro and Gut Microbiota Ex Vivo Treatment" Nutrients 14, no. 11: 2287. https://doi.org/10.3390/nu14112287
APA StyleOlędzka, A., Cichocka, K., Woliński, K., Melzig, M. F., & Czerwińska, M. E. (2022). Potentially Bio-Accessible Metabolites from an Extract of Cornus mas Fruit after Gastrointestinal Digestion In Vitro and Gut Microbiota Ex Vivo Treatment. Nutrients, 14(11), 2287. https://doi.org/10.3390/nu14112287







