Proanthocyanidins Restore the Metabolic Diurnal Rhythm of Subcutaneous White Adipose Tissue According to Time-Of-Day Consumption
Abstract
1. Introduction
2. Materials and Methods
2.1. Proanthocyanidin Extract
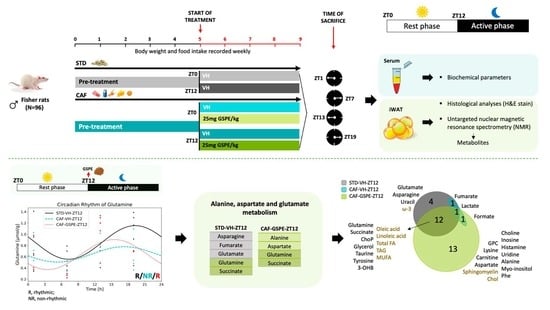
2.2. Study Design and Dosage Information
2.3. Biometric Parameters and Circulating Biomarkers
2.4. Adipose Tissue Histology
2.5. Adipose Tissue Preparation for 1H NMR-Based Metabolomics Assay
2.6. NMR Analysis
2.7. Statistical Analysis
3. Results
3.1. GSPE Administration at ZT12 Reduces Body Weight Gain and Accumulated Food Intake
3.2. Adipocyte Size Is Modified According to Time-of-Day GSPE Administration
3.3. GSPE Administration at ZT12 Recovers the Diurnal Rhythmic Concentration of Serum TAG That Is Lost in Diet-Induced Obesity
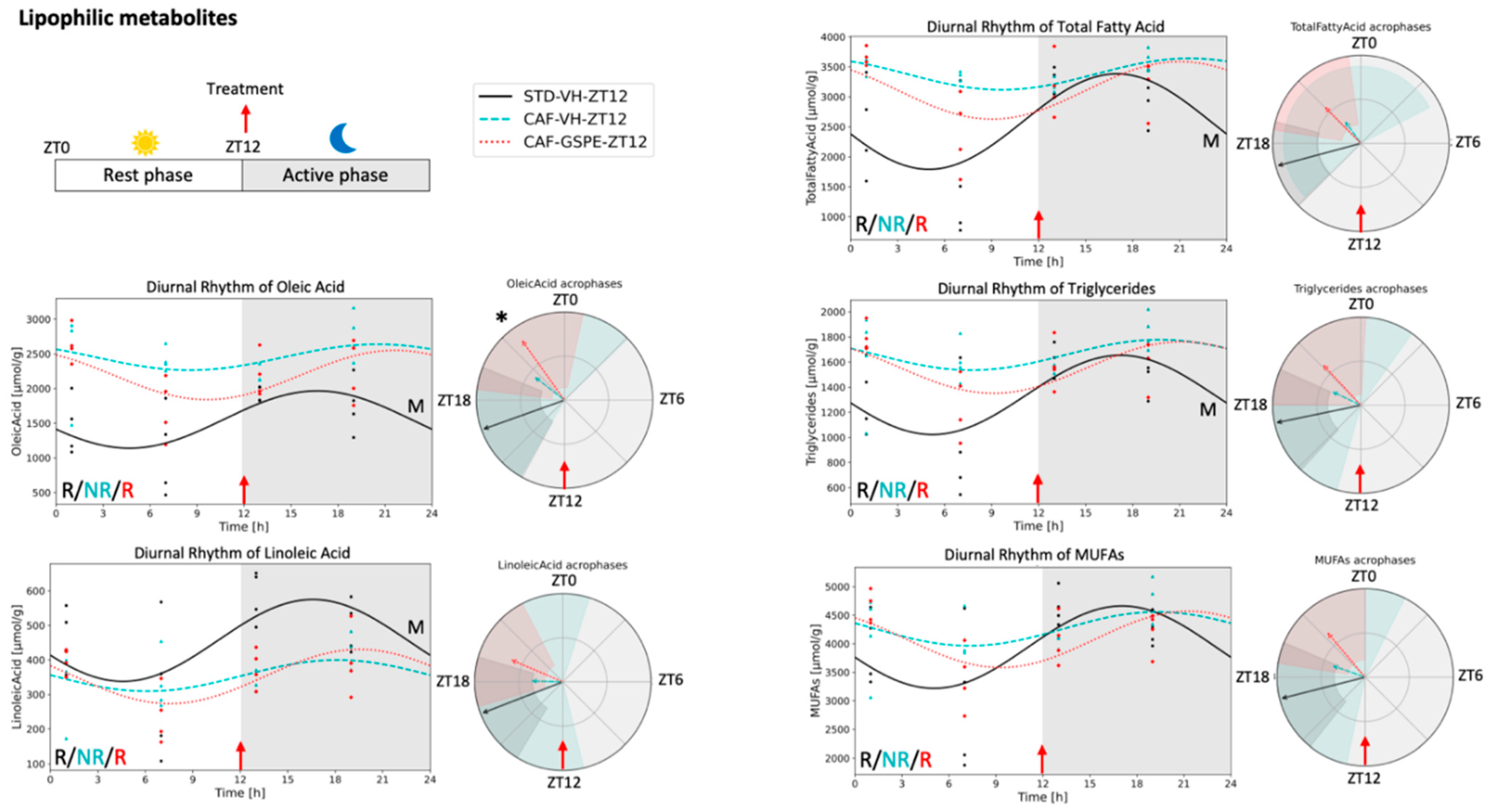
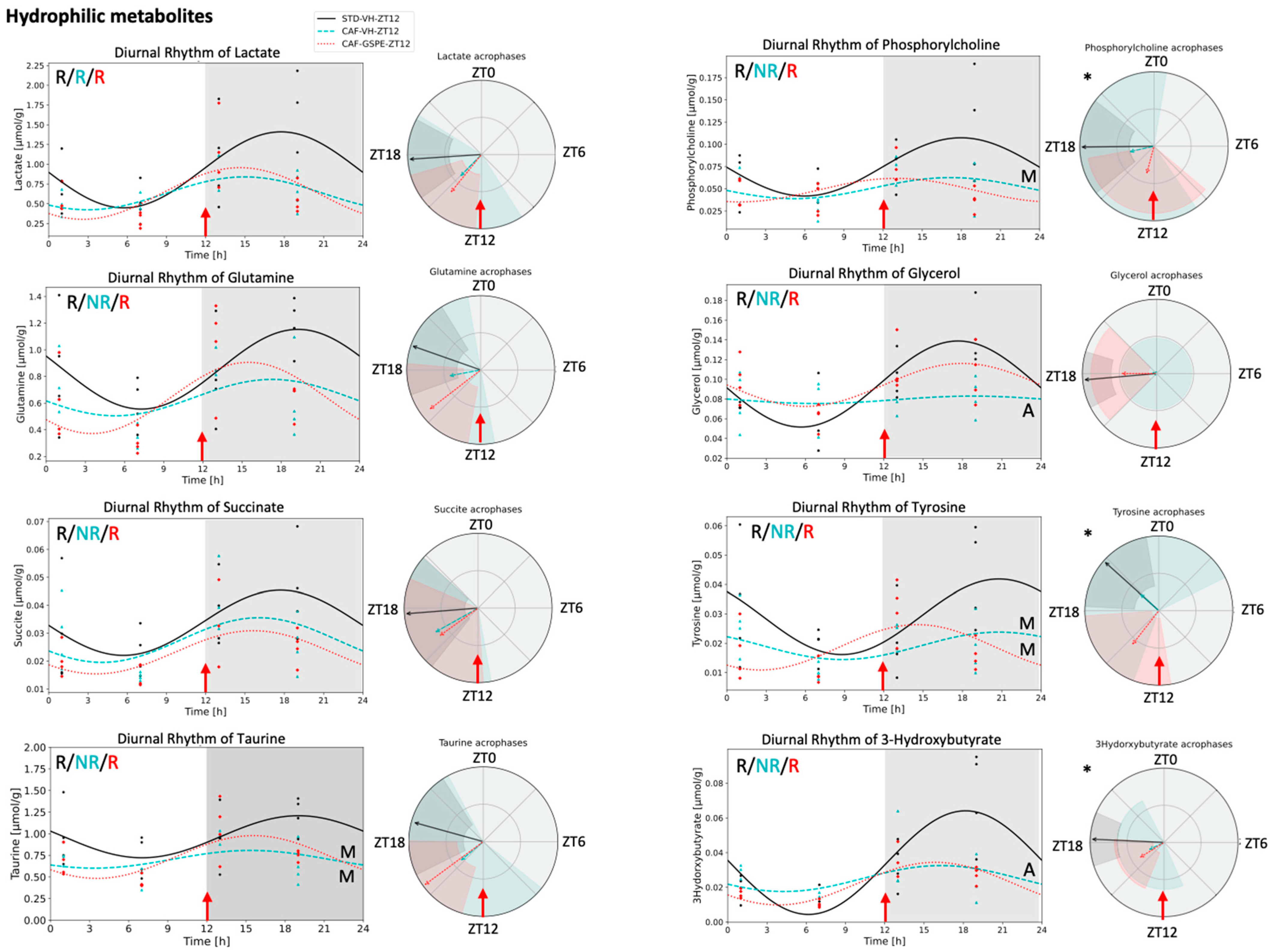
3.4. Metabolite Concentrations in iWAT Are Restored in Response to GSPE Administration in a Time-Dependent Manner
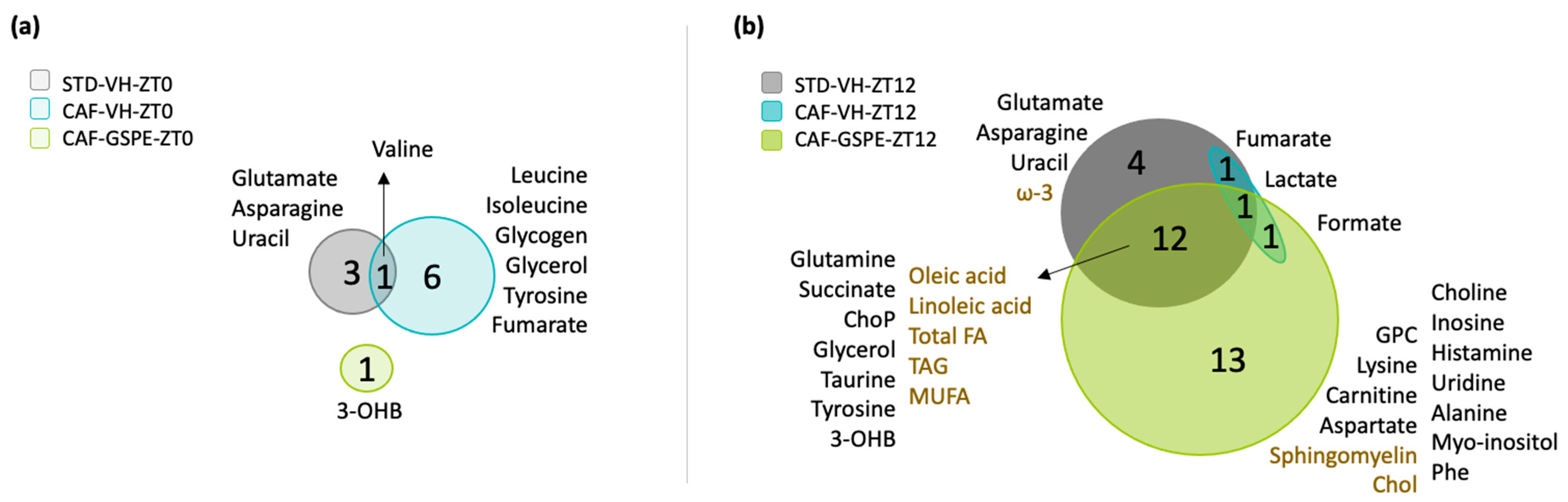
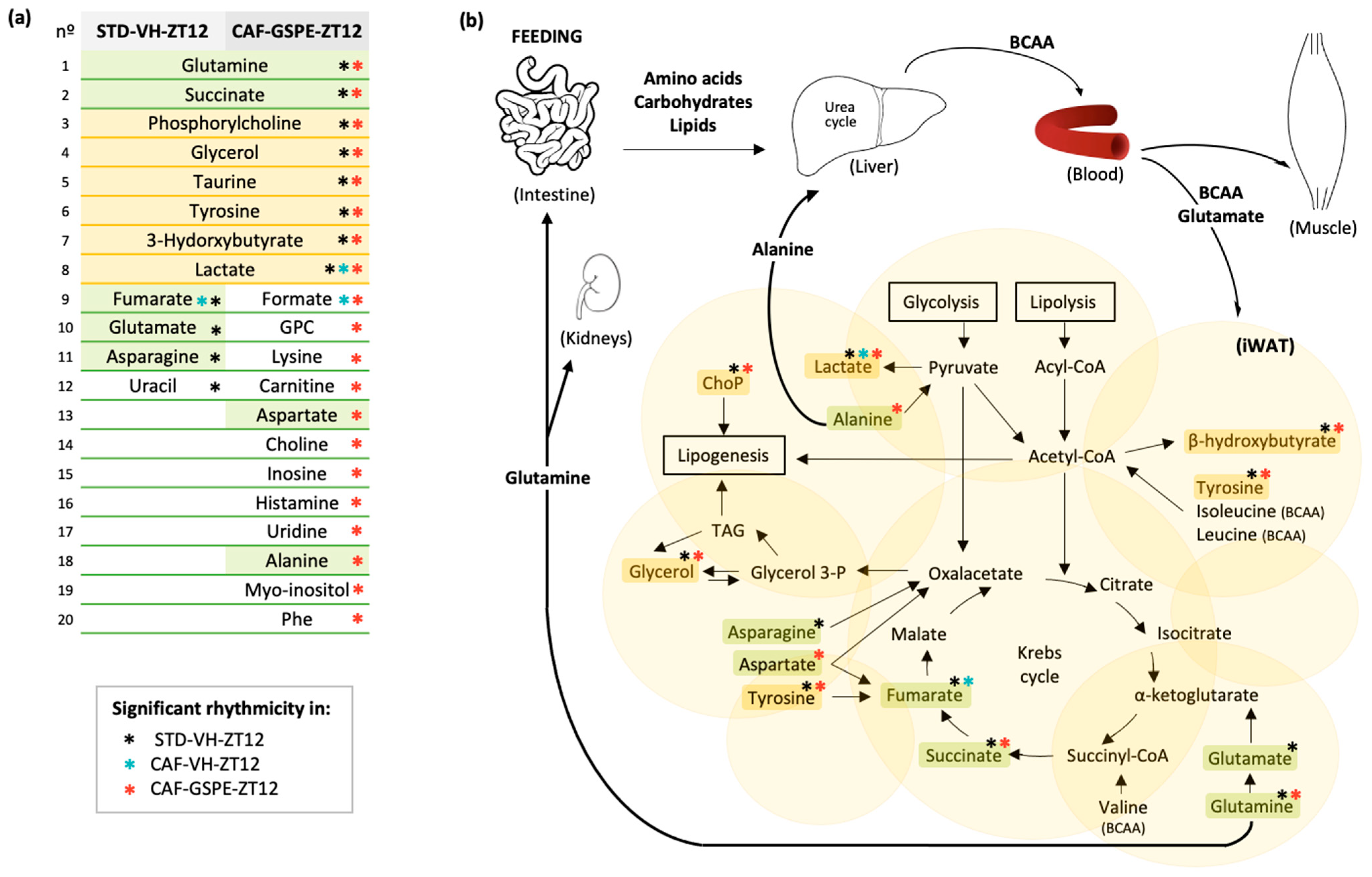
3.5. GSPE Administration at ZT12 Re-Establishes the Number of Rhythmic Metabolites in iWAT
3.6. GSPE Administration at ZT12 Restores the Rhythmicity of Alanine, Aspartate and Glutamate Metabolism Pathways in iWAT
4. Discussion
4.1. Biometric Parameters and Circulating Biomarkers
4.2. Lipophilic Metabolites in Adipose Tissue
4.3. Lipid Metabolism in Adipose Tissue
4.4. Hydrophilic Metabolites in Adipose Tissue
4.5. Alanine, Aspartate and Glutamate Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, S.A. Circadian Metabolism: From Mechanisms to Metabolomics and Medicine. Trends Endocrinol. Metab. 2016, 27, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Gamble, K.L.; Berry, R.; Frank, S.J.; Young, M.E. Circadian clock control of endocrine factors. Nat. Rev. Endocrinol. 2014, 10, 466–475. [Google Scholar] [CrossRef] [PubMed]
- de Assis, L.V.M.; Oster, H. The circadian clock and metabolic homeostasis: Entangled networks. Cell. Mol. Life Sci. 2021, 78, 4563–4587. [Google Scholar] [CrossRef] [PubMed]
- Kiehn, J.-T.; Koch, C.E.; Walter, M.; Brod, A.; Oster, H. Circadian rhythms and clocks in adipose tissues: Current insights. ChronoPhysiology Ther. 2017, 7, 7–17. [Google Scholar] [CrossRef]
- Dyar, K.A.; Lutter, D.; Artati, A.; Ceglia, N.J.; Liu, Y.; Armenta, D.; Jastroch, M.; Schneider, S.; de Mateo, S.; Cervantes, M.; et al. Atlas of Circadian Metabolism Reveals System-wide Coordination and Communication between Clocks. Cell 2018, 174, 1571–1585.e11. [Google Scholar] [CrossRef]
- Bray, M.S.; Ratcliffe, W.F.; Grennet, M.H.; Brewer, R.A.; Gamble, K.L.; Young, M.E. Quantitative Analysis of Light-Phase Restricted Feeding Reveals Metabolic Dyssynchrony in Mice. Int. J. Obes. 2013, 37, 843–852. [Google Scholar] [CrossRef]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian timing of food intake contributes to weight gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef]
- Satoh, Y.; Kawai, H.; Kudo, N.; Kawashima, Y.; Mitsumoto, A. Time-restricted feeding entrains daily rhythms of energy metabolism in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, 1276–1283. [Google Scholar] [CrossRef]
- Arble, D.M.; Vitaterna, M.H.; Turek, F.W. Rhythmic leptin is required for weight gain from circadian desynchronized feeding in the mouse. PLoS ONE 2011, 6, e25079. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef]
- Chaix, A.; Lin, T.; Le, H.D.; Chang, M.W.; Panda, S. Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab. 2019, 29, 303–319.e4. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Timothy Garvey, W.; Newman, J.W.; Lok, K.H.; Hoppel, C.L.; Adams, S.H. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS ONE 2010, 5, e15234. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, P.; Soininen, P.; Kangas, A.J.; Rönnemaa, T.; Lehtimäki, T.; Kähönen, M.; Viikari, J.S.; Raitakari, O.T.; Ala-Korpela, M. Branched-chain and aromatic amino acidsare predictors of insulinresistance in young adults. Diabetes Care 2013, 36, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.A.; She, P.; Peroni, O.D.; Lynch, C.J.; Kahn, B.B. Adipose tissue Branched Chain Amino Acid (BCAA) metabolism modulates circulating BCAA levels. J. Biol. Chem. 2010, 285, 11348–11356. [Google Scholar] [CrossRef]
- Frayn, K.N.; Khan, K.; Coppack, S.W.; Elia, M. Amino acid metabolism in human subcutaneous adipose tissue in vivo. Clin. Sci. 1991, 80, 471–474. [Google Scholar] [CrossRef]
- Cheng, S.; Wiklund, P.; Autio, R.; Borra, R.; Ojanen, X.; Xu, L.; Törmäkangas, T.; Alen, M. Adipose tissue dysfunction and altered systemic amino acid metabolism are associated with non-alcoholic fatty liver disease. PLoS ONE 2015, 10, e0138889. [Google Scholar] [CrossRef]
- Constabel, C. Molecular controls of proanthocyanidin synthesis and structure: Prospects for genetic engineering in crop plants. J. Agric. Food Chem. 2018, 66, 9882–9888. [Google Scholar] [CrossRef]
- Mannino, G.; Chinigò, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and where to find them: A meta-analytic approach to investigate their chemistry, biosynthesis, distribution, and effect on human health. Antioxidants 2021, 10, 1229. [Google Scholar] [CrossRef]
- Pascual-Serrano, A.; Bladé, C.; Suárez, M.; Arola-Arnal, A. Grape Seed Proanthocyanidins Improve White Adipose Tissue Expansion during Diet-Induced Obesity Development in Rats. Int. J. Mol. Sci. 2018, 19, 2632. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvado, J.; Arola, L.; Bladé, C. Chronic consumption of dietary proanthocyanidins modulates peripheral clocks in healthy and obese rats. J. Nutr. Biochem. 2015, 26, 112–119. [Google Scholar] [CrossRef]
- Margalef, M.; Pons, Z.; Iglesias-Carres, L.; Bravo, F.I.; Muguerza, B.; Arola-Arnal, A. Lack of Tissue Accumulation of Grape Seed Flavanols after Daily Long-Term Administration in Healthy and Cafeteria-Diet Obese Rats. J. Agric. Food Chem. 2015, 63, 9996–10003. [Google Scholar] [CrossRef] [PubMed]
- Gibert-Ramos, A.; Palacios-Jordan, H.; Salvadó, M.J.; Crescenti, A. Consumption of out-of-season orange modulates fat accumulation, morphology and gene expression in the adipose tissue of Fischer 344 rats. Eur. J. Nutr. 2020, 59, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Briggs, W.; Paschos, G.K.; Fitzgerald, G.A.; Griffin, J.L. A metabolomic study of adipose tissue in mice with a disruption of the circadian system. Mol. Biosyst. 2015, 11, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Jordan, H.; Martín-González, M.Z.; Suárez, M.; Aragonès, G.; Mugureza, B.; Rodríguez, M.A.; Bladé, C. The disruption of liver metabolic circadian rhythms by a cafeteria diet is sex-dependent in fischer 344 rats. Nutrients 2020, 12, 1085. [Google Scholar] [CrossRef]
- Parsons, R.; Parsons, R.; Garner, N.; Oster, H.; Rawashdeh, O. CircaCompare: A method to estimate and statistically support differences in mesor, amplitude and phase, between circadian rhythms. Bioinformatics 2020, 36, 1208–1212. [Google Scholar] [CrossRef]
- Moškon, M. CosinorPy: A python package for cosinor-based rhythmometry. BMC Bioinform. 2020, 21, 485. [Google Scholar] [CrossRef]
- Foster, M.T.; Softic, S.; Caldwell, J.; Kohli, R.; DeKloet, A.D.; Seeley, R.J. Subcutaneous adipose tissue transplantation in diet-induced obese mice attenuates metabolic dysregulation while removal exacerbates it. Physiol. Rep. 2013, 1, e00015. [Google Scholar] [CrossRef]
- Cox-York, K.; Wei, Y.; Wang, D.; Pagliassotti, M.J.; Foster, M.T. Lower body adipose tissue removal decreases glucose tolerance and insulin sensitivity in mice with exposure to high fat diet. Adipocyte 2015, 4, 32–43. [Google Scholar] [CrossRef][Green Version]
- Tran, T.T.; Yamamoto, Y.; Gesta, S.; Kahn, C.R. Beneficial Effects of Subcutaneous Fat Transplantation on Metabolism. Cell Metab. 2008, 7, 410–420. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Del Bas, J.M.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvado, J.; Arola, L.; Bladé, C. Dietary proanthocyanidins modulate melatonin levels in plasma and the expression pattern of clock genes in the hypothalamus of rats. Mol. Nutr. Food Res. 2015, 59, 865–878. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.; Bladé, C.; Arola, L. Dietary proanthocyanidins modulate BMAL1 acetylation, Nampt expression and NAD levels in rat liver. Sci. Rep. 2015, 5, 10954. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Liu, X.; Feng, H.; Zhao, S.; Gao, H. Grape seed proanthocyanidin extracts enhance endothelial nitric oxide synthase expression through 5’-AMP activated protein kinase/Surtuin 1-Krüpple like factor 2 pathway and modulate blood pressure in ouabain induced hypertensive rats. Biol. Pharm. Bull. 2012, 35, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Colom-Pellicer, M.; Rodríguez, R.M.; Navarro-Masip, È.; Bravo, F.I.; Mulero, M.; Arola, L.; Aragonès, G. Time-of-day dependent effect of proanthocyanidins on adipose tissue metabolism in rats with diet-induced obesity. Int. J. Obes. 2022, in press. [CrossRef] [PubMed]
- Wang, S.; Lin, Y.; Gao, L.; Yang, Z.; Lin, J.; Ren, S.; Li, F.; Chen, J.; Wang, Z.; Dong, Z.; et al. PPAR-γ integrates obesity and adipocyte clock through epigenetic regulation of Bmal1. Theranostics 2022, 12, 1589–1606. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Pan, S.; Xu, P.; Xue, T.; Wang, J.; Guo, Y.; Jia, L.; Qiao, X.; Li, L.; Zhai, Y. Melatonin Orchestrates Lipid Homeostasis through the Hepatointestinal Circadian Clock and Microbiota during Constant Light Exposure. Cells 2020, 9, 489. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Eckel-Mahan, K.L.; Mirbolooki, M.R.; Tjong, I.; Griffey, S.M.; Schmunk, G.; Koehne, A.; Halbout, B.; Iadonato, S.; Pedersen, B.; et al. Selective Kv1.3 channel blocker as therapeutic for obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2013, 110, E2239–E2248. [Google Scholar] [CrossRef]
- Possik, E.; Al-Mass, A.; Peyot, M.L.; Ahmad, R.; Al-Mulla, F.; Madiraju, S.R.M.; Prentki, M. New Mammalian Glycerol-3-Phosphate Phosphatase: Role in β-Cell, Liver and Adipocyte Metabolism. Front. Endocrinol. 2021, 12, 706607. [Google Scholar] [CrossRef]
- Christou, S.; Wehrens, S.M.T.; Isherwood, C.; Möller-Levet, C.S.; Wu, H.; Revell, V.L.; Bucca, G.; Skene, D.J.; Laing, E.E.; Archer, S.N.; et al. Circadian regulation in human white adipose tissue revealed by transcriptome and metabolic network analysis. Sci. Rep. 2019, 9, 2641. [Google Scholar] [CrossRef]
- Lackey, D.E.; Lynch, C.J.; Olson, K.C.; Mostaedi, R.; Ali, M.; Smith, W.H.; Karpe, F.; Humphreys, S.; Bedinger, D.H.; Dunn, T.N.; et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am. J. Physiol. Endocrinol. Metab. 2013, 304, 1175–1187. [Google Scholar] [CrossRef]
- Kowalski, T.J.; Guoyao, W.U.; Watford, M. Rat adipose tissue amino acid metabolism in vivo as assessed by microdialysis and arteriovenous techniques. Am. J. Physiol. 1997, 273, E613. [Google Scholar] [CrossRef]
- Burrill, J.S.; Long, E.K.; Reilly, B.; Deng, Y.; Armitage, I.M.; Scherer, P.E.; Bernlohr, D.A. Inflammation and ER stress regulate branched-chain amino acid uptake and metabolism in adipocytes. Mol. Endocrinol. 2015, 29, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Perriello, G.; Meyer, C.; Gerich, J. Role of glutamine in human carbohydrate metabolism in kidney and other tissues. Kidney Int. 1999, 55, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, T.J.; Watford, M. Production of glutamine and utilization of glutamate by rat subcutaneous adipose tissue in vivo. Am. J. Physiol. Endocrinol. Metab. 1994, 266, 51–54. [Google Scholar] [CrossRef]
- Petrus, P.; Lecoutre, S.; Dollet, L.; Wiel, C.; Sulen, A.; Gao, H.; Tavira, B.; Laurencikiene, J.; Rooyackers, O.; Checa, A.; et al. Glutamine Links Obesity to Inflammation in Human White Adipose Tissue. Cell Metab. 2020, 31, 375–390.e11. [Google Scholar] [CrossRef] [PubMed]
| ZT0 SDT-VH | ZT0 CAF-VH | ZT0 CAF-GSPE | ZT12 STD-VH | ZT12 CAF-VH | ZT12 CAF-GSPE | |
|---|---|---|---|---|---|---|
| Body weight (g) | 514.7 ± 7.5 a | 591.1 ± 7.8 b | 573.1 ± 9.2 b | 481.8 ± 7.1 a* | 569.3 ± 8.4 b | 546.8 ± 7.8 b* |
| Body weight gain (g) | 47.3 ± 3.4 a | 74.4 ± 3.9 b | 65.3 ± 4.3 b | 40.9 ± 3.1 a | 72.7 ± 3.3 b | 50.4 ± 3.1 a* |
| Body fat content (%) | 8.1 ± 0.3 a | 13.7 ± 0.4 b | 12.9 ± 0.6 b | 9.3 ± 0.6 a | 14.4 ± 0.6 b | 13.4 ± 0.5 b |
| iWAT (%) | 0.9 ± 0.1 a | 1.7 ± 0.1 b | 1.8 ± 0.1 b | 1.3 ± 0.1 a* | 1.6 ± 0.2 ab | 2.1 ± 0.2 b |
| Acc. food intake 1 (kJ) | 1239.5± 36.5 a | 2598.3 ± 80.5 b | 2696.9 ± 36.6 b | 1226.5 ± 40.1 a | 2793.0 ± 132.6 b | 2513.5 ± 71.1 b* |
| Food intake 2 (kJ/day) | 338.1 ± 18.2 a | 592.0 ± 12.7 b | 576.1 ± 32.5 b | 308.1 ± 4.9 a | 791.4 ± 77.7 b* | 578.1 ± 20.3 c |
| Group | ZT0 Glucose | ZT0 TAG | ZT0 CHOL | ZT0 NEFA | ZT12 Glucose | ZT12 TAG | ZT12 CHOL | ZT12 NEFA | |
|---|---|---|---|---|---|---|---|---|---|
| Rhythmicity (p value) | STD-VH | NS | 0.001 | NS | NS | NS | 0.002 | 0.035 | NS |
| CAF-VH | 0.017 | 0.001 | NS | 0.048 | NS | NS | NS | NS | |
| CAF-GSPE | NS | 0.007 | 0.002 | 0.001 | NS | 0.019 | NS | NS | |
| Mesor (mg/dL) | STD-VH | 87.19 | 71.38 | 91.37 | 25.49 | 84.87 | 90.71 | 111.98 | 29.98 |
| CAF-VH | 103.64 * | 191.58 * | 110.03 * | 29.29 | 117.33 * | 211.44 * | 116.02 | 30.70 | |
| CAF-GSPE | 102.73 # | 163.68 # | 102.03 | 30.59 | 113.53 # | 228.02 # | 122.68 | 32.91 | |
| Amplitude estimation | STD-VH | 4.92 | 34.38 | 7.89 | 5.37 | 3.14 | 52.06 | 22.48 | 2.13 |
| CAF-VH | 10.51 | 104.54 * | 20.42 | 4.15 | 11.05 | 62.78 | 16.51 | 3.59 | |
| CAF-GSPE | 9.23 | 81.10 | 32.14 # | 6.89 | 9.96 | 93.45 | 17.16 | 2.10 | |
| Acrophase (ZT) | STD-VH | NA | 6.39 | NA | NA | NA | 5.71 | 5.32 | NA |
| CAF-VH | 10.06 | 8.83 | NA | 11.83 | NA | NA | NA | NA | |
| CAF-GSPE | NA | 7.39 | 5.52 | 12.65 | NA | 7.04 | NA | NA |
| ZT0 STD-VH | ZT0 CAF-VH | ZT0 CAF-GSPE | ZT12 STD-VH | ZT12 CAF-VH | ZT12 CAF-GSPE | |
|---|---|---|---|---|---|---|
| Omega-3 | 189.7 ± 8.1 a | 137.0 ± 6.1 b | 156.3 ± 9.3 b | 210.0 ± 10.7 a | 145.5 ± 5.5 b | 161.5 ± 8.8 b |
| Oleic Acid (18:1) | 1641.2 ± 106.7 a | 2402.5 ± 109.3 b | 2285.6 ± 125.7 b | 1553.2 ± 128.7 a | 2457.2 ± 105.8 b | 2196.3 ± 119.7 b |
| Linoleic Acid (18:2) | 528.8 ± 24.8 a | 383.0 ± 19.8 b | 361.5 ± 18.4 b | 456.5 ± 38.6 a | 354.0 ± 20.3 b | 352.2 ± 23.4 b |
| PUFAs | 523.2 ± 26.9 a | 269.2 ± 12.4 b | 276.6 ± 24.0 b | 500.9 ± 21.8 a | 252.1 ± 9.7 b | 294.5 ± 16.5 b |
| Total Fatty Acid | 2905.8 ± 122.9 a | 3262.3 ± 148.9 b | 3121.7 ± 111.8 ab | 2587.7 ± 231.8 a | 3279.1 ± 121.9 b | 3108.5 ± 158.4 ab |
| Diglycerides | 3.4 ± 0.5 | 5.0 ± 0.4 | 3.9 ± 0.6 | 3.3 ± 0.5 | 4.6 ± 0.4 | 4.0 ± 0.4 |
| Triglycerides | 1467.5 ± 62.1 | 1636.0 ± 65.6 | 1601.1 ± 59.7 | 1338.9 ± 93.5 a | 1658.5 ± 62.9 b | 1557.0 ± 66.5 ab |
| Sphingomyelin | 0.3 ± 0.05 | 0.2 ± 0.04 | 0.2 ± 0.03 | 0.3 ± 0.06 | 0.2 ± 0.02 | 0.2 ± 0.07 |
| Cholesterol | 5.9 ± 0.4 | 5.5 ± 0.5 | 6.5 ± 0.8 | 5.8 ± 0.6 | 6.0 ± 0.4 | 6.0 ± 0.4 |
| MUFAs | 4265.6 ± 162.1 | 4271.7 ± 180.1 | 4167.9 ± 132.7 | 3941.3 ± 229.2 | 4263.5 ± 133.6 | 4077.3 ± 147.8 |
| ZT0 STD-VH | ZT0 CAF-VH | ZT0 CAF-GSPE | ZT12 STD-VH | ZT12 CAF-VH | ZT12 CAF-GSPE | |
|---|---|---|---|---|---|---|
| Aspartate | 0.11 ± 0.017 a | 0.04 ± 0.004 b | 0.05 ± 0.006 b | 0.08 ± 0.010 | 0.05 ± 0.006 | 0.05 ± 0.008 |
| Lysine | 0.11 ± 0.016 a | 0.06 ± 0.016 b | 0.07 ± 0.015 ab | 0.09 ± 0.011 | 0.06 ± 0.008 | 0.06 ± 0.010 |
| Carnitine | 0.01 ± 0.001 a | 0.005 ± 0.001 b | 0.01 ± 0.001 b | 0.01 ± 0.001 | 0.00 ± 0.000 | 0.01 ± 0.001 |
| Taurine | 1.05 ± 0.110 a | 0.68 ± 0.058 b | 0.78 ± 0.094 b | 0.97 ± 0.080 | 0.70 ± 0.055 | 0.73 ± 0.072 |
| Propionate | 0.02 ± 0.003 a | 0.01 ± 0.002 b | 0.01 ± 0.002 ab | 0.02 ± 0.002 a | 0.01 ± 0.001 b | 0.01 ± 0.002 ab |
| GPC | 0.11 ± 0.018 a | 0.06 ± 0.012 b | 0.07 ± 0.013 ab | 0.09 ± 0.012 | 0.08 ± 0.011 | 0.07 ± 0.001 |
| Choline | 0.01 ± 0.001 a | 0.01 ± 0.001 b | 0.01 ± 0.001 ab | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0.001 |
| Inosine | 0.08 ± 0.011 a | 0.05 ± 0.006 b | 0.06 ± 0.007 ab | 0.07 ± 0.006 | 0.05 ± 0.005 | 0.05 ± 0.007 |
| Histamine | 0.07 ± 0.011 a | 0.04 ± 0.011 b | 0.04 ± 0.008 ab | 0.06 ± 0.008 | 0.03 ± 0.005 | 0.03 ± 0.007 |
| Glucose | 0.31 ± 0.037 a | 0.19 ± 0.017 b | 0.26 ± 0.04 ab | 0.27 ± 0.034 | 0.22 ± 0.021 | 0.21 ± 0.024 |
| Glutamine | 0.96 ± 0.127 | 0.61 ± 0.078 | 0.72 ± 0.116 | 0.85 ± 0.091 | 0.64 ± 0.069 | 0.64 ± 0.085 |
| Lactate | 1.05 ± 0.149 | 0.64 ± 0.089 | 0.74 ± 0.120 | 0.93 ± 0.145 | 0.71 ± 0.116 | 0.63 ± 0.100 |
| Leucine | 0.07 ± 0.011 | 0.04 ± 0.007 | 0.04 ± 0.008 | 0.06 ± 0.008 | 0.04 ± 0.005 | 0.04 ± 0.005 |
| Creatine | 0.15 ± 0.043 | 0.08 ± 0.011 | 0.10 ± 0.022 | 0.11 ± 0.022 | 0.07 ± 0.006 | 0.09 ± 0.019 |
| Isoleucine | 0.03 ± 0.005 | 0.02 ± 0.003 | 0.02 ± 0.003 | 0.03 ± 0.004 | 0.02 ± 0.002 | 0.02 ± 0.003 |
| Glycogen | 0.46 ± 0.156 | 0.17 ± 0.033 | 0.22 ± 0.06 | 0.20 ± 0.085 * | 0.31 ± 0.114 | 0.20 ± 0.065 |
| Glutamate | 0.23 ± 0.034 | 0.15 ± 0.017 | 0.18 ± 0.022 | 0.21 ± 0.025 | 0.16 ± 0.015 | 0.16 ± 0.019 |
| Succinate | 0.04 ± 0.006 | 0.03 ± 0.003 | 0.03 ± 0.003 | 0.03 ± 0.004 | 0.03 ± 0.003 | 0.03 ± 0.005 |
| Valine | 0.05 ± 0.008 | 0.03 ± 0.004 | 0.04 ± 0.006 | 0.05 ± 0.006 | 0.04 ± 0.003 | 0.03 ± 0.004 |
| Uridine | 0.03 ± 0.005 | 0.02 ± 0.003 | 0.02 ± 0.004 | 0.03 ± 0.004 | 0.02 ± 0.002 | 0.03 ± 0.004 |
| Alanine | 0.31 ± 0.051 | 0.20 ± 0.029 | 0.23 ± 0.033 | 0.28 ± 0.032 | 0.21 ± 0.023 | 0.21 ± 0.027 |
| Myo-inositol | 0.59 ± 0.141 | 0.32 ± 0.078 | 0.37 ± 0.086 | 0.52 ± 0.083 | 0.37 ± 0.074 | 0.34 ± 0.077 |
| Asparagine | 0.03 ± 0.005 | 0.01 ± 0.002 | 0.02 ± 0.004 | 0.03 ± 0.005 | 0.02 ± 0.002 | 0.02 ± 0.003 |
| ChoP | 0.07 ± 0.013 | 0.05 ± 0.007 | 0.06 ± 0.008 | 0.07 ± 0.011 | 0.05 ± 0.006 | 0.06 ± 0.011 |
| Glycerol | 0.09 ± 0.008 | 0.09 ± 0.006 | 0.10 ± 0.009 | 0.10 ± 0.010 | 0.08 ± 0.005 | 0.09 ± 0.007 |
| Phenylalanine | 0.03 ± 0.006 | 0.02 ± 0.003 | 0.02 ± 0.005 | 0.03 ± 0.006 | 0.02 ± 0.002 | 0.02 ± 0.003 |
| Tyrosine | 0.03 ± 0.007 | 0.02 ± 0.004 | 0.02 ± 0.004 | 0.03 ± 0.004 | 0.02 ± 0.002 | 0.02 ± 0.003 |
| Niacinamide | 0.02 ± 0.004 | 0.01 ± 0.002 | 0.01 ± 0.003 | 0.02 ± 0.002 | 0.01 ± 0.002 | 0.02 ± 0.002 |
| AMP | 0.03 ± 0.005 | 0.03 ± 0.008 | 0.03 ± 0.004 | 0.02 ± 0.003 * | 0.02 ± 0.004 | 0.02 ± 0.004 |
| Uracil | 0.01 ± 0.002 | 0.01 ± 0.002 | 0.01 ± 0.003 | 0.01 ± 0.002 | 0.01 ± 0.001 | 0.01 ± 0.002 |
| Fumarate | 0.003 ± 0.001 | 0.003 ± 0.001 | 0.002 ± 0.000 | 0.004 ± 0.001 | 0.002 ± 0.000 | 0.003 ± 0.001 |
| 3-OHB | 0.02 ± 0.003 | 0.03 ± 0.003 | 0.03 ± 0.006 | 0.03 ± 0.007 | 0.02 ± 0.004 | 0.03 ± 0.005 |
| Acetate | 0.05 ± 0.006 | 0.05 ± 0.004 | 0.05 ± 0.008 | 0.06 ± 0.006 | 0.04 ± 0.003 | 0.05 ± 0.004 |
| Sarcosine | 0.02 ± 0.002 | 0.02 ± 0.002 | 0.02 ± 0.002 | 0.02 ± 0.003 | 0.01 ± 0.002 | 0.02 ± 0.005 |
| Formate | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0.002 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0.001 |
| Group | Metabolic Pathway with Diurnal Rhythm | Total | Hits | p Value | FDR | Impact |
|---|---|---|---|---|---|---|
| STD-VH | Alanine, aspartate, and glutamate metabolism | 28 | 5 | 1.10 × 10−6 | 9.20 × 10−5 | 3.10 × 10−1 |
| Arginine biosynthesis | 14 | 3 | 1.30 × 10−4 | 4.60 × 10−3 | 1.20 × 10−1 | |
| D-Glutamine and D-glutamate metabolism | 6 | 2 | 8.50 × 10−4 | 1.20 × 10−2 | 5.00 × 10−1 | |
| CAF-GSPE | Alanine, aspartate, and glutamate metabolism | 28 | 4 | 3.80 × 10−4 | 1.60 × 10−2 | 3.40 × 10−1 |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | 4 | 2 | 9.90 × 10−4 | 2.80 × 10−2 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colom-Pellicer, M.; Rodríguez, R.M.; Soliz-Rueda, J.R.; de Assis, L.V.M.; Navarro-Masip, È.; Quesada-Vázquez, S.; Escoté, X.; Oster, H.; Mulero, M.; Aragonès, G. Proanthocyanidins Restore the Metabolic Diurnal Rhythm of Subcutaneous White Adipose Tissue According to Time-Of-Day Consumption. Nutrients 2022, 14, 2246. https://doi.org/10.3390/nu14112246
Colom-Pellicer M, Rodríguez RM, Soliz-Rueda JR, de Assis LVM, Navarro-Masip È, Quesada-Vázquez S, Escoté X, Oster H, Mulero M, Aragonès G. Proanthocyanidins Restore the Metabolic Diurnal Rhythm of Subcutaneous White Adipose Tissue According to Time-Of-Day Consumption. Nutrients. 2022; 14(11):2246. https://doi.org/10.3390/nu14112246
Chicago/Turabian StyleColom-Pellicer, Marina, Romina M. Rodríguez, Jorge R. Soliz-Rueda, Leonardo Vinícius Monteiro de Assis, Èlia Navarro-Masip, Sergio Quesada-Vázquez, Xavier Escoté, Henrik Oster, Miquel Mulero, and Gerard Aragonès. 2022. "Proanthocyanidins Restore the Metabolic Diurnal Rhythm of Subcutaneous White Adipose Tissue According to Time-Of-Day Consumption" Nutrients 14, no. 11: 2246. https://doi.org/10.3390/nu14112246
APA StyleColom-Pellicer, M., Rodríguez, R. M., Soliz-Rueda, J. R., de Assis, L. V. M., Navarro-Masip, È., Quesada-Vázquez, S., Escoté, X., Oster, H., Mulero, M., & Aragonès, G. (2022). Proanthocyanidins Restore the Metabolic Diurnal Rhythm of Subcutaneous White Adipose Tissue According to Time-Of-Day Consumption. Nutrients, 14(11), 2246. https://doi.org/10.3390/nu14112246