Efficacy of a Novel Therapeutic, Based on Natural Ingredients and Probiotics, in a Murine Model of Multiple Food Intolerance and Maldigestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Experimental Design
2.3.1. HCD Induction
- Group 1
- SD; mice were fed with a SD plus vehicle for three weeks (n = 4);
- Group 2
- SD + NTN mice were fed with a SD for three weeks plus oral administration of NTN for the next two weeks (n = 8);
- Group 3
- HCD; mice were fed with an HCD for three weeks plus oral administration of vehicle for the next two weeks (n = 8);
- Group 4
- HCD + NTN; mice were fed with an HCD for three weeks plus oral administration of NTN for the next two weeks (n = 8);
2.3.2. HFD Induction
- Group 1
- SD; mice were fed with an SD plus vehicle for 14 weeks (n = 4);
- Group 2
- SD + NTN; mice were fed with an SD for 12 weeks plus oral administration of NTN for the next two weeks (n = 8);
- Group 3
- HFD; mice were fed with an HFD for 12 weeks plus oral administration of vehicle for the next two weeks (n = 8);
- Group 4
- HFD + NTN; mice were fed with an HCD for 12 weeks plus oral administration of NTN for the next two weeks (n = 8);
2.3.3. HFrD Induction
- Group 1
- SD; mice were fed with an SD plus vehicle for 15 weeks (n = 4);
- Group 2
- SD + NTN; mice were fed with a SD for 13 weeks plus oral administration of NTN for the next two weeks (n = 8);
- Group 3
- HFrD; mice were fed with an HFrD for 13 weeks plus oral administration of vehicle for the next two weeks (n = 8);
- Group 4
- HFrD + NTN; mice were fed with an HFrD for 13 weeks plus oral administration of NTN for the next two weeks (n = 8);
2.4. Histological Evaluations
2.5. Immunohistochemical Localization of ZO-1 and Occludin
2.6. Gut Permeability
2.7. Plasma Insulin and Glucose Levels
2.8. Analysis of Liver Weight
2.9. Quantification of NEFA and TG
2.10. Statistical Analysis
3. Results
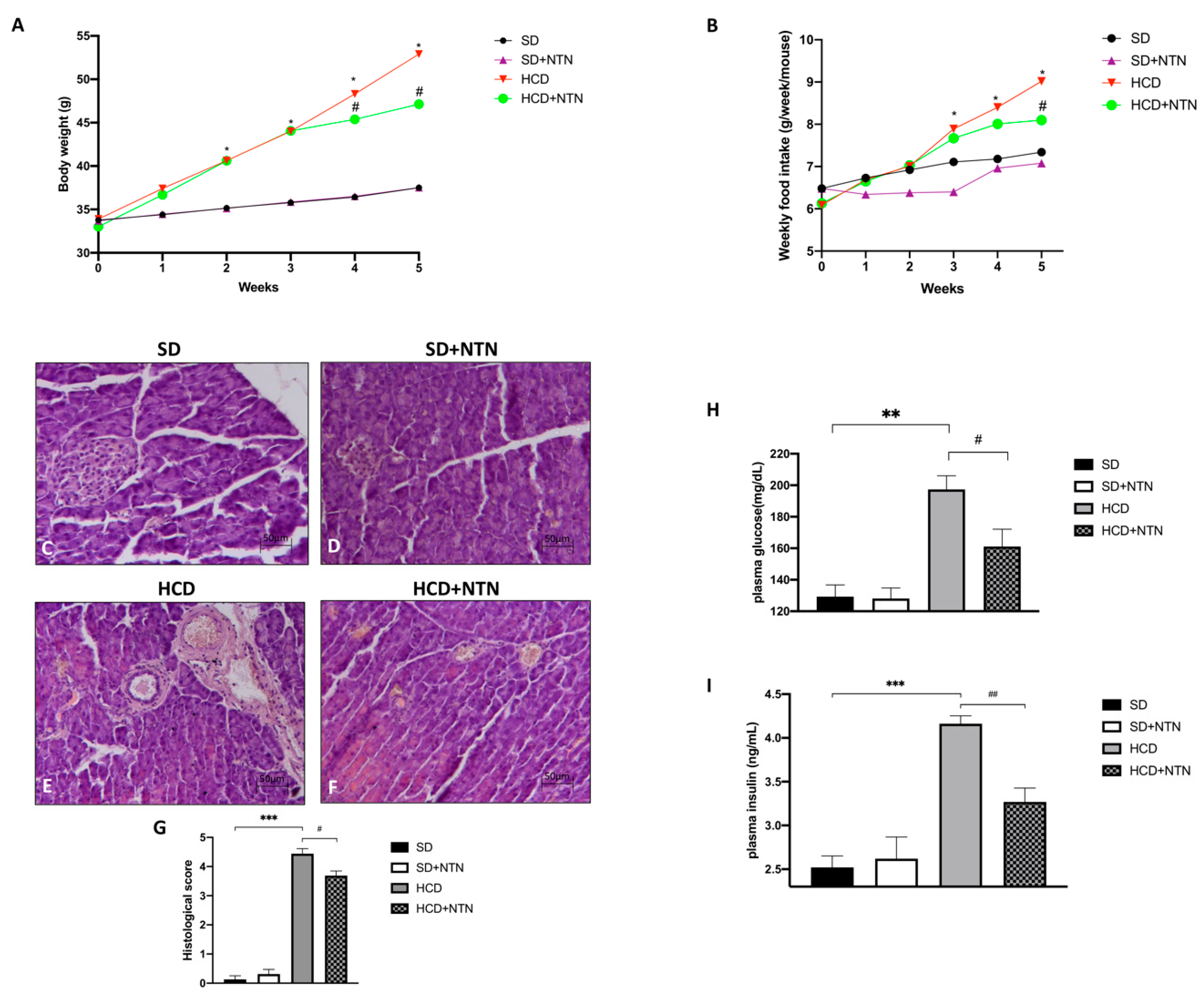
3.1. Effects of NTN Administration on Body Weight, Food Intake, Pancreas Tissue Damage and Glucose-Insulin Levels in HCD Mice
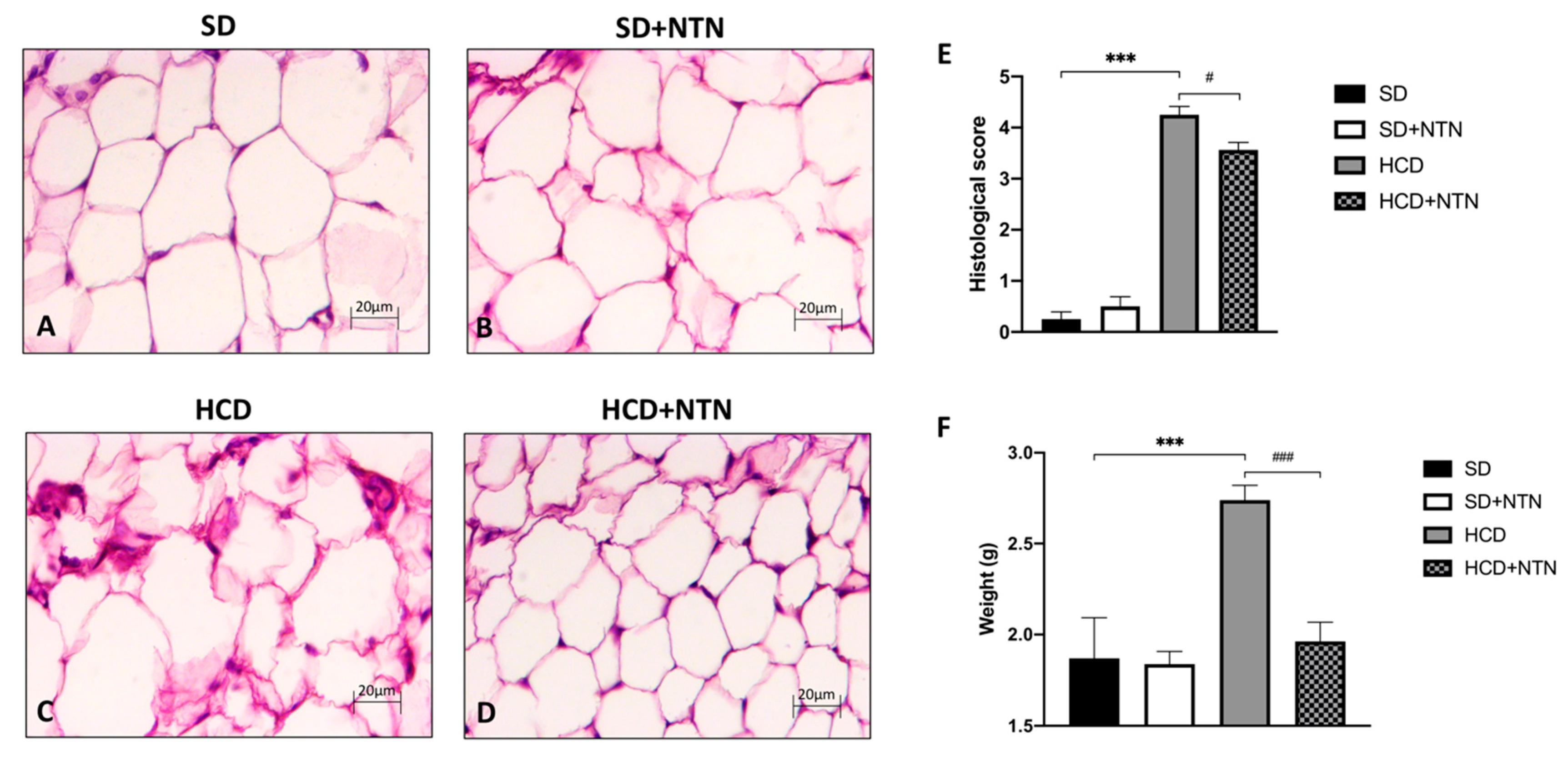
3.2. Effects of NTN Administration on Abdominal Adipose Tissue Damage and Steatosis in HCD Mice
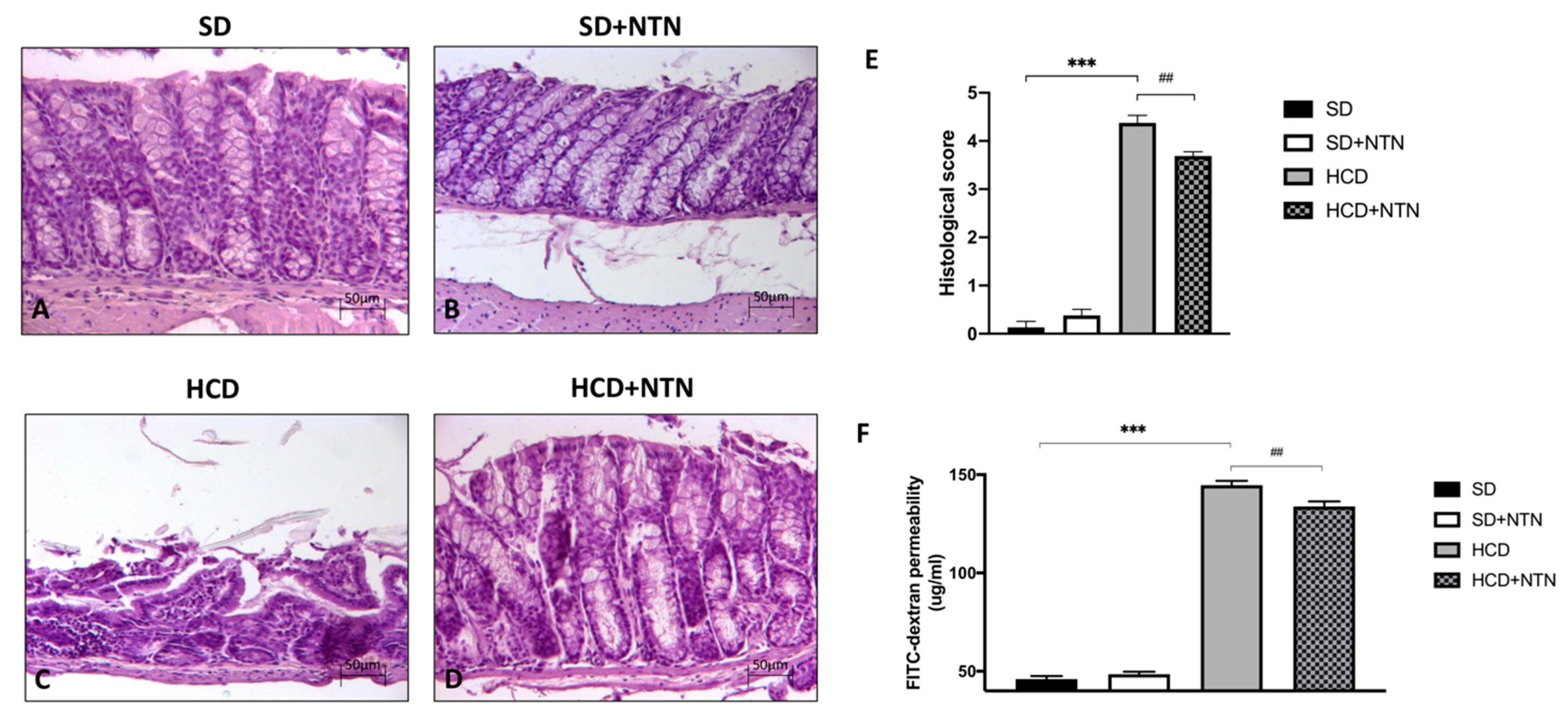
3.3. Effects of NTN Administration on Intestinal Tissue Damage and Permeability
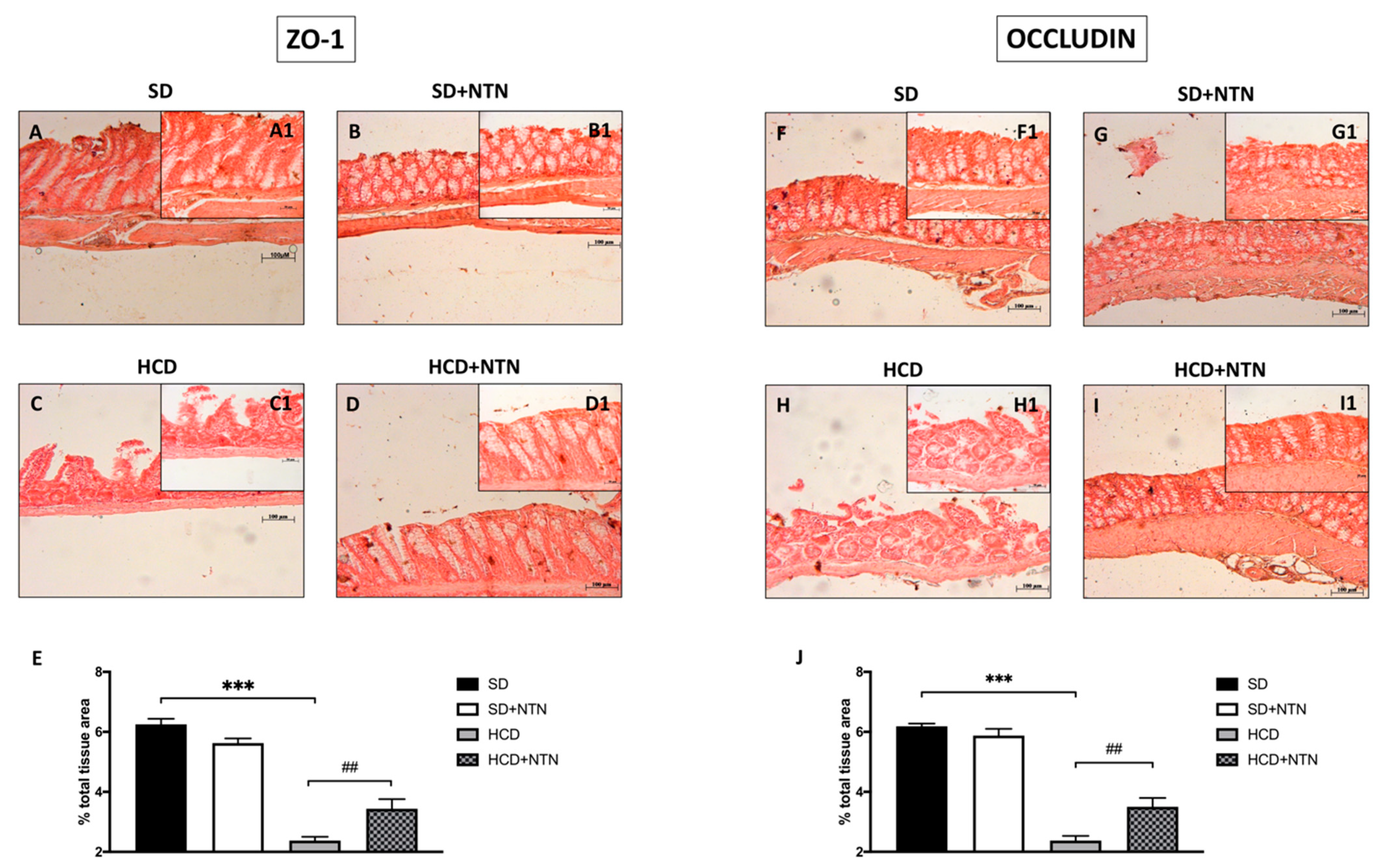
3.4. Effects of NTN Administration on Intestine Epithelial Integrity in HCD Mice
3.5. Effects of NTN Administration on Body Weight, Food Intake, Liver Tissue Damage, Lipid Tolerance Parameters and Gut Permeability in HFD Mice
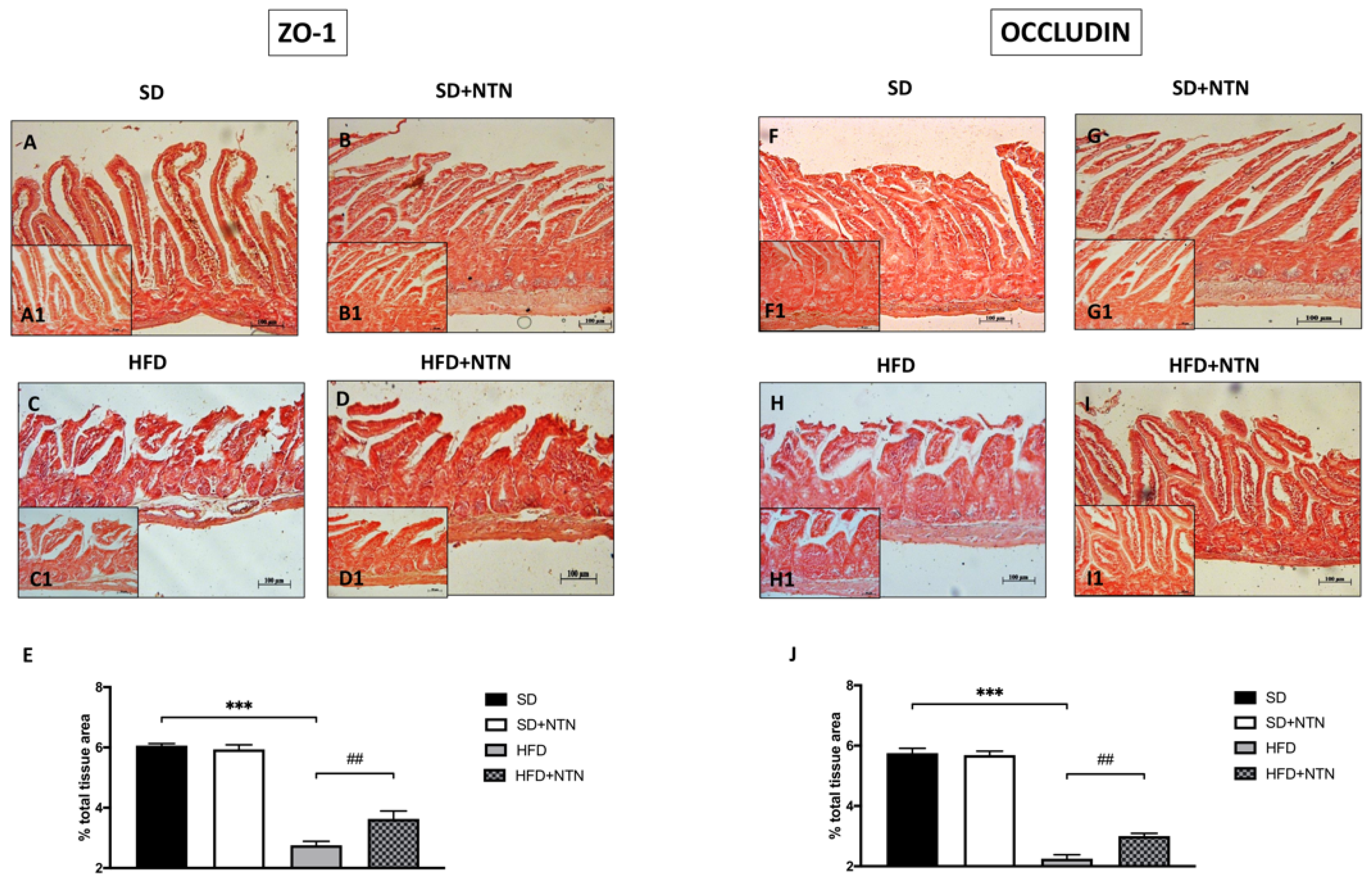
3.6. Effects of NTN Administration on Intestine Epithelial Integrity in HFD Mice
3.7. Effects of NTN Administration on Abdominal Adipose Tissue Damage in HFD Mice
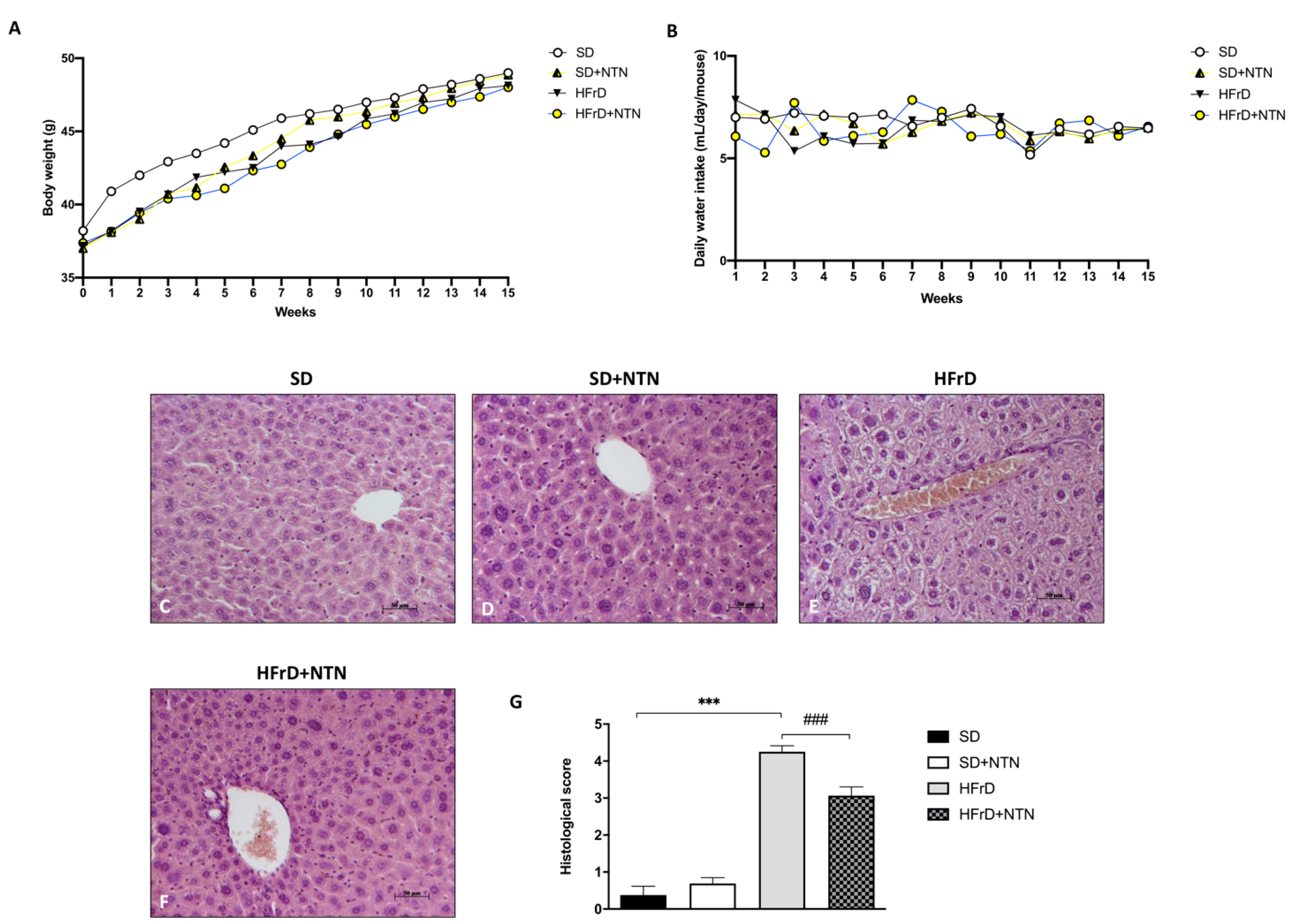
3.8. Effects of NTN Administration on Body Weight, Food Intake and Liver Tissue Damage in HFrD Mice
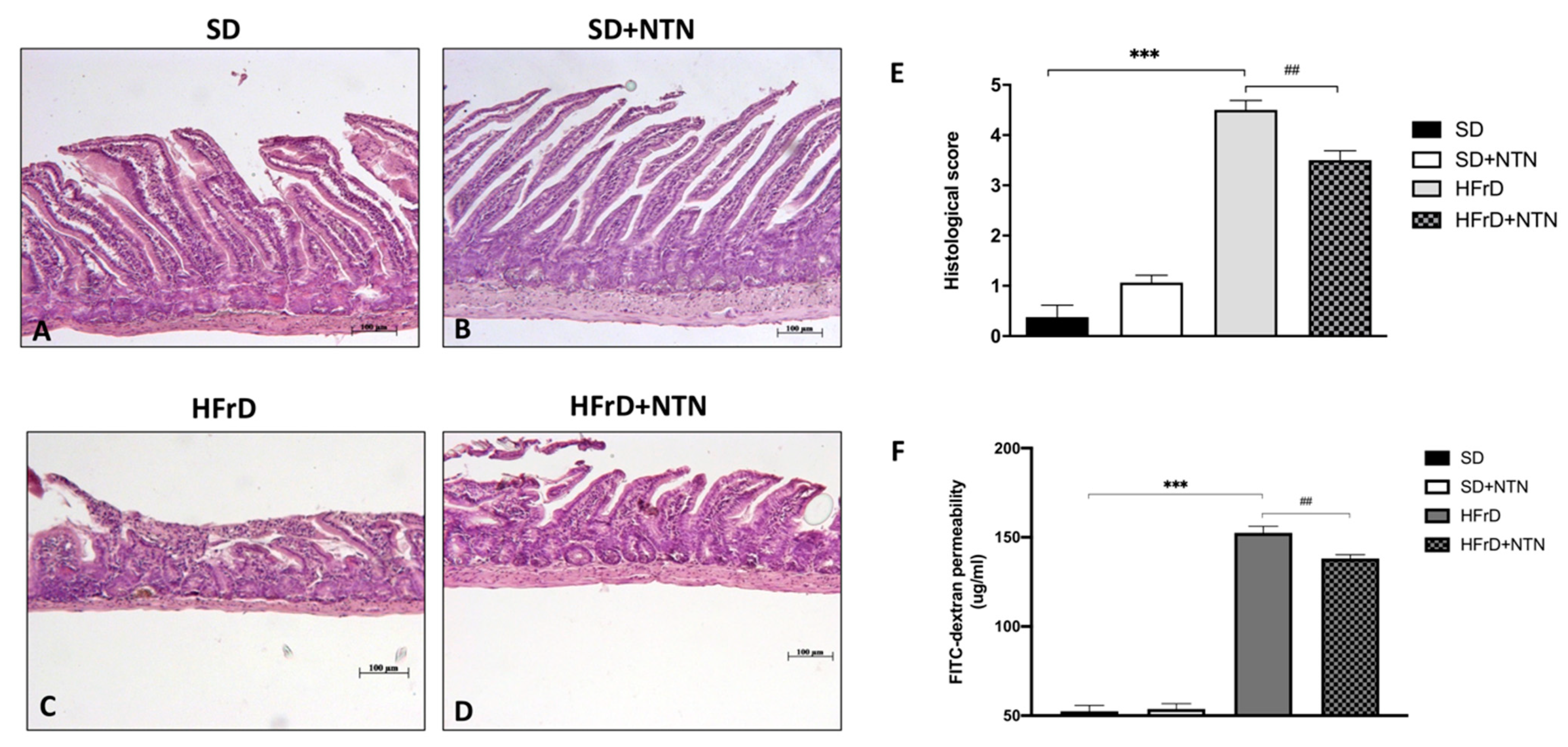
3.9. Effects of NTN Administration on Intestinal Tissue Damage and Permeability in HFrD Mice
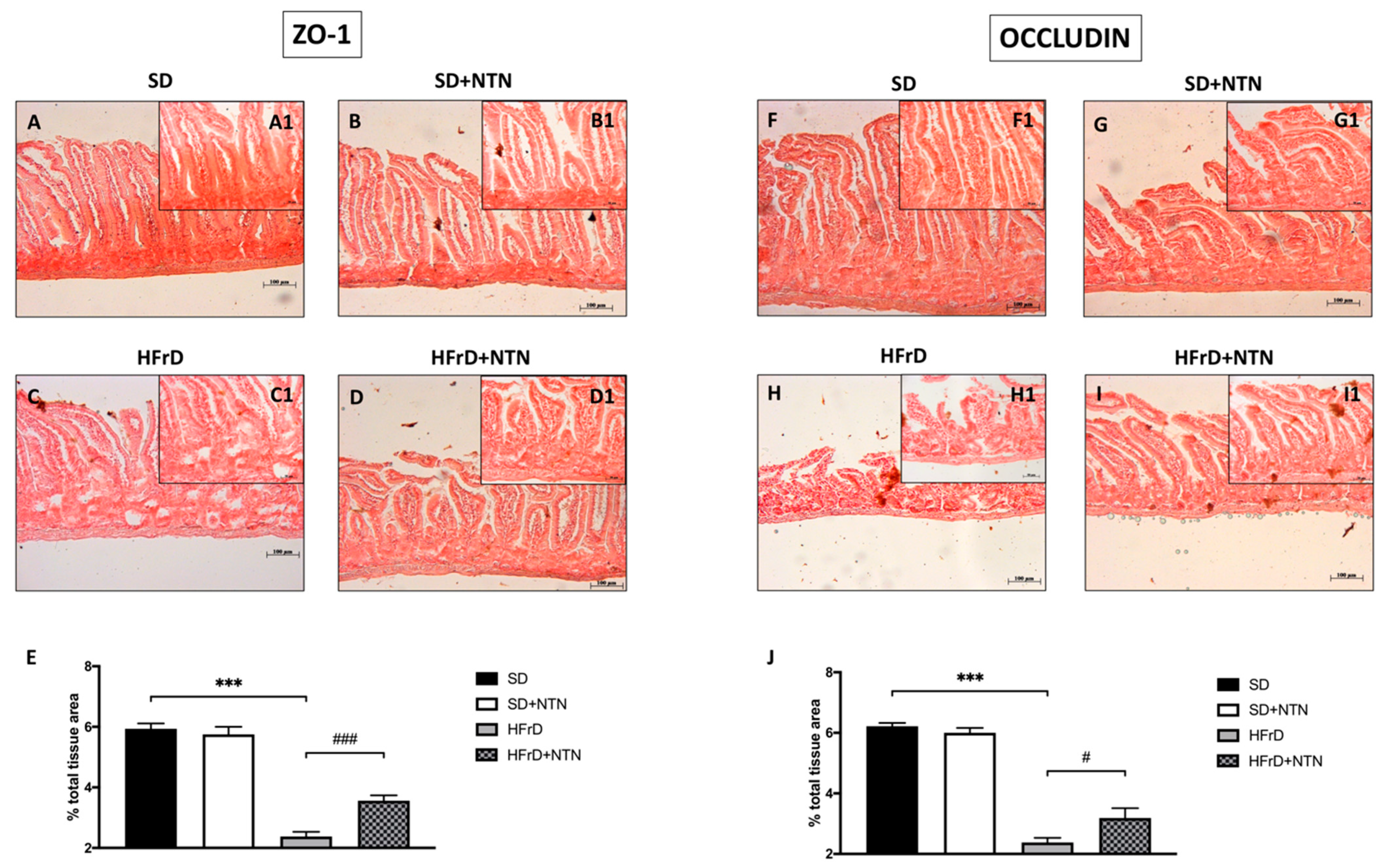
3.10. Effect of NTN on Epithelial Integrity in the Intestines of HFrD Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lomer, M.C. Review article: The aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment. Pharmacol. Ther. 2015, 41, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Zopf, Y.; Baenkler, H.-W.; Silbermann, A.; Hahn, E.G.; Raithel, M. The Differential Diagnosis of Food Intolerance. Dtsch. Arztebl. Int. 2009, 106, 359–370; quiz 369–370, 4p following 370. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Dean, T. Food Intolerance and the Food Industry; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Owen, L.; Corfe, B. The role of diet and nutrition on mental health and wellbeing. Proc. Nutr. Soc. 2017, 76, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B.; Trugo, L.; Finglas, P. Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2003; Volume 1–10. [Google Scholar]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, A.; Diwan, V.; Brown, L. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef]
- Gluvic, Z.; Zaric, B.; Resanovic, I.; Obradovic, M.; Mitrovic, A.; Radak, D.; Isenovic, E. Link between Metabolic Syndrome and Insulin Resistance. Curr. Vasc. Pharmacol. 2017, 15, 30–39. [Google Scholar] [CrossRef]
- Després, J.-P. Cardiovascular Disease Under the Influence of Excess Visceral Fat. Crit. Pathways Cardiol. 2007, 6, 51–59. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, X.J. Effects of a high fat diet on intestinal microbiota and gastrointestinal diseases. World J. Gastroenterol. 2016, 22, 8905–8909. [Google Scholar] [CrossRef]
- Yang, R.-L.; Li, W.; Shi, Y.-H.; Le, G.-W. Lipoic acid prevents high-fat diet–induced dyslipidemia and oxidative stress: A microarray analysis. Nutrition 2008, 24, 582–588. [Google Scholar] [CrossRef]
- Gummesson, A.; Carlsson, L.M.; Storlien, L.H.; Bäckhed, F.; Lundin, P.; Löfgren, L.; Stenlöf, K.; Lam, Y.Y.; Fagerberg, B.; Carlsson, B. Intestinal Permeability Is Associated With Visceral Adiposity in Healthy Women. Obesity 2011, 19, 2280–2282. [Google Scholar] [CrossRef]
- Keim, N.L.; Stanhope, K.; Havel, P. Fructose and High-Fructose Corn Syrup. Encycl. Food Health 2016, 119–124. [Google Scholar] [CrossRef]
- Taskinen, M.-R.; Packard, C.J.; Borén, J. Dietary Fructose and the Metabolic Syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, A.; Rao, S.S. Dietary Fructose Intolerance, Fructan Intolerance and FODMAPs. Curr. Gastroenterol. Rep. 2014, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal barrier function and metabolic/liver diseases. Liver Res. 2020, 4, 81–87. [Google Scholar] [CrossRef]
- Ohtsuka, Y. Food intolerance and mucosal inflammation. Pediatr. Int. 2015, 57, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.C. Intestinal Epithelial Barrier Dysfunction in Food Hypersensitivity. J. Allergy 2012, 2012, 596081. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, A.; Ishayek, N. Lactose Intolerance, Dairy Avoidance, and Treatment Options. Nutrients 2018, 10, 1994. [Google Scholar] [CrossRef] [PubMed]
- Buzas, G.M. Fructose and fructose intolerance. Orv. Hetil. 2016, 157, 1708–1716. [Google Scholar] [PubMed]
- de Vrese, M.; Schrezenmeir, J. Probiotics, prebiotics, and synbiotics. Adv. Biochem. Eng. Biotechnol. 2008, 111, 1–66. [Google Scholar]
- Massot-Cladera, M.; Azagra-Boronat, I.; Franch, À.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. Gut Health-Promoting Benefits of a Dietary Supplement of Vitamins with Inulin and Acacia Fibers in Rats. Nutrients 2020, 12, 2196. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.X.; Corke, H.; Gul, K.; Gan, R.Y.; Fang, Y. The health benefits, functional properties, modifications, and applications of pea (Pisum sativum L.) protein: Current status, challenges, and perspectives. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1835–1876. [Google Scholar] [CrossRef] [PubMed]
- Fassio, F.; Facioni, M.S.; Guagnini, F. Lactose Maldigestion, Malabsorption, and Intolerance: A Comprehensive Review with a Focus on Current Management and Future Perspectives. Nutrients 2018, 10, 1599. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Chaumontet, C.; Azzout-Marniche, D.; Blais, A.; Chalvon-Dermersay, T.; Nadkarni, N.A.; Piedcoq, J.; Fromentin, G.; Tomé, D.; Even, P.C. Rats Prone to Obesity Under a High-Carbohydrate Diet have Increased Post-Meal CCK mRNA Expression and Characteristics of Rats Fed a High-Glycemic Index Diet. Front. Nutr. 2015, 2, 22. [Google Scholar] [CrossRef]
- Peterson, J.M.; Seldin, M.M.; Tan, S.Y.; Wong, G.W. CTRP2 Overexpression Improves Insulin and Lipid Tolerance in Diet-Induced Obese Mice. PLoS ONE 2014, 9, e88535. [Google Scholar] [CrossRef]
- Kuhre, R.E.; Gribble, F.M.; Hartmann, B.; Reimann, F.; Windeløv, J.A.; Rehfeld, J.F.; Holst, J.J. Fructose stimulates GLP-1 but not GIP secretion in mice, rats, and humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G622–G630. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, E.; Oh, M.-J.; Kim, Y.; Park, H.-Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef]
- Casili, G.; Lanza, M.; Campolo, M.; Messina, S.; Scuderi, S.; Ardizzone, A.; Filippone, A.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Therapeutic potential of flavonoids in the treatment of chronic venous insufficiency. Vasc. Pharmacol. 2021, 137, 106825. [Google Scholar] [CrossRef]
- Campolo, M.; Crupi, R.; Cordaro, M.; Cardali, S.M.; Ardizzone, A.; Casili, G.; Scuderi, S.A.; Siracusa, R.; Esposito, E.; Conti, A.; et al. Co-Ultra PEALut Enhances Endogenous Repair Response Following Moderate Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 8717. [Google Scholar] [CrossRef]
- Gowdra, V.S.; Mudgal, J.; Bansal, P.; Nayak, P.G.; Reddy, S.A.M.; Shenoy, G.G.; Valiathan, M.; Chamallamudi, M.R.; Nampurath, G.K. Synthesis, Characterization, and Preclinical Evaluation of New Thiazolidin-4-ones Substituted with p-Chlorophenoxy Acetic Acid and Clofibric Acid against Insulin Resistance and Metabolic Disorder. BioMed Res. Int. 2014, 2014, 620434. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Sun, W.; Liu, L.; Wang, G.; Xiao, Z.; Pei, X.; Wang, M. Chitosan Oligosaccharide Attenuates Nonalcoholic Fatty Liver Disease Induced by High Fat Diet through Reducing Lipid Accumulation, Inflammation and Oxidative Stress in C57BL/6 Mice. Mar. Drugs 2019, 17, 645. [Google Scholar] [CrossRef] [PubMed]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2012, 346, e7492. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, P.K.; Wood, E.L.; Klockars, A.; Levine, A.S. Excessive Consumption of Sugar: An Insatiable Drive for Reward. Curr. Nutr. Rep. 2019, 8, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Deutz, N.E.P.; Biolo, G.; Bischoff, S.; Boirie, Y.; Cederholm, T.; Cuerda, C.; Delzenne, N.; Leon Sanz, M.; Ljungqvist, O.; et al. Carbohydrates and insulin resistance in clinical nutrition: Recommendations from the ESPEN expert group. Clin. Nutr. 2017, 36, 355–363. [Google Scholar] [CrossRef]
- Wolever, T.M.S. Dietary carbohydrates and insulin action in humans. Br. J. Nutr. 2000, 83 (Suppl. S1), S97–S102. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef]
- Sawada, N. Tight junction-related human diseases. Pathol. Int. 2013, 63, 1–12. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High fat diet induced obesity model using four strainsof mice: Kunming, C57BL/6, BALB/c and ICR. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef]
- Yang, Y.; Smith, D.L., Jr.; Keating, K.D.; Allison, D.B.; Nagy, T.R. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity 2014, 22, 2147–2155. [Google Scholar] [CrossRef]
- Karpe, F.; Dickmann, J.R.; Frayn, K.N. Fatty Acids, Obesity, and Insulin Resistance: Time for a Reevaluation. Diabetes 2011, 60, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Rifai, N.; Merrill, J.R.; Holly, R.G. Postprandial Effect of a High Fat Meal on Plasma Lipid, Lipoprotein Cholesterol and Apolipoprotein Measurements. Ann. Clin. Biochem. 1990, 27 Pt 5, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Nemoto, Y.; Takei, Y.; Morikawa, R.; Oshima, S.; Nagaishi, T.; Okamoto, R.; Tsuchiya, K.; Nakamura, T.; Stutte, S.; et al. High-fat diet-derived free fatty acids impair the intestinal immune system and increase sensitivity to intestinal epithelial damage. Biochem. Biophys. Res. Commun. 2020, 522, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Worsley, O.; Yang, E.; Purbojati, R.W.; Liang, A.L.; Tan, W.; Moses, D.I.D.; Hartono, S.; Fan, V.; Lim, T.K.H.; et al. Persistent changes in liver methylation and microbiome composition following reversal of diet-induced non-alcoholic-fatty liver disease. Cell Mol. Life Sci. 2019, 76, 4341–4354. [Google Scholar] [CrossRef]
- Tillman, E.J.; Morgan, D.A.; Rahmouni, K.; Swoap, S.J. Three Months of High-Fructose Feeding Fails to Induce Excessive Weight Gain or Leptin Resistance in Mice. PLoS ONE 2014, 9, e107206. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, C.H.; Materna, A.; Wermelinger, C.; Schuler, J. Fructose and lactose intolerance and malabsorption testing: The relationship with symptoms in functional gastrointestinal disorders. Aliment. Pharmacol. Ther. 2013, 37, 1074–1083. [Google Scholar] [CrossRef]
- Volynets, V.; Louis, S.; Pretz, D.; Lang, L.; Ostaff, M.J.; Wehkamp, J.; Bischoff, S.C. Intestinal Barrier Function and the Gut Microbiome Are Differentially Affected in Mice Fed a Western-Style Diet or Drinking Water Supplemented with Fructose. J. Nutr. 2017, 147, 770–780. [Google Scholar] [CrossRef]
- Zar, S.; Kumar, D.; Benson, M.J. Food hypersensitivity and irritable bowel syndrome. Aliment. Pharmacol. Ther. 2001, 15, 439–449. [Google Scholar] [CrossRef]
- Choung, R.S.; Talley, N.J. Food Allergy and Intolerance in IBS. Gastroenterol. Hepatol. 2006, 2, 756–760. [Google Scholar]
- Tuck, C.J.; Biesiekierski, J.R.; Schmid-Grendelmeier, P. Food Intolerances. Nutrients 2019, 11, 1684. [Google Scholar] [CrossRef]
- Facioni, M.S.; Raspini, B.; Pivari, F.; Dogliotti, E.; Cena, H. Nutritional management of lactose intolerance: The importance of diet and food labelling. J. Transl. Med. 2020, 18, 260. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Méheust, A.; De Vos, W.M.; et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Polimeno, L.; Amoruso, A.; Gatti, F.; Annoscia, E.; Marinaro, M.; Di Leo, E.; Matino, M.; Buquicchio, R.; Bonini, S.; et al. Intestinal permeability in patients with adverse reactions to food. Dig. Liver Dis. 2006, 38, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Colgan, S.P.; Curtis, V.F.; Lanis, J.M.; E Glover, L. Metabolic regulation of intestinal epithelial barrier during inflammation. Tissue Barriers 2015, 3, e970936. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef]
- Piqué, N.; Gómez-Guillén, M.D.C.; Montero, M.P. Xyloglucan, a Plant Polymer with Barrier Protective Properties over the Mucous Membranes: An Overview. Int. J. Mol. Sci. 2018, 19, 673. [Google Scholar] [CrossRef]
- Bron, P.A.; Kleerebezem, M.; Brummer, R.J.; Cani, P.D.; Mercenier, A.; MacDonald, T.T.; Wells, J.M. Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 2017, 117, 93–107. [Google Scholar] [CrossRef]
- Nicoletti, A.; Ponziani, F.R.; Biolato, M.; Valenza, V.; Marrone, G.; Sganga, G.; Gasbarrini, A.; Miele, L.; Grieco, A. Intestinal permeability in the pathogenesis of liver damage: From non-alcoholic fatty liver disease to liver transplantation. World J. Gastroenterol. 2019, 25, 4814–4834. [Google Scholar] [CrossRef]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrio, J.; Lopez-Suarez, P.; Sánchez, B.; González, S.; Gueimonde, M.; Margolles, A.; Reyes-Gavilán, C.G.D.L.; Suarez-Diaz, A.M. Intestinal Dysbiosis Is Associated with Altered Short-Chain Fatty Acids and Serum-Free Fatty Acids in Systemic Lupus Erythematosus. Front. Immunol. 2017, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Stahel, P.; Carreiro, A.L.; Hung, Y.-H.; Dash, S.; Bookman, I.; Buhman, K.K.; Lewis, G.F. Oral Glucose Mobilizes Triglyceride Stores From the Human Intestine. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 313–337. [Google Scholar] [CrossRef] [PubMed]
- Semova, I.; Carten, J.D.; Stombaugh, J.; Mackey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota Regulate Intestinal Absorption and Metabolism of Fatty Acids in the Zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Shuster, A.; Patlas, M.; Pinthus, J.H.; Mourtzakis, M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012, 85, 1–10. [Google Scholar] [CrossRef]
- Bosello, O.; Zamboni, M. Visceral obesity and metabolic syndrome. Obes. Rev. 2000, 1, 47–56. [Google Scholar] [CrossRef]
- Aparecida Silveira, E.; Vaseghi, G.; Santos, A.S.D.C.; Kliemann, N.; Masoudkabir, F.; Noll, M.; Mohammadifard, N.; Sarrafzadegan, N.; De Oliveira, C. Visceral Obesity and Its Shared Role in Cancer and Cardiovascular Disease: A Scoping Review of the Pathophysiology and Pharmacological Treatments. Int. J. Mol. Sci. 2020, 21, 9042. [Google Scholar] [CrossRef]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar]
- Vázquez-Vela, M.E.F.; Torres, N.; Tovar, A.R. White Adipose Tissue as Endocrine Organ and Its Role in Obesity. Arch. Med. Res. 2008, 39, 715–728. [Google Scholar] [CrossRef]
- Baylin, A.; Kabagambe, E.K.; Siles, X.; Campos, H. Adipose tissue biomarkers of fatty acid intake. Am. J. Clin. Nutr. 2002, 76, 750–757. [Google Scholar] [CrossRef]
- Freedland, E.S. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: Implications for controlling dietary carbohydrates: A review. Nutr. Metab. 2004, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.J. Effect of low-carbohydrate diets on cardiometabolic risk, insulin resistance, and metabolic syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; E Corkey, B.E.; Istfan, N.W.; Apovian, C.M. Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction. J. Endocr. Soc. 2019, 3, 1727–1747. [Google Scholar] [CrossRef] [PubMed]
- Basse, A.L.; Dalbram, E.; Larsson, L.; Gerhart-Hines, Z.; Zierath, J.; Treebak, J.T. Skeletal Muscle Insulin Sensitivity Show Circadian Rhythmicity Which Is Independent of Exercise Training Status. Front. Physiol. 2018, 9, 1198. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Scheer, F.A.J.L.; Schrauwen, P.; La Fleur, S.E.; Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Ma, J.; Rayner, C.K.; Jones, K.L.; Horowitz, M. Insulin secretion in healthy subjects and patients with Type 2 diabetes—Role of the gastrointestinal tract. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 413–424. [Google Scholar] [CrossRef]



| INGREDIENTS | QUANTITY (mg) |
|---|---|
| Acacia senegal (L.) Willd. (gummi) | 100 |
| L. acidophilus tyndalized | 10 |
| L. reuteri tyndalized | 7 |
| Pea protein | 50 |
| Grape seed extract | 50 |
| β-galactosidase | 13 |
| Weight Content (g/kg) | HCD |
|---|---|
| Milk proteins | 140.0 |
| Starch | 622.4 |
| Sucrose | 100.3 |
| Soy Oil | 40.0 |
| Minerals | 35.0 |
| Vitamins | 10.0 |
| Cellulose | 50.0 |
| Choline | 2.3 |
| Energy Content (%) | HCD |
| Protein | 14.7 |
| Carbohydrate | 75.9 |
| Fat | 9.4 |
| Energy density (kJ/g) | 15.95 |
| Food quotient | 0.946 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardizzone, A.; Lanza, M.; Casili, G.; Campolo, M.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Efficacy of a Novel Therapeutic, Based on Natural Ingredients and Probiotics, in a Murine Model of Multiple Food Intolerance and Maldigestion. Nutrients 2022, 14, 2251. https://doi.org/10.3390/nu14112251
Ardizzone A, Lanza M, Casili G, Campolo M, Paterniti I, Cuzzocrea S, Esposito E. Efficacy of a Novel Therapeutic, Based on Natural Ingredients and Probiotics, in a Murine Model of Multiple Food Intolerance and Maldigestion. Nutrients. 2022; 14(11):2251. https://doi.org/10.3390/nu14112251
Chicago/Turabian StyleArdizzone, Alessio, Marika Lanza, Giovanna Casili, Michela Campolo, Irene Paterniti, Salvatore Cuzzocrea, and Emanuela Esposito. 2022. "Efficacy of a Novel Therapeutic, Based on Natural Ingredients and Probiotics, in a Murine Model of Multiple Food Intolerance and Maldigestion" Nutrients 14, no. 11: 2251. https://doi.org/10.3390/nu14112251
APA StyleArdizzone, A., Lanza, M., Casili, G., Campolo, M., Paterniti, I., Cuzzocrea, S., & Esposito, E. (2022). Efficacy of a Novel Therapeutic, Based on Natural Ingredients and Probiotics, in a Murine Model of Multiple Food Intolerance and Maldigestion. Nutrients, 14(11), 2251. https://doi.org/10.3390/nu14112251












