Photoprotective and Antiaging Effects of a Standardized Red Orange (Citrus sinensis (L.) Osbeck) Extract in Asian and Caucasian Subjects: A Randomized, Double-Blind, Controlled Study
Abstract
:1. Introduction
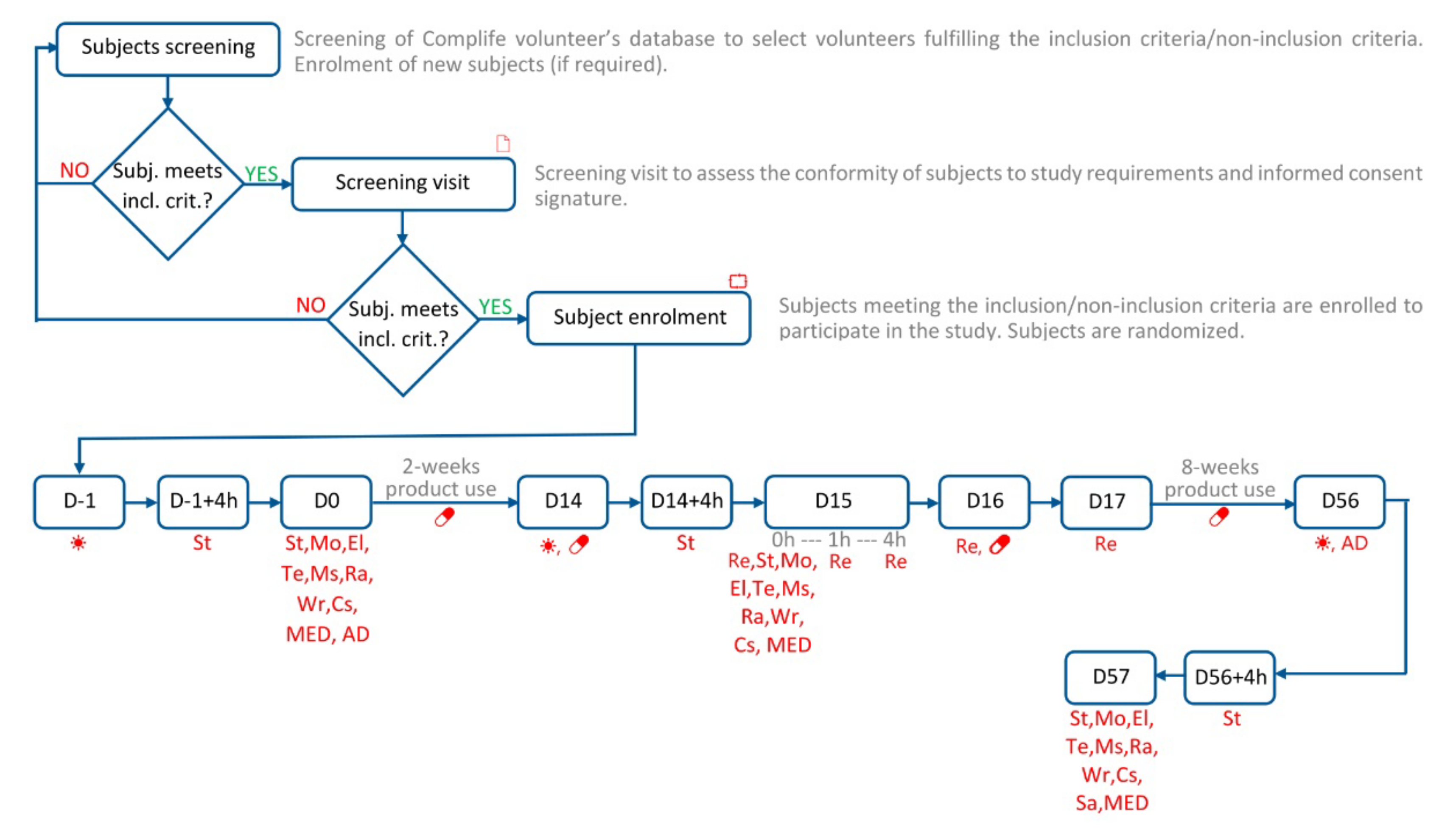
2. Materials and Methods
2.1. Description of Study Design
2.2. Eligibility Criteria for Participants
2.3. Settings and Locations
2.4. Intervention
2.5. Primary and Secondary Objectives and Outcome Measures
2.5.1. UV Exposure
2.5.2. Minimal Erythema Dose (MED) Measurement and UV-Induced Skin Redness
2.5.3. Antioxidant Properties Measurement
2.5.4. Skin Bioengineering Techniques
- The measurement of skin moisturization was based on the Corneometer® method. The corneometer® method is based on the dielectric constant of water. The probe shows changes of capacitance according to the moisture content of the skin. An electric scatter field penetrates the very first layers of the skin (10–20 µm) and determines the dielectricity. The used device was the Corneometer® CM 825 (Courage + Khazaka, electronic GmbH, Cologne, Germany).
- The measurement of skin elasticity was based on the suction method using a negative pressure mechanically deforming the skin (Cutometer® method). A negative pressure (450 mbar) is created in the device and the skin is drawn into the aperture of the probe for 2 s and after a defined time (2 s) it is released again. Inside the probe, the penetration depth is determined by a non-contact optical measuring system. The optical measuring system consists of a light source and a light receptor, as well as two prisms facing each other, which project the light from the transmitter to the receptor. The light intensity varies due to the penetration depth of the skin. The resistance of the skin to the negative pressure and its ability to return into its original position are displayed as curves (penetration depth in mm/time) in real time during the measurement. The used device was the Cutometer® MPA 580 (Courage + Khazaka, electronic GmbH, Cologne, Germany). Skin elasticity was measured in the cheek. R0 (skin distensibility), R2 (overall skin elasticity), R5 (net elasticity), and R9 (skin tiring effect) parameters were measured. More information on skin elasticity parameters can be found in Supplementary Figure S3.
- Transepidermal water loss (TEWL) was measured using a Tewameter® TM 300 (Courage + Khazaka, electronic GmbH, Cologne, Germany). The measurement is based on the diffusion law. The diffusion flow dm/dt indicates the mass of water, which is transported per cm² in a specific period. The resulting density gradient is measured indirectly by two pairs of sensors (temperature and relative humidity) and is analyzed by a microprocessor. The measuring head of the probe was a narrow hollow cylinder (10 mm diameter and 20 mm height) to minimize the influence of air turbulence inside the probe.
- The intensity of melanin staining inside dark spots was assessed by means of the Individual Typology Angle (ITA°) parameter [3]. ITA° is calculated starting from L* and b* values measured in the CIELab space (1976) using a spectrophotometer/colorimeter CM-700D (Konica-Minolta, Japan).
- The skin radiance (or skin brightness) is the ability of the skin to reflect the light and was measured using the gloss parameter taken using a spectrophotometer/colorimeter CM-700D (Konica-Minolta, Japan). The instrument emits diffused light that reaches the skin through an opening located at the extreme of the lighting sphere. A sensor located at 8° compared to the vertical axis of the opening detects then the reflected light and calculates a parameter known as “gloss”. The gloss value is used in the management of the brilliance of the color.
- Wrinkle depth, length, area, and wrinkle count in the “crow’s feet” area. were measured using a real 3D microtopography imaging system (PrimosCR SF, Canfield Scientific, NJ, USA). Skin surface was reconstructed using an algorithm to generate 3D images. Subject repositioning was ensured by a repositioning device (Canfield Scientific, NJ, USA), while before/after image matching was ensured by an overlapping feature of the image analysis software.
- The dermatologists evaluated the skin appearance (wrinkle appearance and dark spot decrease) using a clinical scoring system (−3 greatly worsened, −2 moderately worsened, −1 slightly worsened, 0 no change, +1 slightly improved, +2 moderately improved, +3 greatly improved).
2.5.5. Anthropometric Measurements
2.6. Randomization and Masking
2.7. Statistical Analysis
3. Results
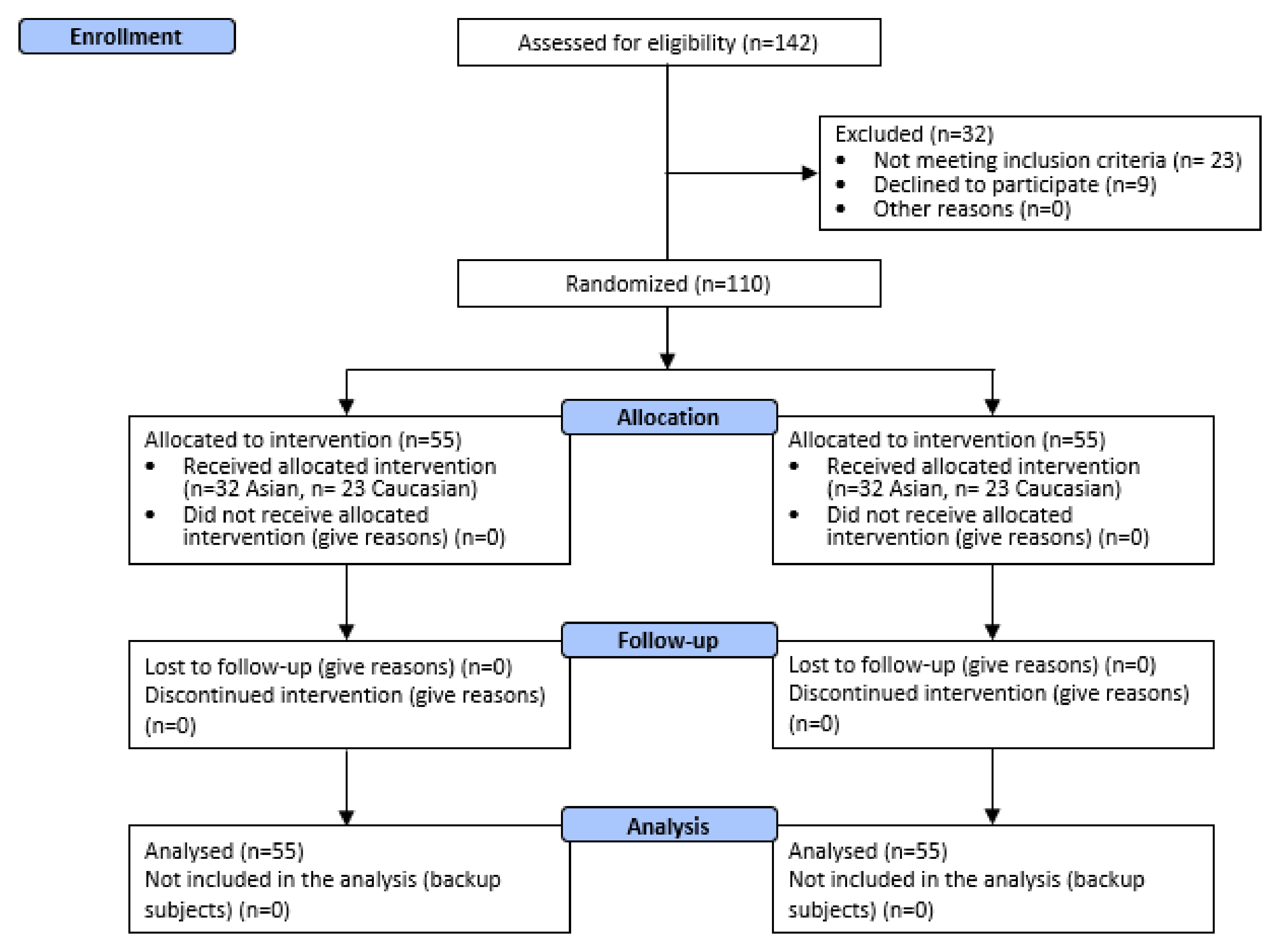
3.1. Participants and Product Tolerability
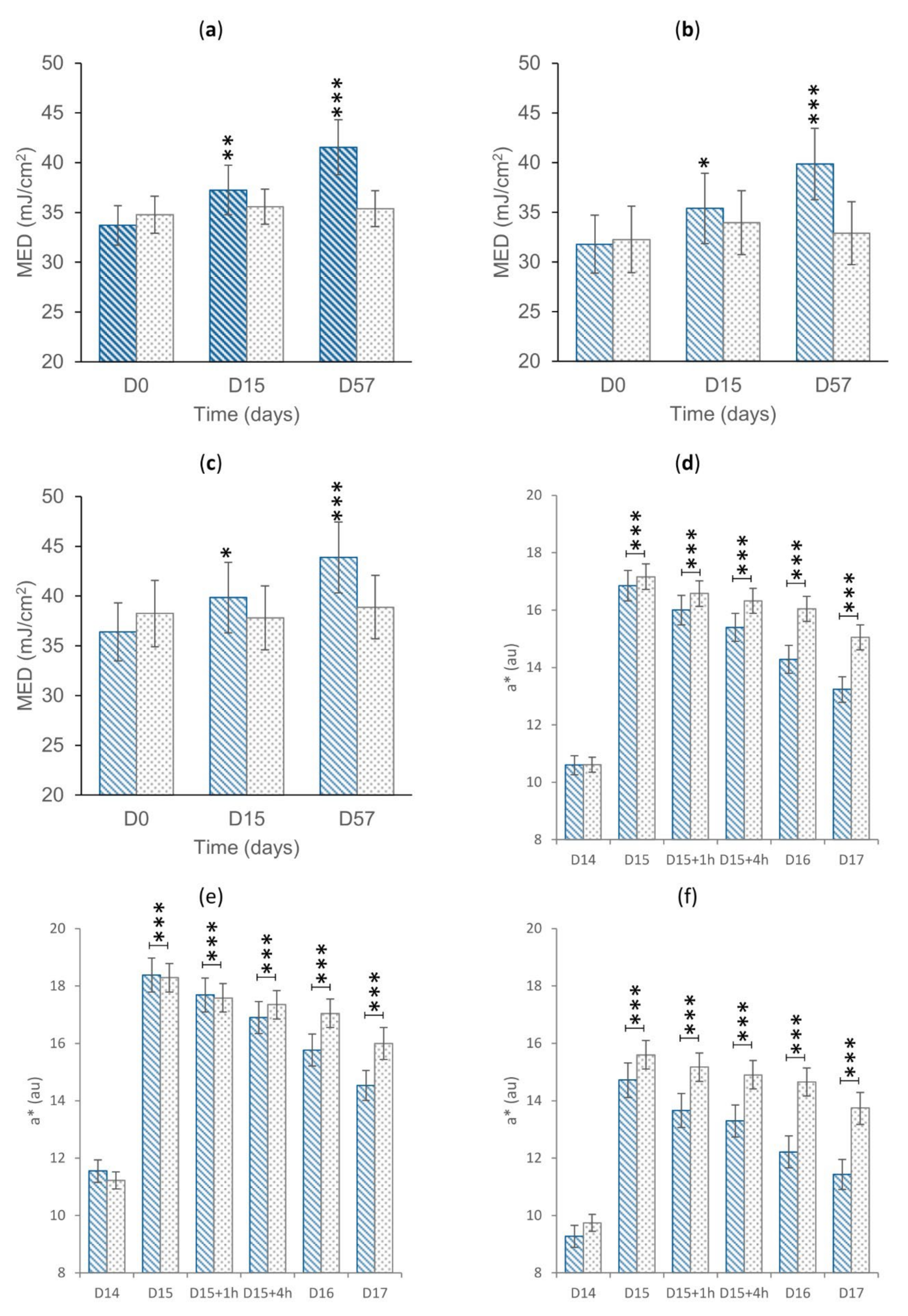
3.2. Primary Endpoints: MED and UV-Induced Erythema
3.3. Secondary Endpoints
3.3.1. Antioxidant Efficacy
3.3.2. Skin Antiaging Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fountoulakis, I.; Diémoz, H.; Siani, A.-M.; Laschewski, G.; Filippa, G.; Arola, A.; Bais, A.F.; De Backer, H.; Lakkala, K.; Webb, A.R.; et al. Solar UV Irradiance in a Changing Climate: Trends in Europe and the Significance of Spectral Monitoring in Italy. Environments 2020, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, R.L.; Aucamp, P.J.; Bais, A.F.; Björn, L.O.; Ilyas, M.; Madronich, S. Ozone Depletion and Climate Change: Impacts on UV Radiation. Photochem. Photobiol. Sci. 2011, 10, 182–198. [Google Scholar] [CrossRef] [PubMed]
- Saewan, N.; Jimtaisong, A. Natural Products as Photoprotection. J. Cosmet Dermatol 2015, 14, 47–63. [Google Scholar] [CrossRef] [PubMed]
- NASA—UV Exposure Has Increased Over the Last 30 Years, but Stabilized Since the Mid-1990s. Available online: https://www.nasa.gov/topics/solarsystem/features/uv-exposure.html (accessed on 29 March 2022).
- Diffey, B.L. Time and Place as Modifiers of Personal UV Exposure. Int. J. Environ. Res. Public Health 2018, 15, E1112. [Google Scholar] [CrossRef] [Green Version]
- Doré, J.-F.; Chignol, M.-C. Tanning Salons and Skin Cancer. Photochem. Photobiol. Sci. 2012, 11, 30–37. [Google Scholar] [CrossRef]
- Choi, K.; Lazovich, D.; Southwell, B.; Forster, J.; Rolnick, S.J.; Jackson, J. Prevalence and Characteristics of Indoor Tanning Use among Men and Women in the United States. Arch. Dermatol. 2010, 146, 1356–1361. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic Effects of Ultraviolet Radiation on the Skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the Skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the Environment: Regulation of Local and Global Homeostasis by the Skin’s Neuroendocrine System. Adv. Anat. Embryol. Cell Biol. 2012, 212, v–115. [Google Scholar] [CrossRef]
- Clydesdale, G.J.; Dandie, G.W.; Muller, H.K. Ultraviolet Light Induced Injury: Immunological and Inflammatory Effects. Immunol. Cell Biol. 2001, 79, 547–568. [Google Scholar] [CrossRef] [PubMed]
- Bayerl, C.; Taake, S.; Moll, I.; Jung, E.G. Characterization of Sunburn Cells after Exposure to Ultraviolet Light. Photodermatol. Photoimmunol. Photomed. 1995, 11, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Sklar, L.R.; Almutawa, F.; Lim, H.W.; Hamzavi, I. Effects of Ultraviolet Radiation, Visible Light, and Infrared Radiation on Erythema and Pigmentation: A Review. Photochem. Photobiol. Sci. 2013, 12, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P.; You, Y.-H.; Besaratinia, A. Mutations Induced by Ultraviolet Light. Mutat. Res. 2005, 571, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Brem, R.; Zhang, X.; Xu, Y.-Z.; Karran, P. UVA Photoactivation of DNA Containing Halogenated Thiopyrimidines Induces Cytotoxic DNA Lesions. J. Photochem. Photobiol. B 2015, 145, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tyrrell, R.M. Ultraviolet Radiation and Free Radical Damage to Skin. Biochem. Soc. Symp. 1995, 61, 47–53. [Google Scholar] [CrossRef]
- Sander, C.S.; Chang, H.; Hamm, F.; Elsner, P.; Thiele, J.J. Role of Oxidative Stress and the Antioxidant Network in Cutaneous Carcinogenesis. Int. J. Dermatol. 2004, 43, 326–335. [Google Scholar] [CrossRef]
- Burke, K.E. Photoaging: The Role of Oxidative Stress. G. Ital. Dermatol. Venereol. 2010, 145, 445–459. [Google Scholar]
- Nichols, J.A.; Katiyar, S.K. Skin Photoprotection by Natural Polyphenols: Anti-Inflammatory, Antioxidant and DNA Repair Mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Marcílio Cândido, T.; Bueno Ariede, M.; Vieira Lima, F.; de Souza Guedes, L.; Robles Velasco, M.V.; Rolim Baby, A.; Rosado, C. Dietary Supplements and the Skin: Focus on Photoprotection and Antioxidant Activity-A Review. Nutrients 2022, 14, 1248. [Google Scholar] [CrossRef]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, K.G.; Kim, N. Dietary Supplements in Dermatology: A Review of the Evidence for Zinc, Biotin, Vitamin D, Nicotinamide, and Polypodium. J. Am. Acad. Dermatol. 2021, 84, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Morini, F.; Dusatti, F.; Bonina, F.P.; Saija, A.; Ferro, M. Iron-Induced Lipid Peroxidation in Human Skin-Derived Cell Lines: Protection by a Red Orange Extract. Altern. Lab. Anim. 2000, 28, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Cimino, F.; Cristani, M.; Saija, A.; Bonina, F.P.; Virgili, F. Protective Effects of a Red Orange Extract on UVB-Induced Damage in Human Keratinocytes. BioFactors 2007, 30, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Cardile, V.; Frasca, G.; Rizza, L.; Rapisarda, P.; Bonina, F. Antiinflammatory Effects of a Red Orange Extract in Human Keratinocytes Treated with Interferon-Gamma and Histamine. Phytother Res. 2010, 24, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Bonina, F.P.; Puglia, C.; Frasca, G.; Cimino, F.; Trombetta, D.; Tringali, G.; Roccazzello, A.; Insiriello, E.; Rapisarda, P.; Saija, A. Protective Effects of a Standardised Red Orange Extract on Air Pollution-Induced Oxidative Damage in Traffic Police Officers. Nat. Prod. Res. 2008, 22, 1544–1551. [Google Scholar] [CrossRef]
- Puglia, C.; Offerta, A.; Saija, A.; Trombetta, D.; Venera, C. Protective Effect of Red Orange Extract Supplementation against UV-Induced Skin Damages: Photoaging and Solar Lentigines. J. Cosmet. Dermatol. 2014, 13, 151–157. [Google Scholar] [CrossRef]
- Tomasello, B.; Malfa, G.A.; Acquaviva, R.; La Mantia, A.; Di Giacomo, C. Phytocomplex of a Standardized Extract from Red Orange (Citrus sinensis L. Osbeck) against Photoaging. Cells 2022, 11, 1447. [Google Scholar] [CrossRef]
- Bazin, R.; Doublet, E. Skin Aging Atlas; Med’com: Paris, France, 2007; Volume 1. [Google Scholar]
- Bazin, R.; Flament, F. Skin Aging Atlas; Med’com: Paris, France, 2010; Volume 2. [Google Scholar]
- COLIPA. Guideline for the Colorimetric Determination of Skin Colour Typing and Prediction of the Minimal Erythemal Dose (MED) without UV Exposure; COLIPA: Brussels, Belgium, 2007. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Ribet, V.; Nobile, V.; Rossi, A.B. In Situ Antioxidant Activity of a Dermo-Cosmetic Product: A Randomized Controlled Clinical Study. Exp. Dermatol. 2019, 28, 1219–1226. [Google Scholar] [CrossRef] [Green Version]
- Erdelmeier, I.; Gérard-Monnier, D.; Yadan, J.C.; Chaudière, J. Reactions of N-Methyl-2-Phenylindole with Malondialdehyde and 4-Hydroxyalkenals. Mechanistic Aspects of the Colorimetric Assay of Lipid Peroxidation. Chem. Res. Toxicol. 1998, 11, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; Candore, G.; Scapagnini, G.; Caruso, C. Aging and Antiaging Strategies. In Textbook of Aging Skin, 2nd ed.; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1817–1827. [Google Scholar]
- Marionnet, C.; Tricaud, C.; Bernerd, F. Exposure to Non-Extreme Solar UV Daylight: Spectral Characterization, Effects on Skin and Photoprotection. Int. J. Mol. Sci. 2014, 16, 68–90. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.; Kang, S.-G.; Huang, K.; Tong, T. Boosting the Photoaged Skin: The Potential Role of Dietary Components. Nutrients 2021, 13, 1691. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, R.B.; Weimer, P.; Rossi, R.C. Effects of Hydrolyzed Collagen Supplementation on Skin Aging: A Systematic Review and Meta-Analysis. Int. J. Dermatol. 2021, 60, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-Y.; Ko, H.-C.; Ko, S.-Y.; Hwang, J.-H.; Park, J.-G.; Kang, S.-H.; Han, S.-H.; Yun, S.-H.; Kim, S.-J. Correlation between Flavonoid Content and the NO Production Inhibitory Activity of Peel Extracts from Various Citrus Fruits. Biol. Pharm. Bull. 2007, 30, 772–778. [Google Scholar] [CrossRef] [Green Version]
- Thiele, J.J.; Schroeter, C.; Hsieh, S.N.; Podda, M.; Packer, L. The Antioxidant Network of the Stratum Corneum. Curr. Probl. Dermatol. 2001, 29, 26–42. [Google Scholar] [CrossRef]
- Tamaru, E.; Watanabe, M.; Nomura, Y. Dietary Immature Citrus Unshiu Alleviates UVB- Induced Photoaging by Suppressing Degradation of Basement Membrane in Hairless Mice. Heliyon 2020, 6, e04218. [Google Scholar] [CrossRef]
- Hashmi, F.; Malone-Lee, J. Measurement of Skin Elasticity on the Foot. Skin Res. Technol. 2007, 13, 252–258. [Google Scholar] [CrossRef]
- Dobrev, H. In Vivo Study of Skin Mechanical Properties in Raynaud’s Phenomenon. Skin Res. Technol. 2007, 13, 91–94. [Google Scholar] [CrossRef]
- Koch, R.J.; Cheng, E.T. Quantification of Skin Elasticity Changes Associated with Pulsed Carbon Dioxide Laser Skin Resurfacing. Arch. Facial Plast. Surg. 1999, 1, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Hinek, A.; Kim, H.J.; Wang, Y.; Wang, A.; Mitts, T.F. Sodium L-Ascorbate Enhances Elastic Fibers Deposition by Fibroblasts from Normal and Pathologic Human Skin. J. Dermatol. Sci. 2014, 75, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of Antioxidants in the Skin: Anti-Aging Effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Deng, N.; Wang, H.; Li, T.; Chen, L.; Zheng, B.; Liu, R.H. Effects of Orange Extracts on Longevity, Healthspan, and Stress Resistance in Caenorhabditis Elegans. Molecules 2020, 25, 351. [Google Scholar] [CrossRef] [Green Version]
 .
.
 .
.
 (from −500 µm [black] to 350 µm [yellow]).
(from −500 µm [black] to 350 µm [yellow]).
 (from −500 µm [black] to 350 µm [yellow]).
(from −500 µm [black] to 350 µm [yellow]).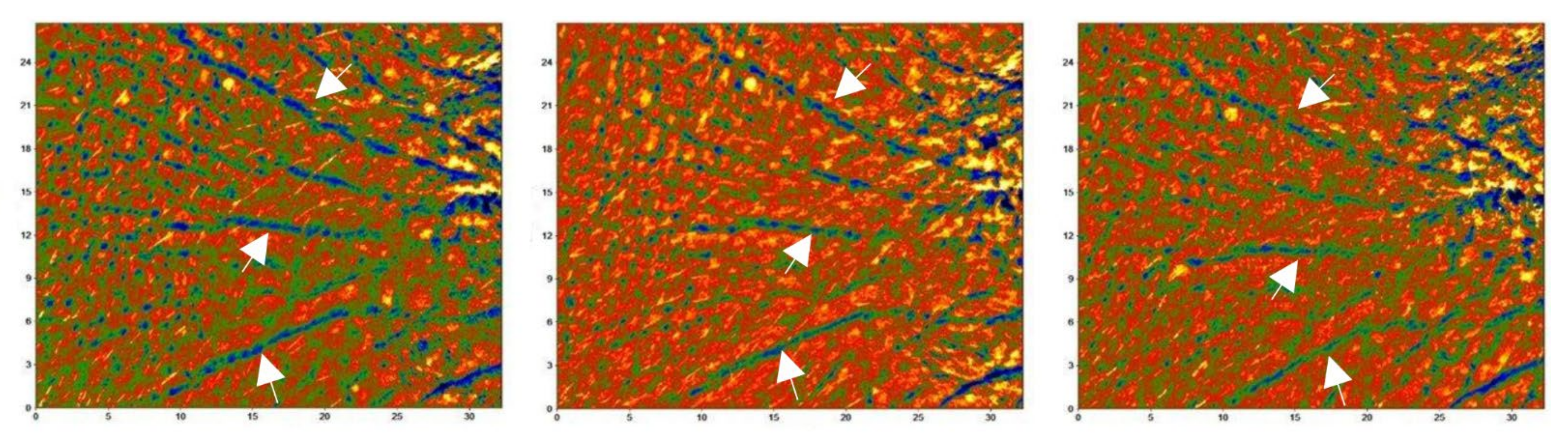
| Active | Placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | D15 | Δ% | D57 | Δ% | D0 | D15 | Δ% | D57 | Δ% | ||
| FRAP (μM Fe2+) | O | 329.0 ± 8.4 | 356.7 ± 9.5 *** | +8.6% *** | 402.3 ± 12.6 *** | +22.3% *** | 333.8 ± 9.3 | 335.1 ± 10.5 | +0.4% | 339.3 ± 10.2 | +2.0% |
| A | 321.2 ± 11.3 | 347.1 ± 11.9 *** | +8.5% ** | 391.9 ± 17.0 *** | +22.0% *** | 331.3 ± 10.5 | 331.4 ± 12.1 | +0.1% | 337.7 ± 11.4 | +2.2% | |
| C | 339.9 ± 12.6 | 370.0 ± 15.4 *** | +8.8% *** | 416.8 ± 18.8 *** | +22.6% *** | 337.2 ± 17.0 | 340.4 ± 18.9 | +0.8% | 341.5 ± 18.9 | +1.7% | |
| D−1 | D14 | D56 | D−1 | D14 | D56 | ||||||
| MDA (μM MDA) | O | 16.8 ± 0.6 | 16.5 ± 0.6 | −1.5% ** | 15.4 ± 0.6 *** | −8.2% *** | 16.9 ± 0.6 | 17.4 ± 0.6 | +3.7% | 17.3 ± 0.6 | +3.1% |
| A | 16.4 ± 0.8 | 16.1 ± 0.8 | −1.2% | 15.0 ± 0.8 *** | −7.7% *** | 17.3 ± 0.8 | 17.6 ± 0.8 | +2.4% | 17.7 ± 0.8 | +3.2% | |
| C | 17.5 ± 0.9 | 17.1 ± 0.8 | −1.9% * | 16.0 ± 0.8 *** | −8.9% *** | 16.4 ± 0.8 | 17.1 ± 0.8 | +5.5% | 16.7 ± 0.8 | +2.9% | |
| Active | Placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | D15 | Δ% | D57 | Δ% | D0 | D15 | Δ% | D57 | Δ% | ||
| Moist. (cu) | O | 53.7 ± 2.2 | 55.9 ± 2.2 *** | +5.3% ** | 59.3 ± 2.2 *** | +12.3% *** | 55.1 ± 2.2 | 51.6 ± 2.0 * | −3.6% | 53.2 ± 2.0 | −1.9% |
| A | 61.7 ± 2.1 | 64.3 ± 2.0 *** | +4.8% * | 67.9 ± 2.0 *** | +11.0% *** | 62.6 ± 2.3 | 58.3 ± 2.1 | −4.1% | 60.8 ± 1.9 | −1.8% | |
| C | 42.6 ± 3.3 | 44.3 ± 3.1 | +5.9% | 47.3 ± 3.0 *** | +14.2% *** | 44.7 ± 3.0 | 42.2 ± 2.8 | −2.8% | 42.8 ± 2.9 | −2.0% | |
| Rad. (au) | O | 10.9 ± 0.5 | 11.5 ± 0.5 | +9.2% | 12.5 ± 0.6 *** | +18.5% *** | 11.6 ± 0.5 | 11.6 ± 0.5 | +1.8% | 11.7 ± 0.5 | +2.5% |
| A | 10.5 ± 0.7 | 10.8 ± 0.5 | +8.0% | 12.1 ± 0.7 *** | +18.2% *** | 10.6 ± 0.6 | 10.6 ± 0.7 | +1.3% | 10.9 ± 0.7 | +2.4% | |
| C | 11.4 ± 0.9 | 12.4 ± 1.0 * | +10.9% | 13.2 ± 1.0 *** | +18.8% ** | 13.0 ± 0.9 | 12.9 ± 0.7 | +2.5% | 12.9 ± 0.7 | +2.4% | |
| ITA° | O | 23.0 ± 1.3 | 25.7 ± 1.4 *** | +14.8% | 29.3 ± 1.5 *** | +32.9% *** | 23.5 ± 1.1 | 24.3 ± 1.2 | +6.8% | 25.7 ± 1.2 ** | +12.5% |
| A | 23.4 ± 1.8 | 26.1 ± 2.0 *** | +15.3% | 29.7 ± 2.1 *** | +33.2% ** | 24.1 ± 1.7 | 25.0 ± 1.8 | +7.4% | 26.4 ± 1.9 * | +13.2% | |
| C | 22.4 ± 1.7 | 25.2 ± 2.0 | +14.1% *** | 28.9 ± 2.1 *** | +32.4% ** | 22.8 ± 1.4 | 23.2 ± 1.3 | +5.8% | 24.7 ± 1.4 * | +11.5% | |
| TEWL (g/h/m2) | O | 12.7 ± 0.5 | 12.2 ± 0.4 | −2.7% ** | 10.8 ± 0.4 *** | −14.5% ** | 12.2 ± 0.5 | 12.9 ± 0.5 | +8.0% | 12.6 ± 0.4 | +4.8% |
| A | 13.3 ± 0.7 | 12.5 ± 0.5 | −4.0% * | 11.2 ± 0.5 *** | −14.3% *** | 13.2 ± 0.7 | 13.7 ± 0.6 | +6.4% | 13.4 ± 0.6 | +3.5% | |
| C | 11.9 ± 0.6 | 11.7 ± 0.6 | −1.0% | 10.1 ± 0.6 *** | −14.9% *** | 10.9 ± 0.5 | 11.9 ± 0.7 | +10.3% | 11.4 ± 0.5 | +6.6% | |
| Wr. depth (μm) | O | 349.8 ± 14.1 | 350.4 ± 14.4 | +0.1% | 325.0 ± 13.4 *** | −6.9% *** | 345.7 ± 16.7 | 347.8 ± 16.7 | +0.8% | 349.1 ± 16.5 | +1.4% |
| A | 291.0 ± 9.5 | 289.3 ± 9.9 | −0.6% | 269.9 ± 10.3 *** | −7.5% *** | 277.3 ± 12.8 | 282.7 ± 13.3 * | +2.0% | 281.1 ± 12.4 | +1.8% | |
| C | 431.6 ± 21.5 | 435.4 ± 21.4 | +1.1% | 401.6 ± 19.5 ** | −6.2% ** | 440.9 ± 24.7 | 438.4 ± 25.4 | −0.8% | 443.7 ± 24.5 | +0.8% | |
| Active | Placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | D15 | Δ% | D57 | Δ% | D0 | D15 | Δ% | D57 | Δ% | ||
| R0 (mm) | O | 0.321 ± 0.013 | 0.294 ± 0.012 *** | −8.2% *** | 0.269 ± 0.011 *** | −15.6% | 0.322 ± 0.013 | 0.327 ± 0.013 * | +2.0% | 0.330 ± 0.014 | +3.1% |
| A | 0.257 ± 0.006 | 0.254 ± 0.006 *** | −9.3% *** | 0.215 ± 0.007 *** | −15.2% *** | 0.251 ± 0.007 | 0.257 ± 0.008 | +2.5% | 0.260 ± 0.010 | +3.8% | |
| C | 0.413 ± 0.017 | 0.384 ± 0.014 ** | −6.5% *** | 0.343 ± 0.012 *** | −16.0% *** | 0.420 ± 0.012 | 0.425 ± 0.013 | +1.2% | 0.429 ± 0.014 | +3.8% | |
| R2 (%) | O | 0.593 ± 0.015 | 0.626 ± 0.016 *** | +5.9% *** | 0.671 ± 0.015 *** | +13.9% *** | 0.582 ± 0.013 | 0.583 ± 0.013 | +0.1% | 0.577 ± 0.014 | −0.9% |
| A | 0.520 ± 0.010 | 0.551 ± 0.010 *** | +6.2% *** | 0.598 ± 0.011 *** | +15.5% *** | 0.522 ± 0.010 | 0.523 ± 0.011 | 0.0% | 0.518 ± 0.014 | −0.9% | |
| C | 0.695 ± 0.017 | 0.730 ± 0.018 ** | +5.5% ** | 0.773 ± 0.018 *** | +11.6% *** | 0.665 ± 0.014 | 0.667 ± 0.014 | +0.2% | 0.0659 ± 0.015 | −0.8% | |
| R5 (%) | O | 0.449 ± 0.016 | 0.514 ± 0.018 *** | +16.8% *** | 0.587 ± 0.022 *** | +33.4% *** | 0.447 ± 0.016 | 0.438 ± 0.017 | −2.6% | 0.441 ± 0.016 | −1.2% |
| A | 0.504 ± 0.012 | 0.579 ± 0.016 *** | +15.9% *** | 0.668 ± 0.019 *** | +34.3% *** | 0.500 ± 0.011 | 0.485 ± 0.015 | −3.1% | 0.491 ± 0.015 | −1.8% | |
| C | 0.372 ± 0.025 | 0.424 ± 0.025 ** | +17.1% *** | 0.475 ± 0.029 *** | +31.0% *** | 0.375 ± 0.024 | 0.372 ± 0.026 | −1.3% | 0.372 ± 0.024 | −0.6% | |
| R9 (mm) | O | 0.040 ± 0.001 | 0.035 ± 0.001 *** | −12.1% *** | 0.030 ± 0.001 *** | −25.4% *** | 0.039 ± 0.001 | 0.041 ± 0.002 | 6.5% | 0.041 ± 0.002 | +5.9% |
| A | 0.039 ± 0.002 | 0.034 ± 0.002 *** | −12.1% *** | 0.029 ± 0.002 *** | −25.8% *** | 0.039 ± 0.001 | 0.042 ± 0.002 * | +10.3% | 0.041 ± 0.002 | +8.2% | |
| C | 0.041 ± 0.002 | 0.037 ± 0.003 ** | −11.9% * | 0.031 ± 0.002 *** | −23.8% *** | 0.039 ± 0.002 | 0.039 ± 0.002 | +10.3% | 0.041 ± 0.003 | +8.2% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nobile, V.; Burioli, A.; Yu, S.; Zhifeng, S.; Cestone, E.; Insolia, V.; Zaccaria, V.; Malfa, G.A. Photoprotective and Antiaging Effects of a Standardized Red Orange (Citrus sinensis (L.) Osbeck) Extract in Asian and Caucasian Subjects: A Randomized, Double-Blind, Controlled Study. Nutrients 2022, 14, 2241. https://doi.org/10.3390/nu14112241
Nobile V, Burioli A, Yu S, Zhifeng S, Cestone E, Insolia V, Zaccaria V, Malfa GA. Photoprotective and Antiaging Effects of a Standardized Red Orange (Citrus sinensis (L.) Osbeck) Extract in Asian and Caucasian Subjects: A Randomized, Double-Blind, Controlled Study. Nutrients. 2022; 14(11):2241. https://doi.org/10.3390/nu14112241
Chicago/Turabian StyleNobile, Vincenzo, Andrea Burioli, Sara Yu, Shi Zhifeng, Enza Cestone, Violetta Insolia, Vincenzo Zaccaria, and Giuseppe Antonio Malfa. 2022. "Photoprotective and Antiaging Effects of a Standardized Red Orange (Citrus sinensis (L.) Osbeck) Extract in Asian and Caucasian Subjects: A Randomized, Double-Blind, Controlled Study" Nutrients 14, no. 11: 2241. https://doi.org/10.3390/nu14112241
APA StyleNobile, V., Burioli, A., Yu, S., Zhifeng, S., Cestone, E., Insolia, V., Zaccaria, V., & Malfa, G. A. (2022). Photoprotective and Antiaging Effects of a Standardized Red Orange (Citrus sinensis (L.) Osbeck) Extract in Asian and Caucasian Subjects: A Randomized, Double-Blind, Controlled Study. Nutrients, 14(11), 2241. https://doi.org/10.3390/nu14112241








