1. Introduction
Ascorbic acid (AscA), commonly known as vitamin C, is a water-soluble vitamin, antioxidant and immune-response enhancer essential for human survival, commonly associated with the development of scurvy and its complications if AscA-deficient [
1,
2,
3,
4,
5,
6]. Due to their inability to produce AscA, humans entirely rely on external sources, mainly diet, to maintain adequate plasma levels and prevent a state of deficiency [
7,
8,
9]. This becomes of utmost importance to neonates, who completely rely on the active transfer of AscA in utero and postnatally through breastmilk amongst other sources [
10,
11,
12,
13,
14].
Initial observational studies assessing plasma AscA (pAscA) in neonates have reported higher cord-blood levels of pAscA measured in healthy-term newborns at birth compared to their mothers, followed by a significant drop in plasma concentrations over the first 24 h of life [
15,
16,
17], suggesting an active transport system within the placenta to preferentially increase circulating AscA in the developing infant that is postnatally dissociated. Likewise, higher cord-blood levels in premature infants compared to those at term have been identified (146 ± 93 μM/L vs. 102 ± 27 μM/L, respectively;
p = 0.03), with a rapid decrease seen over a few days in preterm infants, which suggests a continued need for AscA supplementation in preterm infants [
18,
19,
20]. These studies found the need for further understanding of supplementation requirements, particularly for preterm infants with increasing survival at the lowest gestational age of viability. Few reports have investigated the influence of maternal health, gestational age, body mass or risk of morbidity development based on pAscA concentrations during the postnatal maturation of premature infants. Silvers et al. through a prospective longitudinal observational study reported significantly higher concentrations of pAscA within two hours of life in premature infants who died vs. those who survived. An increased risk of bronchopulmonary dysplasia (BPD) was also seen with higher pAscA levels measured on day 2 of life [
20]. On the contrary, lower pAscA levels measured at 10 days of life (Mosion, RM) and at 28 days of life (Sluis et al.) were seen in premature infants who developed BPD [
21,
22]. Subsequently, a randomized control trial comparing the combined administration of parenteral and enteral low-dose AscA (10 mg/kg/day) vs. high dose (30–40 mg/kg/day) vs. placebo to premature infants reported no significant difference in clinical outcome amongst the treatment groups; although no levels were identified, these data suggested improved respiratory outcome in those who received high-dose AscA [
23]. Similarly, studies have demonstrated a relationship between maternal smoking and breastmilk AscA levels as well as maternal smoking and offspring pAscA levels in relation to newborn pulmonary function test results, with higher pAscA levels found in breastmilk and offspring when mothers were supplemented with AscA [
24,
25].
Current recommendations suggest that premature infants receive higher doses of AscA (25–31 mg/kg/day) to approximate concentrations seen in the third trimester in utero, with pAscA levels of <15 mg/dL associated with deficiency as per adult and term infant data [
14,
26,
27]. This creates a challenge, as most initially receive AscA parenterally or enterally through multivitamin supplements that can increase other nutrients unintentionally. There are limited data available to guide optimal plasma concentrations postnatally by gestational age or during sickness. Additionally, the diagnosis of scurvy is rare in children and may be uniquely challenged in extremely preterm infants, yet it is this population that is at a high risk for inflammatory processes and/or mortality [
28].
In this study, we aim to detail the relationship of gestational age (GA), birthweight, race, maternal health and evolving preterm hepatic and renal maturation on infant pAscA levels during infant development in the neonatal intensive care unit (NICU). Furthermore, we detail pAscA levels on influencing later preterm-infant common morbidities (bronchopulmonary dysplasia (BPD), infection, necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP) and intraventricular hemorrhage (IVH)) in infants ≤34 weeks of gestation, where we hypothesize that lower-than-expected pAscA levels are associated with greater morbidity risk.
4. Discussion
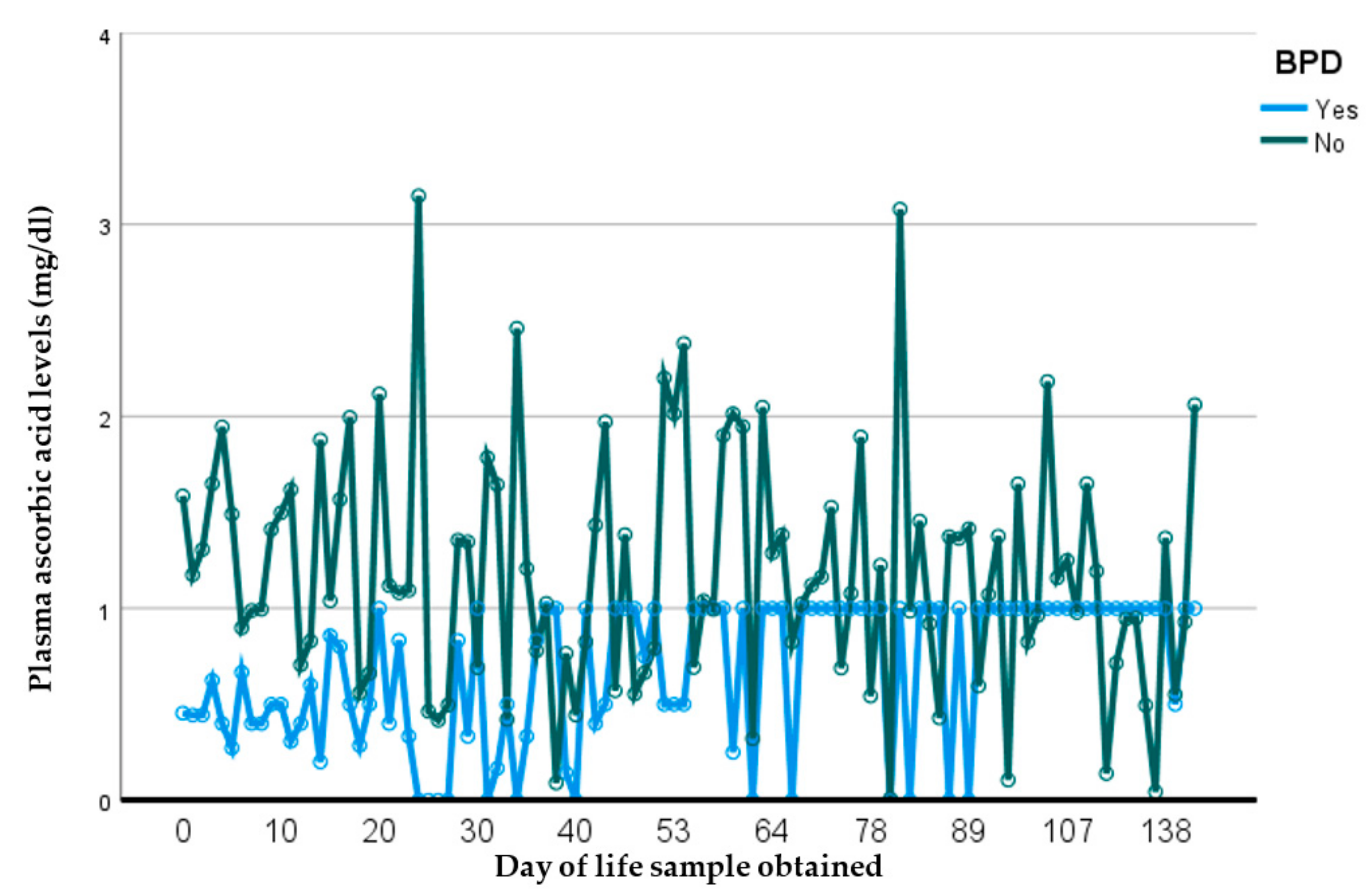
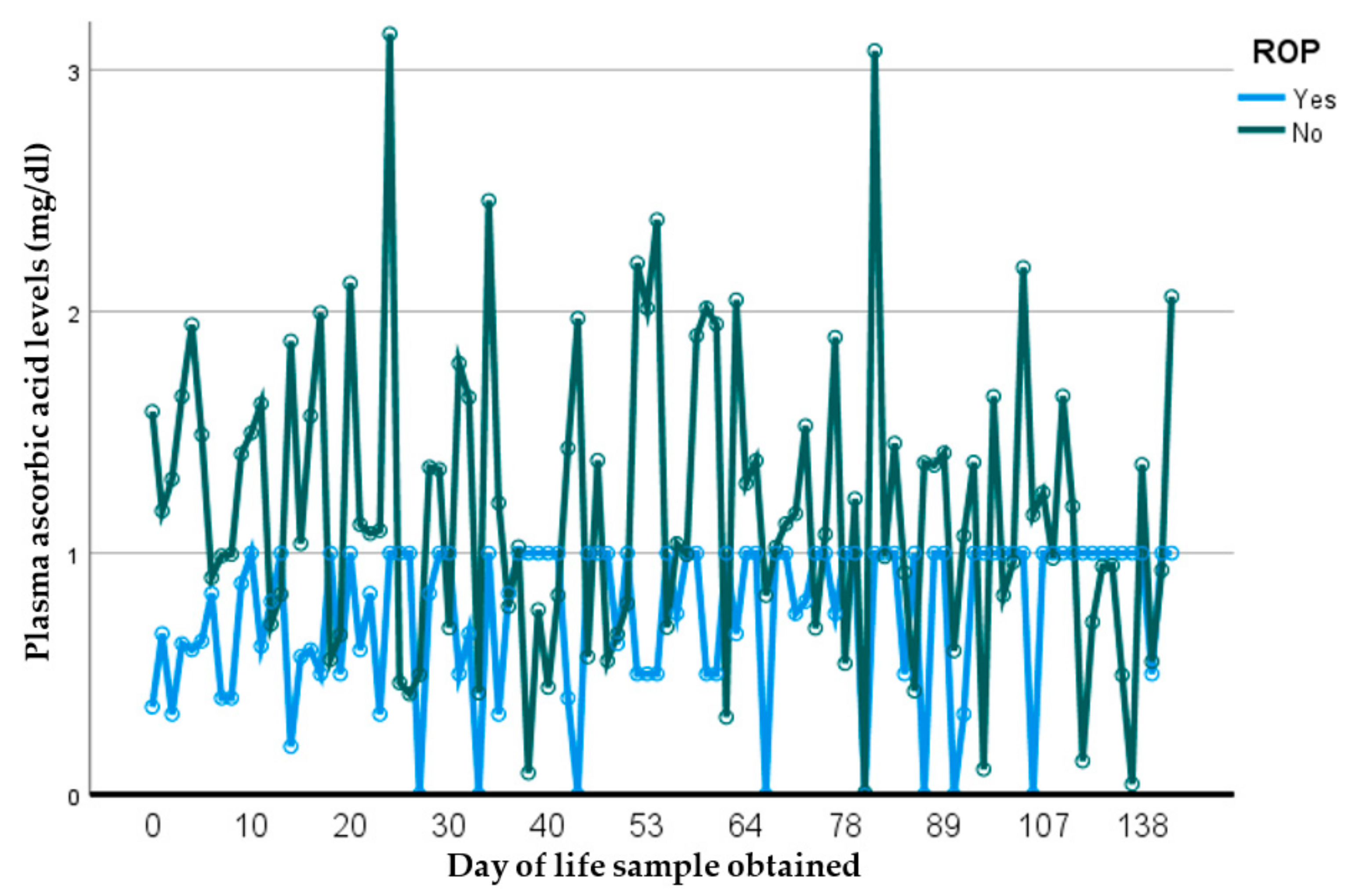
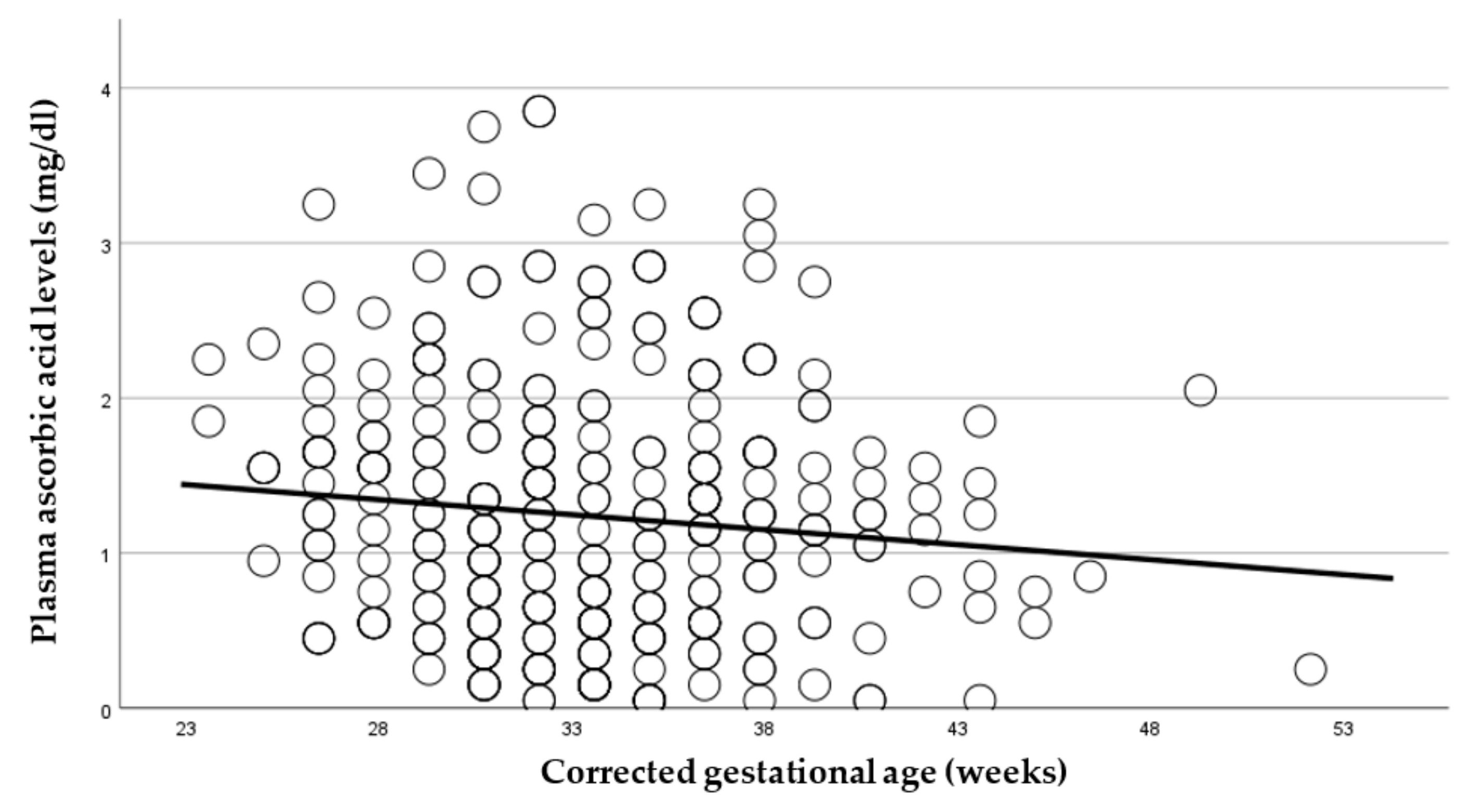
In this prospective observational longitudinal study, we address current gaps that exist to outline the important relationship between plasma ascorbic acid (pAscA) and premature infant health and disease. To our knowledge, there is limited information regarding pAscA levels in premature infants at birth and longitudinally, to address current normative levels for nutrient requirements in this at-risk population. Additionally, earlier information is not relevant to be inclusive of extremely preterm infants and micro preemies, with no studies showing the potential influence of sociodemographic racial determinants of maternal health on infant pAscA levels as well as risk for morbidity outcomes in premature infants. As seen with earlier studies, we found that as gestational age increases, pAscA levels decrease in the first week of life for preterm infants and continues to decrease with increasing gestational age. However, we saw that early and later AscA levels were associated with specific later severe chronic lung disease (BPD) and retinopathy of prematurity. We noted no associations of AscA levels in later infections, IVH or NEC. However, in those preterm infants who developed BPD, we noted that pAscA levels started at lower “normal” normative levels that remained lower throughout the study period compared to those who did not develop BPD. We believe this finding to be important, as it could suggest that an already immature anti-inflammatory system could be worsened by an ineffective antioxidant response, and that expected normative levels of ascorbic acid may be insufficient in infants at risk of BPD. This relationship was noted by Vyas et al., who assessed bronchoalveolar lavage fluid (BALF) ascorbate levels and risk for BPD in premature infants, reporting a delayed increase in BALF ascorbate levels to be associated with an increased risk for BPD, most likely due to a lack of antioxidant protection [
37]. Furthermore, it could find that certain medications provided to infants with developing chronic lung disease may influence circulating AscA levels. Similarly, through its antioxidant effect, ascorbic acid could play a role in the prevention of ROP, with data being controversial and scarce [
38]. To our knowledge, this is the first study to report ascorbic-acid levels in premature infants, specifically those ≤30 weeks and risk for ROP.
The observed higher levels of pAscA at birth and/or in the first day of life aligns with those observations made by Awoyelu et al. [
15] and Silvers et al. [
20]. Given the dependency of active transport of AscA through the placenta to maintain physiologic levels in the fetus, these results suggest that mothers in our studies consumed prenatal vitamins during the perinatal period and highlight the importance of maternal nutritional status during pregnancy as a significant influence on fetal AscA levels. This protective phenomenon persists in the postnatal life, where maternal health status and dietary intake of AscA influences its availability in breastmilk and is another avenue of communication to advance levels of AscA in infants of breastfeeding mothers. Although not reported in our study, breastmilk AscA levels measured in our lab were higher in colostrum, with a decrease in levels over the course of lactation as described by others [
14,
39]. Another major observation from our study focused on racial differences among black, white and Hispanic infants in both the first week of life and over the entire study period. The striking increase in plasma AscA levels in white infants at birth is likely due to maternal differences in prenatal vitamin intake related to social determinants of health, although genetic variant predispositions affecting the function of the sodium-dependent AscA transporters in the body and/or the antioxidant response in times of growth and disease cannot be excluded [
40,
41,
42]. Additionally, our study uncovered that maternal exposure to perinatal magnesium and prenatal smoking impacted infant pAscA levels, as seen by McEvoy et al. [
25], while the burden of other maternal diseases such as preeclampsia, hypertension, chorioamnionitis and maternal obesity were not influential in affecting AscA levels in the infant. To date, the exact mechanism through which magnesium sulfate impacts on vitamin C levels remains unclear, with some suggesting that it enhances the expression of the sodium-dependent AscA transporter at the cellular level and increases cellular AscA uptake [
43]. Our results found that preterm infant renal and hepatic maturational changes over time influenced AscA levels, suggesting that postnatal requirement of ascorbic acid may need to be augmented based on gestational maturity and/or renal and hepatic maturation.
We recognize that the subject sample size may limit the potential result, given possible sample bias and subsequently the external validity of the study, but to our knowledge, this is the largest sample collection and assessment of pAscA levels in a cohort of premature infants to date for assessing AscA changes over time, gestational maturity and during illness. We were also limited with the routine collection of cord-blood samples and day-one-of-life samples from all subjects enrolled in part due to the timing of and circumstances surrounding enrollment, despite efforts to keep consistency. Although our goal was to collect weekly samples until discharge, sample numbers per patient differed due to the expected difference in length of stay.
Ascorbic acid by nature is a very unstable molecule, with its degradation being accelerated in the presence of light, potentially leading to significantly lower measured levels than those truly representative of the actual state during growth and sickness. To reduce the amount of potential degradation and obtain samples most representative of the clinical scenario at time of collection, all samples were shielded from light using photoprotective sample-collection bags and stabilized in our lab for storage as quickly as possible. Additionally, laboratory standards were maintained to limit degradation. Current dietary vitamin recommendations are often overestimated and targeted to prevent certain pathologies no longer met in premature infants, as seen with ascorbic-acid deficiency and the prevention of scurvy, or in trying to mimic levels seen in utero. Our results suggest that the preterm infants rarely experienced deficiency levels that are currently in place. Instead, infants experienced commonly low “normal” levels, suggesting that current expected recommendations may need revision to higher dosing levels in the extremely preterm as well as potentially be increased during postnatal period in those infants most at risk for debilitating chronic lung disease. To more effectively and appropriately dose, supply and utilize AscA in premature infants, further studies are urgently needed to target better intake assessments and identify potential biomarkers of health to better understand appropriate dosing requirements of ascorbic acid during critical times of growth in regulating health and risk for inflammatory conditions in the vulnerable preterm infant.