The Role of Phytosterols in Nonalcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Phytosterols: Composition, Occurrence, and Biological Activities
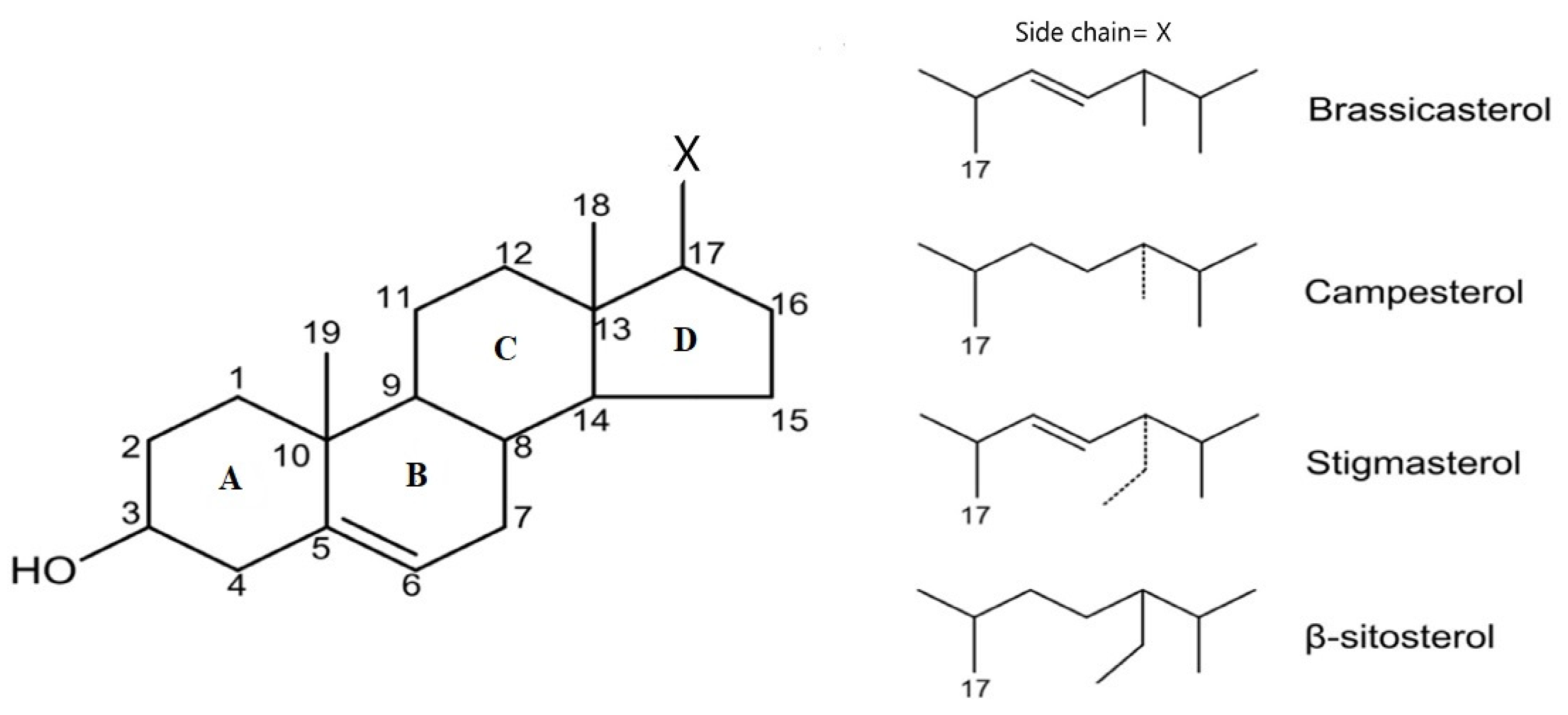
2.1. Chemical Structures
2.2. Sources
| Product | Weight | Phytosterol Content | |
| Sesame oil | 14 g (1 spoon) | 118 mg | |
| Sunflower oil | 14 g (1 spoon) | 60 mg | |
| Olive oil | 14 g (1 spoon) | 30 mg | |
| Dried soybean seeds | 100 g | 300 mg | |
| Pumpkin seeds | 100 g | 94–265 mg | |
| Sesame seeds | 100 g | 400 mg | |
| Sunflower seeds | 100 g | 176–322 mg | |
| Flaxseed | 100 g | 197–214 mg | |
| Raw peas | 75 g | 133 mg | |
| Pistachio | 100 g | 279–297 mg | |
| Cashew | 100 g | 80–158 mg | |
| Nuts | 100 g | 63–206 mg | |
| Almond | 100 g | 89–208 mg | |
| PSs in oils as percentage of total sterol fraction | |||
| Product | β-Sitosterol | Campesterol | Stigmasterol |
| Sunflower oil | 56–63 | 7–13 | 8–11 |
| Olive oil | 75.6–90 | 2.3–3.6 | 0.6–2 |
| Coconut oil | 50–70 | 7–10 | 12–18 |
| Corn oil | 55–67 | 7.2–8.4 | 1.2–1.8 |
| Peanut oil | 48–65 | 12–20 | 5–13 |
| Soy bean oil | 52–58 | 16–24 | 16–19 |
2.3. Biological Properties of PSs
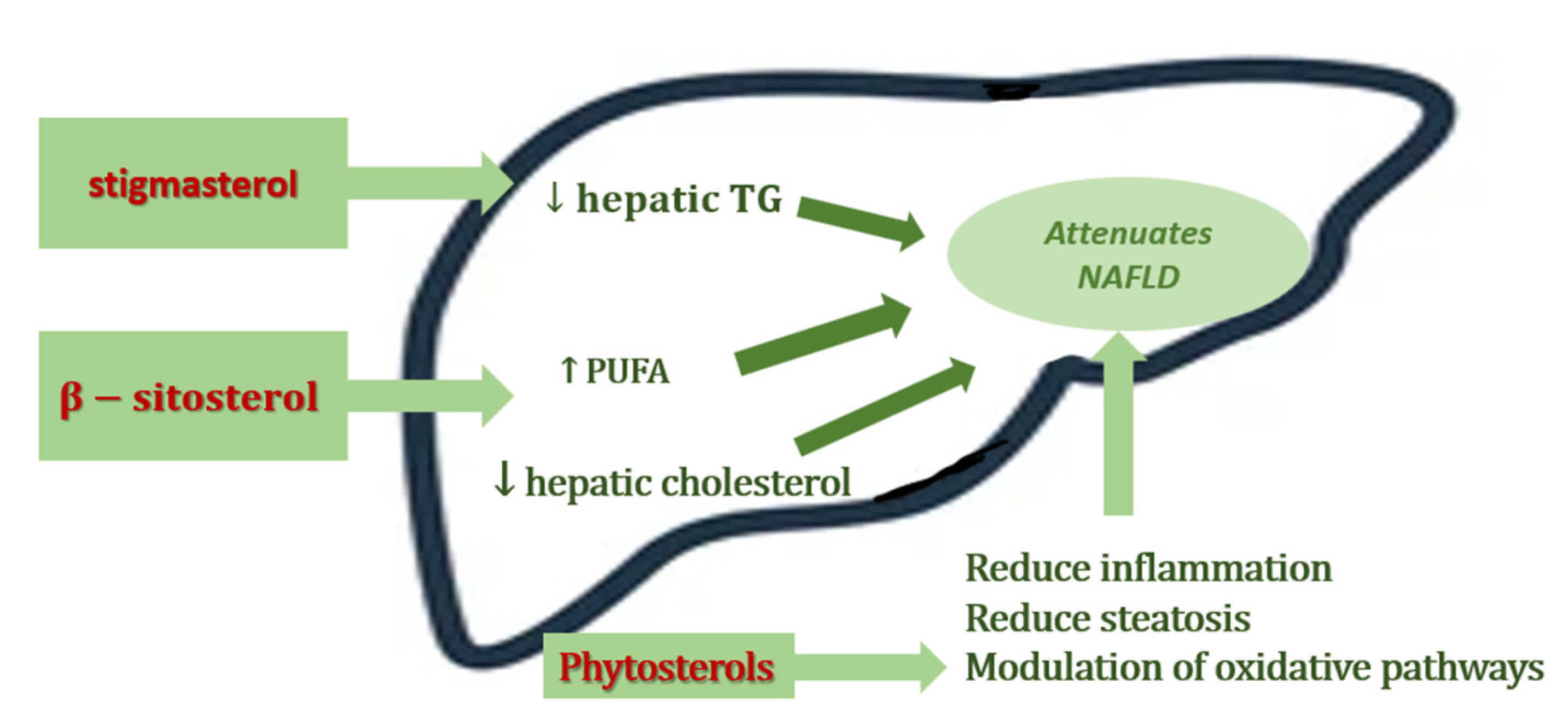
3. The Role of Phytosterols in NAFLD
| Animal Models Studies | |||||
|---|---|---|---|---|---|
| Study Design | Dietary Phytosterols Supplementation | Intervention | Biological Activity of Phytosterol | Reference | |
| 1 | 10–12 weeks old female low-density lipoprotein (LDL) receptor deficient mice, high-fat diet (HFD) | Plant sterol and stanols | Plant sterol esters (2%) or plant stanol esters (2%) | Lowered hepatic inflammation ↓ serum and hepatic cholesterol ↓ serum TAG concentrations | Plat J et al., 2014 [39] |
| 2 | Male apolipoprotein-E knockout mice, high fat diet | Flaxseed oil combined with its ester of plant sterols-treated (FO-PS) | 3.3% (w/w) flaxseed oil ester of plant sterols mixture with amount of flaxseed oil | Improving hepatic steatosis ↓ ROS production ↓ inflammatory markers (IL-6, TNF, MCP-1, and ICAM-1 | Han H et al., 2014 [40] |
| 3 | Five-week-old male Sprague Dawley rats, high-fat diet | Phytosterols esters (PSEs) | low and medium doses of PSEs (equal to 3 or 6 g/d in humans) | ↓ LDL-C serum level; ↓ ALT, AST serum level; Modulating key cytokines; improved oxidative status ameliorate hepatic lesions | Song L et al., 2017 [41] |
| 4 | Adult Sprague Dawley rats, high-fat diet | Phytosterol esters (PSEs) | low-dose PSE (0.05 g per 100 g body weight) and high-dose PSE (0.10 g per 100 g body weight), | high-dose PSE treatment: - effectively inhibited the increase in liver and abdominal fat indexes and hepatic lipids - increased the relative abundance of Bacteroidetes and Anaerostipes | Song L et al., 2020 [42] |
| 5 | 8-week old male C57BL/6 mice, high-fat modified Western-style diet | Stigmasterol β-sitosterol | 0.4% stigmasterol or 0.4% β-sitosterol (comparable to that suggested for humans by the FDA) | ↓ body weight gain ↓ serum cholesterol ↓ histological score ↓ ALT levels ↑ hepatic HMGCoAR mRNA expression ↓ the expression of lipogenic genes, FAS and SCD1 ↓ circulating CM levels | Feng S et al., 2018 [43] |
| 6 | 78 week old male C57BL/6 mice, high-fat modified Western-style diet | Stigmasterol β-sitosterol | 0.4% stigmasterol or 0.4% β-sitosterol | ↓ triacylglycerols with polyunsaturated fatty acids decreased of free hepatic fatty acids increased MUFA and PUFA levels | Feng S et al. 2018 [44] |
| 7 | Growing male Sprague Dawley rats, high-fructose diet | β- sitosterol | + 20 mg/kg β-sitosterol in a gelatine cube | ↓ liver lipids ↓in liver mass ↓ areas of inflammation lesions of micro- and macrovesicular hepatic steatosis | Gumede NM et al., 2020 [45] |
| 8 | 21-day-old female Sprague Dawley rat pups, high-fructose diet | β- sitosterol | + 20 mg/kg β-sitosterol in a gelatine cube | ↓ hypertriglyceridemia ↑ plasma adiponectin concentration ↓ plasma insulin concentration prevented the high-fructose diet-induced visceral obesity | Gumede NM et al., 2020 [46] |
| 9 | C57BL/6J mice, high fat and high cholesterol diet | Plant sterol ester of α-linolenic acid (PS-ALA) | 3.3% PS-ALA | suppressed hepatic steatosis ↓ mitochondrial damage Inhibited Srebp Pathway Activation ↓ fatty acid accumulation ↓ oxidative stress ↓ lipid disorder ↓ release of IL-1β and IL-18 ↓ ALT, AST | Han H et al., 2019 [47] |
| Clinical Trials | |||||
| Study Design | Dietary Phytosterols Supplementation | Intervention | Results | Reference | |
| 1 | double-blind clinical trial 38 patients with NAFLD | phytosterols | 1.6 g phytosterol supplementation daily | ↓ LDL-C, TNF-α, ALT, AST | Javanmardi MA et al., 2018 [48] |
| 2 | cross-over clinical trial, 40 NAFLD subjects | phytosterols | 1.8 g PSs supplementation daily for 4 weeks | ↓ LDL-C ↓ systemic inflammation Improved insulin resistance | Chen et al., 2015 [49] |
| 3 | double-blind placebo-controlled trial 96 NAFLD subjects, overweight or dyslipidaemic | phytosterol esters + n-3 fatty acids | PS-enriched soy milk powder containing 3·3 g of PS plus fish oil capsules containing highly concentrated EPA and DHA (450 mg EPA + 1500 mg DHA), 12 weeks | ↑ the liver: spleen attenuation ratio ↓ TAG levels ↓ TGF-β levels ↓ TNF-α levels ameliorate hepatic lesions | Song L et al., 2020 [50] |
3.1. Animal Models’ Studies
3.2. Clinical Trials
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maurice, J.; Manousou, P. Non-alcoholic fatty liver disease. Clin. Med. 2018, 18, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Citro, V.; Capone, D. Nonalcoholic Fatty Liver Disease: A Challenge from Mechanisms to Therapy. J. Clin. Med. 2019, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslam, M.; Sanyal, A.J.; George, J.; on behalf of theInternational Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1991. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Alkhouri, N.; Vajro, P.; Baumann, U.; Weiss, R.; Socha, P.; Marcus, C.; Lee, W.S.; Kelly, D.; Porta, G.; et al. Defining paediatric metabolic (dysfunction)-associated fatty liver disease: An international expert consensus statement. Lancet Gastroenterol. Hepatol. 2021, 6, 864–873. [Google Scholar] [CrossRef]
- Flisiak-Jackiewicz, M.; Bobrus-Chociej, A.; Wasilewska, N.; Lebensztejn, D. From Nonalcoholic Fatty Liver Disease (NAFLD) to Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)—New Terminology in Pediatric Patients as a Step in Good Scientific Direction? J. Clin. Med. 2021, 10, 924. [Google Scholar] [CrossRef]
- Drescher, H.K.; Weiskirchen, S.; Weiskirchen, R. Current Status in Testing for Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH). Cells 2019, 8, 845. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.; Tacke, F.; Arrese, M.; Sharma, B.C.; Mostafa, I.; Bugianesi, E.; Wong, V.W.-S.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E.L.; Howe, L.; Jones, H.; Higgins, J.; Lawlor, D.A.; Fraser, A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0140908. [Google Scholar] [CrossRef] [Green Version]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, X.; Wang, Q.; Li, J.Z. New insight into inter-organ crosstalk contributing to the pathogenesis of non-alcoholic fatty liver disease (NAFLD). Protein Cell 2017, 9, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-L.; Chen, H.; Wang, C.-L.; Liang, L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From “two hit theory” to “multiple hit model”. World J. Gastroenterol. 2018, 24, 2974–2983. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Guo, R.; Fung, M.L.; Liong, E.C.; Tipoe, G.L. Therapeutic approaches to non-alcoholic fatty liver disease: Past achievements and future challenges. Hepatobiliary Pancreat. Dis. Int. 2013, 12, 125–135. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Singal, A.K. Emerging medical therapies for non-alcoholic fatty liver disease and for alcoholic hepatitis. Transl. Gastroenterol. Hepatol. 2019, 4, 53. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Alisi, A.; Nobili, V. Non-alcoholic fatty liver disease in children now: Lifestyle changes and pharmacologic treatments. Nutrition 2012, 28, 722–726. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef]
- Ratziu, V.; Bellentani, S.; Cortez-Pinto, H.; Day, C.; Marchesini, G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol. 2010, 53, 372–384. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Chen, S.; Ji, X.; Shen, X.; You, J.; Liang, X.; Yin, H.; Zhao, L. Current innovations in nutraceuticals and functional foods for intervention of non-alcoholic fatty liver disease. Pharmacol. Res. 2021, 166, 105517. [Google Scholar] [CrossRef]
- Bellentani, S.; Grave, R.D.; Suppini, A.; Marchesini, G. Behavior therapy for nonalcoholic fatty liver disease: The need for a multidisciplinary approach. Hepatology 2007, 47, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Berardo, C.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Vairetti, M.; Ferrigno, A. Nonalcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis: Current Issues and Future Perspectives in Preclinical and Clinical Research. Int. J. Mol. Sci. 2020, 21, 9646. [Google Scholar] [CrossRef] [PubMed]
- Salvoza, N.; Giraudi, P.J.; Tiribelli, C.; Rosso, N. Natural Compounds for Counteracting Nonalcoholic Fatty Liver Disease (NAFLD): Advantages and Limitations of the Suggested Candidates. Int. J. Mol. Sci. 2022, 23, 2764. [Google Scholar] [CrossRef] [PubMed]
- Plat, J.; Baumgartner, S.; Mensink, R.P. Mechanisms Underlying the Health Benefits of Plant Sterol and Stanol Ester Consumption. J. AOAC Int. 2015, 98, 697–700. [Google Scholar] [CrossRef]
- Ogbe, R.J.; Ochalefu, D.O.; Mafulul, S.G.; Olaniru, O.B. A review on dietary phytosterols: Their occurrence, metabolism and health benefits. Asian J. Plant Sci. Res. 2015, 5, 10–21. [Google Scholar]
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The Bioavailability and Biological Activities of Phytosterols as Modulators of Cholesterol Metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From Preclinical Evidence to Potential Clinical Applications. Front. Pharmacol. 2021, 11, 599959. [Google Scholar] [CrossRef]
- Benesch, M.; McElhaney, R.N. A comparative calorimetric study of the effects of cholesterol and the plant sterols campesterol and brassicasterol on the thermotropic phase behavior of dipalmitoylphosphatidylcholine bilayer membranes. Biochim. et Biophys. Acta (BBA)-Biomembr. 2014, 1838, 1941–1949. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.-W.; Lu, B.-Y.; Zhao, Y.-J.; Luo, J.-Y.; Hong, X. Formation of phytosterol photooxidation products: A chemical reaction mechanism for light-induced oxidation. Food Chem. 2020, 333, 127430. [Google Scholar] [CrossRef]
- Kaur, N.; Chaudhary, J.; Jain, A.; Kishore, L. Stigmasterol: A Comprehensive Review. Int. J. Pharm. Sci. Res. 2011, 2, 2259–2265. [Google Scholar]
- Babu, S.; Jayaraman, S. An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef] [PubMed]
- Cabral, C.E.; Klein, M.R.S.T. Phytosterols in the Treatment of Hypercholesterolemia and Prevention of Cardiovascular Diseases. Arq. Bras. de Cardiol. 2017, 109, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A. Fats and Fatty Oils. Ullmann’s Encycl. Ind. Chem. 2000. [Google Scholar] [CrossRef]
- Chen, J.; Li, D.; Tang, G.; Zhou, J.; Liu, W.; Bi, Y. Thermal-Oxidation Stability of Soybean Germ Phytosterols in Different Lipid Matrixes. Molecules 2020, 25, 4079. [Google Scholar] [CrossRef] [PubMed]
- Kwan, B.C.; Kronenberg, F.; Beddhu, S.; Cheung, A.K. Lipoprotein Metabolism and Lipid Management in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2007, 18, 1246–1261. [Google Scholar] [CrossRef]
- Poli, A.; Marangoni, F.; Corsini, A.; Manzato, E.; Marrocco, W.; Martini, D.; Medea, G.; Visioli, F. Phytosterols, Cholesterol Control, and Cardiovascular Disease. Nutrients 2021, 13, 2810. [Google Scholar] [CrossRef]
- Cunningham, R.P.; Sheldon, R.D.; Rector, R.S. The Emerging Role of Hepatocellular eNOS in Non-alcoholic Fatty Liver Disease Development. Front. Physiol. 2020, 11, 767. [Google Scholar] [CrossRef]
- Buca, B.R.; Tartau, L.; Rezus, C.; Filip, C.; Pinzariu, A.C.; Rezus, E.; Popa, G.E.; Panainte, A.; Lupusoru, C.E.; Bogdan, M.; et al. The Effects of Two Nitric Oxide Donors in Acute Inflammation in Rats Experimental data. Rev. Chim. 2018, 69, 2899–2903. [Google Scholar] [CrossRef]
- Plat, J.; Hendrikx, T.; Bieghs, V.; Jeurissen, M.L.J.; Walenbergh, S.M.A.; Van Gorp, P.J.; De Smet, E.; Konings, M.; Vreugdenhil, A.C.E.; Guichot, Y.D.; et al. Protective Role of Plant Sterol and Stanol Esters in Liver Inflammation: Insights from Mice and Humans. PLoS ONE 2014, 9, e110758. [Google Scholar] [CrossRef]
- Han, H.; Ma, H.; Rong, S.; Chen, L.; Shan, Z.; Xu, J.; Zhang, Y.; Liu, L. Flaxseed oil containing flaxseed oil ester of plant sterol attenuates high-fat diet-induced hepatic steatosis in apolipoprotein-E knockout mice. J. Funct. Foods 2015, 13, 169–182. [Google Scholar] [CrossRef]
- Song, L.; Qu, D.; Zhang, Q.; Jiang, J.; Zhou, H.; Jiang, R.; Li, Y.; Zhang, Y.; Yan, H. Phytosterol esters attenuate hepatic steatosis in rats with non-alcoholic fatty liver disease rats fed a high-fat diet. Sci. Rep. 2017, 7, srep41604. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Li, Y.; Qu, D.; Ouyang, P.; Ding, X.; Wu, P.; Guan, Q.; Yang, L. The regulatory effects of phytosterol esters (PSEs) on gut flora and faecal metabolites in rats with NAFLD. Food Funct. 2019, 11, 977–991. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Dai, Z.; Liu, A.B.; Huang, J.; Narsipur, N.; Guo, G.; Kong, B.; Reuhl, K.; Lu, W.; Luo, Z.; et al. Intake of stigmasterol and β-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. Biochim. et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 1274–1284. [Google Scholar] [CrossRef]
- Feng, S.; Gan, L.; Yang, C.S.; Liu, A.B.; Lu, W.; Shao, P.; Dai, Z.; Sun, P.; Luo, Z. Effects of Stigmasterol and β-Sitosterol on Nonalcoholic Fatty Liver Disease in a Mouse Model: A Lipidomic Analysis. J. Agric. Food Chem. 2018, 66, 3417–3425. [Google Scholar] [CrossRef] [PubMed]
- Gumede, N.M.; Lembede, B.W.; Nkomozepi, P.; Brooksbank, R.L.; Erlwanger, K.H.; Chivandi, E. β-Sitosterol mitigates the development of high-fructose diet-induced nonalcoholic fatty liver disease in growing male Sprague–Dawley rats. Can. J. Physiol. Pharmacol. 2020, 98, 44–50. [Google Scholar] [CrossRef]
- Gumede, N.M.; Lembede, B.W.; Brooksbank, R.L.; Erlwanger, K.H.; Chivandi, E. β-Sitosterol Shows Potential to Protect Against the Development of High-Fructose Diet-Induced Metabolic Dysfunction in Female Rats. J. Med. Food 2020, 23, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Guo, Y.; Li, X.; Shi, D.; Xue, T.; Wang, L.; Li, Y.; Zheng, M. Plant Sterol Ester of α-Linolenic Acid Attenuates Nonalcoholic Fatty Liver Disease by Rescuing the Adaption to Endoplasmic Reticulum Stress and Enhancing Mitochondrial Biogenesis. Oxidative Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahi, M.M.; Javanmardi, M.A.; Seyedian, S.S.; Haghighizadeh, M.H. Effects of Phytosterol Supplementation on Serum Levels of Lipid Profiles, Liver Enzymes, Inflammatory Markers, Adiponectin, and Leptin in Patients Affected by Nonalcoholic Fatty Liver Disease: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. J. Am. Coll. Nutr. 2018, 37, 651–658. [Google Scholar] [CrossRef]
- Chen, D.-L.; Huang, P.-H.; Chiang, C.-H.; Leu, H.-B.; Chen, J.-W.; Lin, S.-J. Phytosterols increase circulating endothelial progenitor cells and insulin-like growth factor-1 levels in patients with nonalcoholic fatty liver disease: A randomized crossover study. J. Funct. Foods 2015, 13, 148–157. [Google Scholar] [CrossRef]
- Song, L.; Zhao, X.G.; Ouyang, P.L.; Guan, Q.; Yang, L.; Peng, F.; Du, H.; Yin, F.; Yan, W.; Yu, W.J.; et al. Combined effect of n-3 fatty acids and phytosterol esters on alleviating hepatic steatosis in non-alcoholic fatty liver disease subjects: A double-blind placebo-controlled clinical trial. Br. J. Nutr. 2020, 123, 1148–1158. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Ras, R.T.; Gagliardi, A.C.; Mangili, L.C.; Trautwein, E.A.; Santos, R.D. Effects of phytosterols on markers of inflammation: A systematic review and meta-analysis. Atherosclerosis 2016, 248, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Tauriainen, M.-M.; Männistö, V.; Kaminska, D.; Vaittinen, M.; Kärjä, V.; Käkelä, P.; Venesmaa, S.; Gylling, H.; Pihlajamäki, J. Serum, liver and bile sitosterol and sitostanol in obese patients with and without NAFLD. Biosci. Rep. 2018, 38, BSR2017127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plat, J.; Baumgartner, S.; Houben, T.; Vreugdenhil, A.C.; Mensink, R.P.; Lütjohann, D.; Shiri-Sverdlov, R. Comment on Tauriainen et al.: Serum, liver and bile sitosterol and sitostanol in obese patients with and without NAFLD. Biosci. Rep. 2018, 38, BSR20180505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Xu, Y.; Nie, P.; Zhong, L.; Feng, L.; Guan, Q.; Song, L. Changes in the serum metabolomic profiles of subjects with NAFLD in response to n-3 PUFAs and phytosterol ester: A double-blind randomized controlled trial. Food Funct. 2022, 13, 5189–5201. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frasinariu, O.; Serban, R.; Trandafir, L.M.; Miron, I.; Starcea, M.; Vasiliu, I.; Alisi, A.; Temneanu, O.R. The Role of Phytosterols in Nonalcoholic Fatty Liver Disease. Nutrients 2022, 14, 2187. https://doi.org/10.3390/nu14112187
Frasinariu O, Serban R, Trandafir LM, Miron I, Starcea M, Vasiliu I, Alisi A, Temneanu OR. The Role of Phytosterols in Nonalcoholic Fatty Liver Disease. Nutrients. 2022; 14(11):2187. https://doi.org/10.3390/nu14112187
Chicago/Turabian StyleFrasinariu, Otilia, Roxana Serban, Laura Mihaela Trandafir, Ingrith Miron, Magdalena Starcea, Ioana Vasiliu, Anna Alisi, and Oana Raluca Temneanu. 2022. "The Role of Phytosterols in Nonalcoholic Fatty Liver Disease" Nutrients 14, no. 11: 2187. https://doi.org/10.3390/nu14112187
APA StyleFrasinariu, O., Serban, R., Trandafir, L. M., Miron, I., Starcea, M., Vasiliu, I., Alisi, A., & Temneanu, O. R. (2022). The Role of Phytosterols in Nonalcoholic Fatty Liver Disease. Nutrients, 14(11), 2187. https://doi.org/10.3390/nu14112187






