On the Pathogenicity of the Oral Biofilm: A Critical Review from a Biological, Evolutionary, and Nutritional Point of View
Abstract
1. Introduction
- I.
- The formation of biofilms (such as dental plaque) is a normal process in nature and applies to all species where hard surfaces are in contact with fluids (such as water or saliva).
- II.
- Since bacterial biofilms were already colonizing planet earth long before the successful coevolution of animals and humans, oral health must principally be possible even in the presence of biofilms.
- III.
- In human evolution, the principal process of biofilm formation has not changed, but rather, several harmful lifestyle factors in whole societies, such as poor diet, sedentary behavior, overweight, smoking, and chronic stress, have become prevalent.
2. Evolution of Biofilms in Nature, Animals, and Humans
3. Human Evolution, Nutrition, and the Oral Microbial Colonization
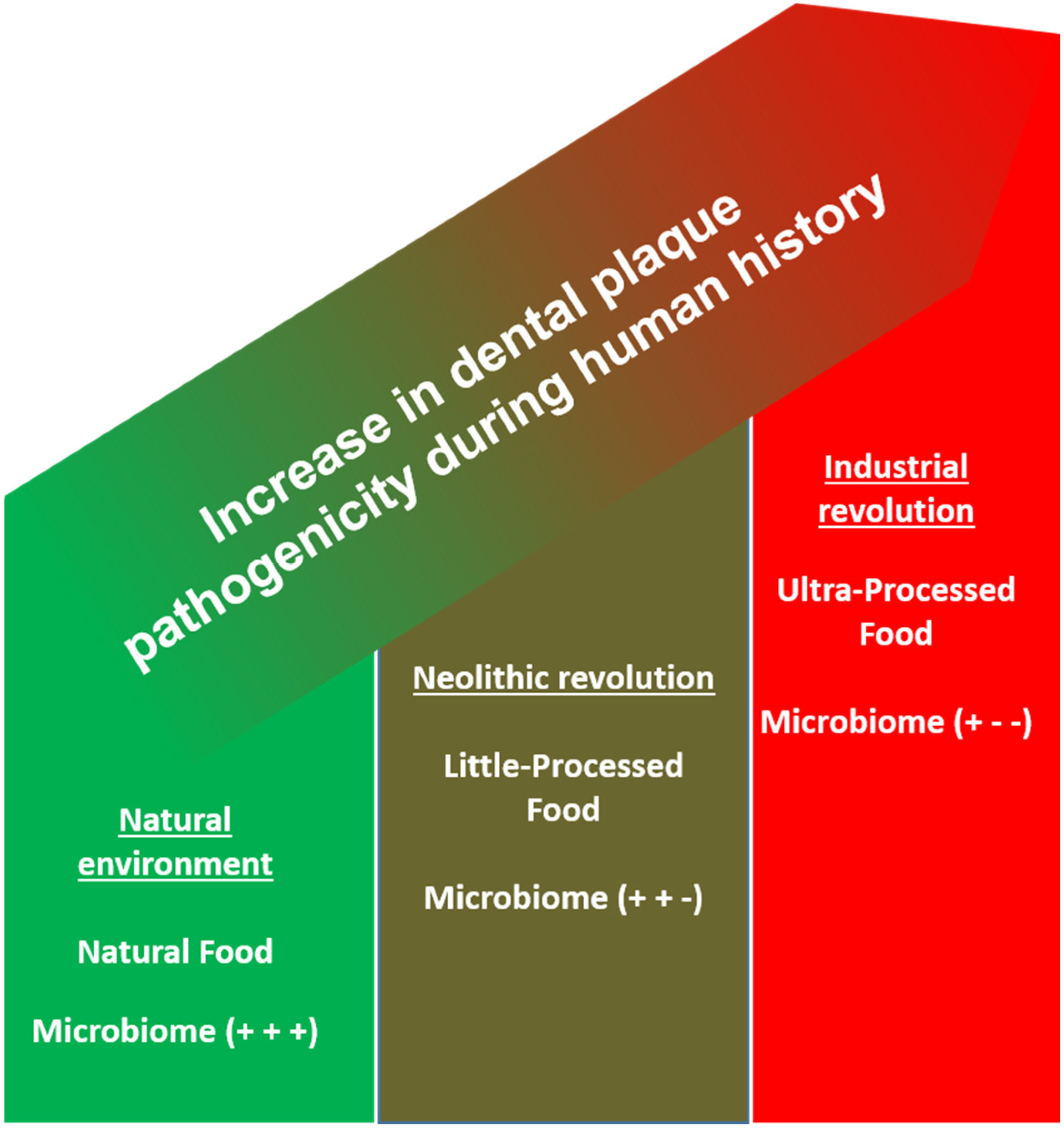
4. Neolithization and Industrial Revolution, Cultural Evolution, and the Role of Plaque in a Dramatic Change in the Nutritional Environment
5. Symptomatic and Causal Approaches against Caries and Periodontitis and Evolutionary and Lifestyle Dentistry
- -
- Dietary patterns focusing on a higher intake of non-processed (fibrous and antioxidant-rich) and an avoidance of processed foods were consistently shown to reduce gingival inflammation—even despite constant or higher plaque values [99,100,101,102]. Whole-food diets were shown to reduce periodontal pathogens without any additional mechanical therapy [103,104]. Interventional trials showed that avoiding sugar can significantly reduce the amount of S. mutans [105,106]. Low-sugar diet patterns are significantly associated with a lower caries experience [107].
- -
- -
- -
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. EFP Workshop Participants and Methodological Consultants Treatment of Stage I-III Periodontitis-The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef]
- How to Practice Good Oral Hygiene|FDI. Available online: https://www.fdiworlddental.org/how-practice-good-oral-hygiene (accessed on 3 January 2022).
- Chapple, I.L.C.; Van der Weijden, F.; Doerfer, C.; Herrera, D.; Shapira, L.; Polak, D.; Madianos, P.; Louropoulou, A.; Machtei, E.; Donos, N.; et al. Primary Prevention of Periodontitis: Managing Gingivitis. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S71–S76. [Google Scholar] [CrossRef]
- Thomassen, T.M.J.A.; Van der Weijden, F.G.A.; Slot, D.E. The Efficacy of Powered Toothbrushes: A Systematic Review and Network Meta-Analysis. Int. J. Dent. Hyg. 2021, 20, 3–17. [Google Scholar] [CrossRef]
- Yaacob, M.; Worthington, H.V.; Deacon, S.A.; Deery, C.; Walmsley, A.D.; Robinson, P.G.; Glenny, A.-M. Powered versus Manual Toothbrushing for Oral Health. Cochrane Database Syst. Rev. 2014, 2014, CD002281. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Antunes, L.; Namorado, S.; Kislaya, I.; João Santos, A.; Rodrigues, A.P.; Braz, P.; Gaio, V.; Barreto, M.; Lyshol, H.; et al. Oral Hygiene Habits in Portugal: Results from the First Health Examination Survey (INSEF 2015). Acta Odontol. Scand. 2019, 77, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.A.; Bodechtel, C.; Hertrampf, K.; Hoffmann, T.; Kocher, T.; Nitschke, I.; Schiffner, U.; Stark, H.; Zimmer, S.; Micheelis, W.; et al. The Fifth German Oral Health Study (Fünfte Deutsche Mundgesundheitsstudie, DMS V)—Rationale, Design, and Methods. BMC Oral Health 2014, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.L.; Rams, T.E.; Andersen, R.M. Socio-Behavioral Determinants of Oral Hygiene Practices among USA Ethnic and Age Groups. Adv. Dent. Res. 1997, 11, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Marcenes, W.; Kassebaum, N.J.; Bernabé, E.; Flaxman, A.; Naghavi, M.; Lopez, A.; Murray, C.J.L. Global Burden of Oral Conditions in 1990-2010: A Systematic Analysis. J. Dent. Res. 2013, 92, 592–597. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Periodontitis in 1990-2010: A Systematic Review and Meta-Regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Fischman, S.L. The History of Oral Hygiene Products: How Far Have We Come in 6000 Years? Periodontol. 2000 1997, 15, 7–14. [Google Scholar] [CrossRef]
- Hofer, K. Try Hippocrates’ Home Made Tooth Powder. Certif. Akers Lab. 1978, 42, 27–28. [Google Scholar]
- Tal, M. Periodontal Disease and Oral Hygiene. Described by Antoni van Leeuwenhoek. J. Periodontol. 1980, 51, 668–669. [Google Scholar] [CrossRef] [PubMed]
- Löe, H.; Theilade, E.; Jensen, S.B. Experimental Gingivitis in Man. J. Periodontol. 1965, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Hamp, S.; Löe, H. Plaque Induced Periodontal Disease in Beagle Dogs. A 4-Year Clinical, Roentgenographical and Histometrical Study. J. Periodontal Res. 1975, 10, 243–255. [Google Scholar] [CrossRef]
- Hamp, S.; Lindhe, J. Long Term Effect of Chlorhexidine on Developing Gingivitis in the Beagle Dog. J. Periodontal Res. 1973, 8, 63–70. [Google Scholar] [CrossRef]
- Hamp, S.; Lindhe, J.; Heyden, G. Experimental Gingivitis in the Dog. An Enzyme Histochemical Study. Arch. Oral Biol. 1972, 17, 329–336. [Google Scholar] [CrossRef]
- Von der Fehr, F.R.; Löe, H.; Theilade, E. Experimental Caries in Man. Caries Res. 1970, 4, 131–148. [Google Scholar] [CrossRef]
- Mathiesen, A.T.; Ogaard, B.; Rølla, G. Oral Hygiene as a Variable in Dental Caries Experience in 14-Year-Olds Exposed to Fluoride. Caries Res. 1996, 30, 29–33. [Google Scholar] [CrossRef]
- Hujoel, P.P.; Hujoel, M.L.A.; Kotsakis, G.A. Personal Oral Hygiene and Dental Caries: A Systematic Review of Randomised Controlled Trials. Gerodontology 2018, 35, 282–289. [Google Scholar] [CrossRef]
- Woelber, J.P.; Tennert, C. Chapter 13: Diet and Periodontal Diseases. Monogr. Oral Sci. 2020, 28, 125–133. [Google Scholar] [CrossRef]
- Hujoel, P.P.; Kato, T.; Hujoel, I.A.; Hujoel, M.L.A. Bleeding Tendency and Ascorbic Acid Requirements: Systematic Review and Meta-Analysis of Clinical Trials. Nutr. Rev. 2021, 79, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, P. Dietary Carbohydrates and Dental-Systemic Diseases. J. Dent. Res. 2009, 88, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Listl, S. What Is Health Economics? Community Dent. Health 2019, 39, 262–274. [Google Scholar] [CrossRef]
- Sheiham, A.; Watt, R.G. The Common Risk Factor Approach: A Rational Basis for Promoting Oral Health. Community Dent. Oral Epidemiol. 2000, 28, 399–406. [Google Scholar] [CrossRef]
- Javaux, E.J. Challenges in Evidencing the Earliest Traces of Life. Nature 2019, 572, 451–460. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wuertz, S. Bacteria and Archaea on Earth and Their Abundance in Biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial Biofilms in Nature and Disease. Ann. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as Complex Differentiated Communities. Ann. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Wolcott, R.; Costerton, J.W.; Raoult, D.; Cutler, S.J. The Polymicrobial Nature of Biofilm Infection. Clin. Microbiol. Infect. 2013, 19, 107–112. [Google Scholar] [CrossRef]
- Madsen, J.S.; Burmølle, M.; Hansen, L.H.; Sørensen, S.J. The Interconnection between Biofilm Formation and Horizontal Gene Transfer. FEMS Immunol. Med. Microbiol. 2012, 65, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the Human Oral Microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Moye, Z.D.; Zeng, L.; Burne, R.A. Fueling the Caries Process: Carbohydrate Metabolism and Gene Regulation by Streptococcus Mutans. J. Oral Microbiol. 2014, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Hannig, M.; Rehmer, O.; Braun, G.; Hellwig, E.; Al-Ahmad, A. Fluorescence Microscopic Visualization and Quantification of Initial Bacterial Colonization on Enamel in Situ. Arch. Oral Biol. 2007, 52, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Follo, M.; Selzer, A.-C.; Hellwig, E.; Hannig, M.; Hannig, C. Bacterial Colonization of Enamel in Situ Investigated Using Fluorescence in Situ Hybridization. J. Med. Microbiol. 2009, 58, 1359–1366. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Carvalho, C.; Lang, M.; Follo, M.; Braun, G.; Wittmer, A.; Mülhaupt, R.; Hellwig, E. Bacterial and Candida Albicans Adhesion on Rapid Prototyping-Produced 3D-Scaffolds Manufactured as Bone Replacement Materials. J. Biomed. Mater. Res. A 2008, 87, 933–943. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M. The Oral Cavity—A Key System to Understand Substratum-Dependent Bioadhesion on Solid Surfaces in Man. Clin. Oral Investig. 2009, 13, 123–139. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Xiao, J.; Klein, M.I.; Falsetta, M.L.; Lu, B.; Delahunty, C.M.; Yates, J.R.; Heydorn, A.; Koo, H. The Exopolysaccharide Matrix Modulates the Interaction between 3D Architecture and Virulence of a Mixed-Species Oral Biofilm. PLoS Pathog. 2012, 8, e1002623. [Google Scholar] [CrossRef]
- Anderson, A.C.; Rothballer, M.; Altenburger, M.J.; Woelber, J.P.; Karygianni, L.; Lagkouvardos, I.; Hellwig, E.; Al-Ahmad, A. In-Vivo Shift of the Microbiota in Oral Biofilm in Response to Frequent Sucrose Consumption. Sci. Rep. 2018, 8, 14202. [Google Scholar] [CrossRef]
- Anderson, A.C.; Rothballer, M.; Altenburger, M.J.; Woelber, J.P.; Karygianni, L.; Vach, K.; Hellwig, E.; Al-Ahmad, A. Long-Term Fluctuation of Oral Biofilm Microbiota Following Different Dietary Phases. Appl. Environ. Microbiol. 2020, 86, e01421-20. [Google Scholar] [CrossRef] [PubMed]
- Rosier, B.T.; De Jager, M.; Zaura, E.; Krom, B.P. Historical and Contemporary Hypotheses on the Development of Oral Diseases: Are We There Yet? Front. Cell Infect. Microbiol. 2014, 4, 92. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Microbial Ecology of Dental Plaque and Its Significance in Health and Disease. Adv. Dent. Res. 1994, 8, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nyvad, B. The Role of Bacteria in the Caries Process: Ecological Perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.J.; Dobney, K.; Weyrich, L.S.; Kaidonis, J.; Walker, A.W.; Haak, W.; Bradshaw, C.J.; Townsend, G.; Soltysiak, A.; Alt, K.W. Sequencing Ancient Calcified Dental Plaque Shows Changes in Oral Microbiota with Dietary Shifts of the Neolithic and Industrial Revolutions. Nat. Genet. 2013, 45, 450. [Google Scholar] [CrossRef] [PubMed]
- Sawaswong, V.; Praianantathavorn, K.; Chanchaem, P.; Khamwut, A.; Kemthong, T.; Hamada, Y.; Malaivijitnond, S.; Payungporn, S. Comparative Analysis of Oral-Gut Microbiota between Captive and Wild Long-Tailed Macaque in Thailand. Sci. Rep. 2021, 11, 14280. [Google Scholar] [CrossRef] [PubMed]
- Boehlke, C.; Rupf, S.; Tenniswood, M.; Chittur, S.V.; Hannig, C.; Zierau, O. Caries and Periodontitis Associated Bacteria Are More Abundant in Human Saliva Compared to Other Great Apes. Arch. Oral Biol. 2020, 111, 104648. [Google Scholar] [CrossRef]
- Ottoni, C.; Guellil, M.; Ozga, A.T.; Stone, A.C.; Kersten, O.; Bramanti, B.; Porcier, S.; Van Neer, W. Metagenomic Analysis of Dental Calculus in Ancient Egyptian Baboons. Sci. Rep. 2019, 9, 19637. [Google Scholar] [CrossRef]
- Richter, D.; Grün, R.; Joannes-Boyau, R.; Steele, T.E.; Amani, F.; Rué, M.; Fernandes, P.; Raynal, J.-P.; Geraads, D.; Ben-Ncer, A.; et al. The Age of the Hominin Fossils from Jebel Irhoud, Morocco, and the Origins of the Middle Stone Age. Nature 2017, 546, 293–296. [Google Scholar] [CrossRef]
- Hublin, J.-J.; Ben-Ncer, A.; Bailey, S.E.; Freidline, S.E.; Neubauer, S.; Skinner, M.M.; Bergmann, I.; Le Cabec, A.; Benazzi, S.; Harvati, K. New Fossils from Jebel Irhoud, Morocco and the Pan-African Origin of Homo Sapiens. Nature 2017, 546, 289–292. [Google Scholar] [CrossRef]
- Sansonetti, P.J. To Be or Not to Be a Pathogen: That Is the Mucosally Relevant Question. Mucosal. Immunol. 2011, 4, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Wilks, M. Bacteria and Early Human Development. Early Hum. Dev. 2007, 83, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Lif Holgerson, P.; Harnevik, L.; Hernell, O.; Tanner, A.C.R.; Johansson, I. Mode of Birth Delivery Affects Oral Microbiota in Infants. J. Dent. Res. 2011, 90, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the Human Gut Microbiome: An International Review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.B.; Tolonen, A.C.; Xavier, R.J. Human Genetic Variation and the Gut Microbiome in Disease. Nat. Rev. Genet. 2017, 18, 690–699. [Google Scholar] [CrossRef]
- Hublin, J.-J.; Richards, M.P. The Evolution of Hominin Diets: Integrating Approaches to the Study of Palaeolithic Subsistence; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Benz, M. Die Neolithisierung Im Vorderen Orient; Ex Oriente: Berlin, Germany, 2000. [Google Scholar]
- Oxilia, G.; Peresani, M.; Romandini, M.; Matteucci, C.; Spiteri, C.D.; Henry, A.G.; Schulz, D.; Archer, W.; Crezzini, J.; Boschin, F. Earliest Evidence of Dental Caries Manipulation in the Late Upper Palaeolithic. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Larsen, C.S. Bioarchaeology: Interpreting Behavior from the Human Skeleton; Cambridge University Press: Cambridge, MA, USA, 2015; Volume 69. [Google Scholar]
- Bailey, S.E.; Hublin, J.-J. Dental Perspectives on Human Evolution: State of the Art Research in Dental Paleoanthropology; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Hillson, S. Dental Pathology. In Iological Anthropology of the Human Skeleton; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 301–340. [Google Scholar]
- Gibbons, A. An Evolutionary Theory of Dentistry. Science 2012, 6084, 973–975. [Google Scholar] [CrossRef]
- Humphrey, L.T.; De Groote, I.; Morales, J.; Barton, N.; Collcutt, S.; Ramsey, C.B.; Bouzouggar, A. Earliest Evidence for Caries and Exploitation of Starchy Plant Foods in Pleistocene Hunter-Gatherers from Morocco. Proc. Natl. Acad. Sci. USA 2014, 111, 954–959. [Google Scholar] [CrossRef]
- Mateiciucová, I. Talking Stones. In The Chipped Stone Industry in Lower Austria and Moravia and the Beginnings of the Neolithic in Central Europe (LBK); Muni Press: Brno, Czech Republic, 2008; p. 5700-4900 BC. [Google Scholar]
- Gronenborn, D. Beyond the Models: Neolithisation’in Central Europe. In Proceedings of the Proceedings-British Academy; Oxford University Press Inc.: Oxford, UK, 2007; Volume 144, p. 73. [Google Scholar] [CrossRef]
- Haak, W.; Lazaridis, I.; Patterson, N.; Rohland, N.; Mallick, S.; Llamas, B.; Brandt, G.; Nordenfelt, S.; Harney, E.; Stewardson, K. Massive Migration from the Steppe Was a Source for Indo-European Languages in Europe. Nature 2015, 522, 207–211. [Google Scholar] [CrossRef]
- Lazaridis, I.; Patterson, N.; Mittnik, A.; Renaud, G.; Mallick, S.; Kirsanow, K.; Sudmant, P.H.; Schraiber, J.G.; Castellano, S.; Lipson, M. Ancient Human Genomes Suggest Three Ancestral Populations for Present-Day Europeans. Nature 2014, 513, 409–413. [Google Scholar] [CrossRef]
- Sayers, K.; Lovejoy, C.O. Blood, bulbs, and bunodonts: On evolutionary ecology and the diets of ardipithecus, australopithecus, and early homo. Q. Rev. Biol. 2014, 89, 319–357. [Google Scholar] [CrossRef]
- Cordain, L.; Miller, J.B.; Eaton, S.B.; Mann, N.; Holt, S.H.; Speth, J.D. Plant-Animal Subsistence Ratios and Macronutrient Energy Estimations in Worldwide Hunter-Gatherer Diets. Am. J. Clin. Nutr. 2000, 71, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, M.; Kruger, A.C.; Ratner, H.H. Cultural Learning. Behav. Brain. Sci. 1993, 16, 495–511. [Google Scholar] [CrossRef]
- Wrangham, R.; Conklin-Brittain, N. Cooking as a Biological Trait. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 136, 35–46. [Google Scholar] [CrossRef]
- Hancock, S.; Zinn, C.; Schofield, G. The Consumption of Processed Sugar- and Starch-Containing Foods, and Dental Caries: A Systematic Review. Eur. J. Oral Sci. 2020, 128, 467–475. [Google Scholar] [CrossRef]
- Moynihan, P.; Makino, Y.; Petersen, P.E.; Ogawa, H. Implications of WHO Guideline on Sugars for Dental Health Professionals. Community Dent. Oral Epidemiol. 2018, 46, 1–7. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and Evolution of the Western Diet: Health Implications for the 21st Century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Falbe, J.; Thompson, H.R.; Patel, A.; Madsen, K.A. Potentially Addictive Properties of Sugar-Sweetened Beverages among Adolescents. Appetite 2019, 133, 130–137. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Abraham, J.; Ali, M.K.; Alvarado, M.; Atkinson, C.; Baddour, L.M.; Bartels, D.H.; Benjamin, E.J.; Bhalla, K.; Birbeck, G. The State of US Health, 1990-2010: Burden of Diseases, Injuries, and Risk Factors. JAMA 2013, 310, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.D. From Coffeehouse to Parlour: The Consumption of Coffee, Tea and Sugar in North-Western Europe in the Seventeenth and Eighteenth Centuries. In Consuming Habits; Routledge: London, UK, 2007; ISBN 978-0-203-96411-8. [Google Scholar]
- Coe, K.; Benitez, T.; Tasevska, N.; Arriola, A.; Keller, C. The use of family rituals in eating behaviors in hispanic mothers. Fam. Community Health 2018, 41, 28–36. [Google Scholar] [CrossRef]
- Cossez, E.; Baker, P.; Mialon, M. “The Second Mother”: How the Baby Food Industry Captures Science, Health Professions and Civil Society in France. Matern. Child. Nutr. 2021, 18, e13301. [Google Scholar] [CrossRef]
- Garton, K.; Swinburn, B.; Thow, A.M. Who Influences Nutrition Policy Space Using International Trade and Investment Agreements? A Global Stakeholder Analysis. Glob. Health 2021, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Coleman, P.C.; Hanson, P.; van Rens, T.; Oyebode, O. A Rapid Review of the Evidence for Children’s TV and Online Advertisement Restrictions to Fight Obesity. Prev. Med. Rep. 2022, 26, 101717. [Google Scholar] [CrossRef] [PubMed]
- Kearns, C.E.; Glantz, S.A.; Schmidt, L.A. Sugar Industry Influence on the Scientific Agenda of the National Institute of Dental Research’s 1971 National Caries Program: A Historical Analysis of Internal Documents. PLoS Med. 2015, 12, e1001798. [Google Scholar] [CrossRef]
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.-H.; Gersch, M.S.; Benner, S.; Sánchez-Lozada, L.G. Potential Role of Sugar (Fructose) in the Epidemic of Hypertension, Obesity and the Metabolic Syndrome, Diabetes, Kidney Disease, and Cardiovascular Disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar] [CrossRef]
- Rook, G.A.W. Hygiene Hypothesis and Autoimmune Diseases. Clin. Rev. All. Immunol. 2012, 42, 5–15. [Google Scholar] [CrossRef]
- Huang, C.; Shi, G. Smoking and Microbiome in Oral, Airway, Gut and Some Systemic Diseases. J. Transl. Med. 2019, 17, 225. [Google Scholar] [CrossRef]
- Bosma-den Boer, M.M.; van Wetten, M.-L.; Pruimboom, L. Chronic Inflammatory Diseases Are Stimulated by Current Lifestyle: How Diet, Stress Levels and Medication Prevent Our Body from Recovering. Nutr. Metab. 2012, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Gianaros, P.J.; Manuck, S.B. A Stage Model of Stress and Disease. Perspect. Psychol. Sci. 2016, 11, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Nicolaidis, S. Environment and Obesity. Metabolism 2019, 100S, 153942. [Google Scholar] [CrossRef] [PubMed]
- Bullon, P.; Newman, H.N.; Battino, M. Obesity, Diabetes Mellitus, Atherosclerosis and Chronic Periodontitis: A Shared Pathology via Oxidative Stress and Mitochondrial Dysfunction? Periodontol. 2000 2014, 64, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Haber, J.; Wattles, J.; Crowley, M.; Mandell, R.; Joshipura, K.; Kent, R.L. Evidence for Cigarette Smoking as a Major Risk Factor for Periodontitis. J. Periodontol. 1993, 64, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Bernabé, E.; Vehkalahti, M.M.; Sheiham, A.; Lundqvist, A.; Suominen, A.L. The Shape of the Dose-Response Relationship between Sugars and Caries in Adults. J. Dent. Res. 2016, 95, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.; Eliasson, S. Cigarette Smoking and Alveolar Bone Height in Subjects with a High Standard of Oral Hygiene. J. Clin. Periodont. 1987, 14, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Ganten, D.; Nesse, R. The Evolution of Evolutionary Molecular Medicine: Genomics Are Transforming Evolutionary Biology into a Science with New Importance for Modern Medicine. J. Mol. Med. 2012, 90, 467–470. [Google Scholar] [CrossRef][Green Version]
- Meier, T.; Deumelandt, P.; Christen, O.; Stangl, G.I.; Riedel, K.; Langer, M. Global Burden of Sugar-Related Dental Diseases in 168 Countries and Corresponding Health Care Costs. J. Dent. Res. 2017, 96, 845–854. [Google Scholar] [CrossRef]
- Baumgartner, S.; Imfeld, T.; Schicht, O.; Rath, C.; Persson, R.E.; Persson, G.R. The Impact of the Stone Age Diet on Gingival Conditions in the Absence of Oral Hygiene. J. Periodontol. 2009, 80, 759–768. [Google Scholar] [CrossRef]
- Woelber, J.P.; Bremer, K.; Vach, K.; König, D.; Hellwig, E.; Ratka-Krüger, P.; Al-Ahmad, A.; Tennert, C. An Oral Health Optimized Diet Can Reduce Gingival and Periodontal Inflammation in Humans—A Randomized Controlled Pilot Study. BMC Oral Health 2016, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Woelber, J.P.; Gärtner, M.; Breuninger, L.; Anderson, A.; König, D.; Hellwig, E.; Al-Ahmad, A.; Vach, K.; Dötsch, A.; Ratka-Krüger, P.; et al. The Influence of an Anti-Inflammatory Diet on Gingivitis. A Randomized Controlled Trial. J. Clin. Periodontol. 2019, 46, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Bartha, V.; Exner, L.; Schweikert, D.; Woelber, J.P.; Vach, K.; Meyer, A.-L.; Basrai, M.; Bischoff, S.C.; Meller, C.; Wolff, D. Effect of the Mediterranean Diet on Gingivitis: A Randomized Controlled Trial. J. Clin. Periodontol. 2022, 49, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Jenzsch, A.; Eick, S.; Rassoul, F.; Purschwitz, R.; Jentsch, H. Nutritional Intervention in Patients with Periodontal Disease: Clinical, Immunological and Microbiological Variables during 12 Months. Br. J. Nutr. 2009, 101, 879–885. [Google Scholar] [CrossRef]
- Tennert, C.; Reinmuth, A.-C.; Bremer, K.; Al-Ahmad, A.; Karygianni, L.; Hellwig, E.; Vach, K.; Ratka-Krüger, P.; Wittmer, A.; Woelber, J.P. An Oral Health Optimized Diet Reduces the Load of Potential Cariogenic and Periodontal Bacterial Species in the Supragingival Oral Plaque: A Randomized Controlled Pilot Study. Microbiologyopen 2020, 9, e1056. [Google Scholar] [CrossRef]
- Wennerholm, K.; Birkhed, D.; Emilson, C.G. Effects of Sugar Restriction on Streptococcus Mutans and Streptococcus Sobrinus in Saliva and Dental Plaque. Caries Res. 1995, 29, 54–61. [Google Scholar] [CrossRef]
- Kristoffersson, K.; Birkhed, D. Effects of Partial Sugar Restriction for 6 Weeks on Numbers of Streptococcus Mutans in Saliva and Interdental Plaque in Man. Caries Res. 1987, 21, 79–86. [Google Scholar] [CrossRef]
- Stein, C.; Cunha-Cruz, J.; Hugo, F.N. Is Dietary Pattern a Mediator of the Relationship between Socioeconomic Status and Dental Caries? Clin. Oral Investig. 2021, 25, 5441–5447. [Google Scholar] [CrossRef]
- Bergström, J.; Eliasson, S.; Dock, J. A 10-Year Prospective Study of Tobacco Smoking and Periodontal Health. J. Periodon. 2000, 71, 1338–1347. [Google Scholar] [CrossRef]
- Ramseier, C.A.; Anerud, A.; Dulac, M.; Lulic, M.; Cullinan, M.P.; Seymour, G.J.; Faddy, M.J.; Bürgin, W.; Schätzle, M.; Lang, N.P. Natural History of Periodontitis: Disease Progression and Tooth Loss over 40 Years. J. Clin. Periodontol. 2017, 44, 1182–1191. [Google Scholar] [CrossRef]
- Omori, S.; Uchida, F.; Oh, S.; So, R.; Tsujimoto, T.; Yanagawa, T.; Sakai, S.; Shoda, J.; Tanaka, K.; Bukawa, H. Exercise Habituation Is Effective for Improvement of Periodontal Disease Status: A Prospective Intervention Study. Clin. Risk Manag. 2018, 14, 565–574. [Google Scholar] [CrossRef] [PubMed]
- de Ferreira, R.O.; Corrêa, M.G.; Magno, M.B.; Almeida, A.P.C.P.S.C.; Fagundes, N.C.F.; Rosing, C.K.; Maia, L.C.; Lima, R.R. Physical Activity Reduces the Prevalence of Periodontal Disease: Systematic Review and Meta-Analysis. Front. Physiol. 2019, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Merle, C.L.; Richter, L.; Challakh, N.; Haak, R.; Schmalz, G.; Needleman, I.; Wolfarth, B.; Ziebolz, D.; Wüstenfeld, J. Orofacial Conditions and Oral Health Behavior of Young Athletes—A Comparison of Amateur and Competitive Sports. Scand. J. Med. Sci. Sports 2022. [Google Scholar] [CrossRef] [PubMed]
- Sudhanshu, A.; Sharma, U.; Vadiraja, H.S.; Rana, R.K.; Singhal, R. Impact of Yoga on Periodontal Disease and Stress Management. Int. J. Yoga 2017, 10, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Katuri, K.K.; Dasari, A.B.; Kurapati, S.; Vinnakota, N.R.; Bollepalli, A.C.; Dhulipalla, R. Association of Yoga Practice and Serum Cortisol Levels in Chronic Periodontitis Patients with Stress-Related Anxiety and Depression. J. Int. Soc. Prev. Community Dent. 2016, 6, 7–14. [Google Scholar] [CrossRef]
- Ananthalakshmi, R.; Mahendra, J.; Jayamathi, P.; Mahendra, L.; Kareem, N.; Subramaniam, S. Effect of Sudarshan Kriya Pranayama on Periodontal Status and Human Salivary Beta-Defensin-2: An Interventional Study. Dent. Res. J. 2018, 15, 327–333. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woelber, J.P.; Al-Ahmad, A.; Alt, K.W. On the Pathogenicity of the Oral Biofilm: A Critical Review from a Biological, Evolutionary, and Nutritional Point of View. Nutrients 2022, 14, 2174. https://doi.org/10.3390/nu14102174
Woelber JP, Al-Ahmad A, Alt KW. On the Pathogenicity of the Oral Biofilm: A Critical Review from a Biological, Evolutionary, and Nutritional Point of View. Nutrients. 2022; 14(10):2174. https://doi.org/10.3390/nu14102174
Chicago/Turabian StyleWoelber, Johan Peter, Ali Al-Ahmad, and Kurt Werner Alt. 2022. "On the Pathogenicity of the Oral Biofilm: A Critical Review from a Biological, Evolutionary, and Nutritional Point of View" Nutrients 14, no. 10: 2174. https://doi.org/10.3390/nu14102174
APA StyleWoelber, J. P., Al-Ahmad, A., & Alt, K. W. (2022). On the Pathogenicity of the Oral Biofilm: A Critical Review from a Biological, Evolutionary, and Nutritional Point of View. Nutrients, 14(10), 2174. https://doi.org/10.3390/nu14102174






