The Impact of Omega-3 Supplements on Non-Surgical Periodontal Therapy: A Systematic Review
Abstract
1. Introduction
2. Methodology
2.1. Search Strategy
- Population: patients >18 years old with a diagnosis of periodontitis
- Intervention: non-surgical periodontal treatment with oral intake of omega-3 as the sole supplement
- Comparison: non-surgical periodontal treatment with or without a control (i.e., use of a placebo)
- Main outcome: periodontal probing depth (PPD)
2.2. Screening and Selection
- Randomized clinical trials
- Studies of non-surgical treatment of periodontitis with omega-3 supplementation, with or without a control treatment
- A minimum of 3 months of experimental period and supplementation length
- No language restriction
- Study length of <3 months
- Association of omega-3 with other forms of supplementation or medication
- Absence of periodontal treatment
- Absence of supplementation with omega-3
- Pilot studies
- Restricted to evaluation of gingivitis
- Use of ineligible study design
- Use of surgical periodontal therapy
2.3. Risk of Bias Assessment
2.4. Data Extraction
2.5. Data Analysis
2.6. Classification of Evidence
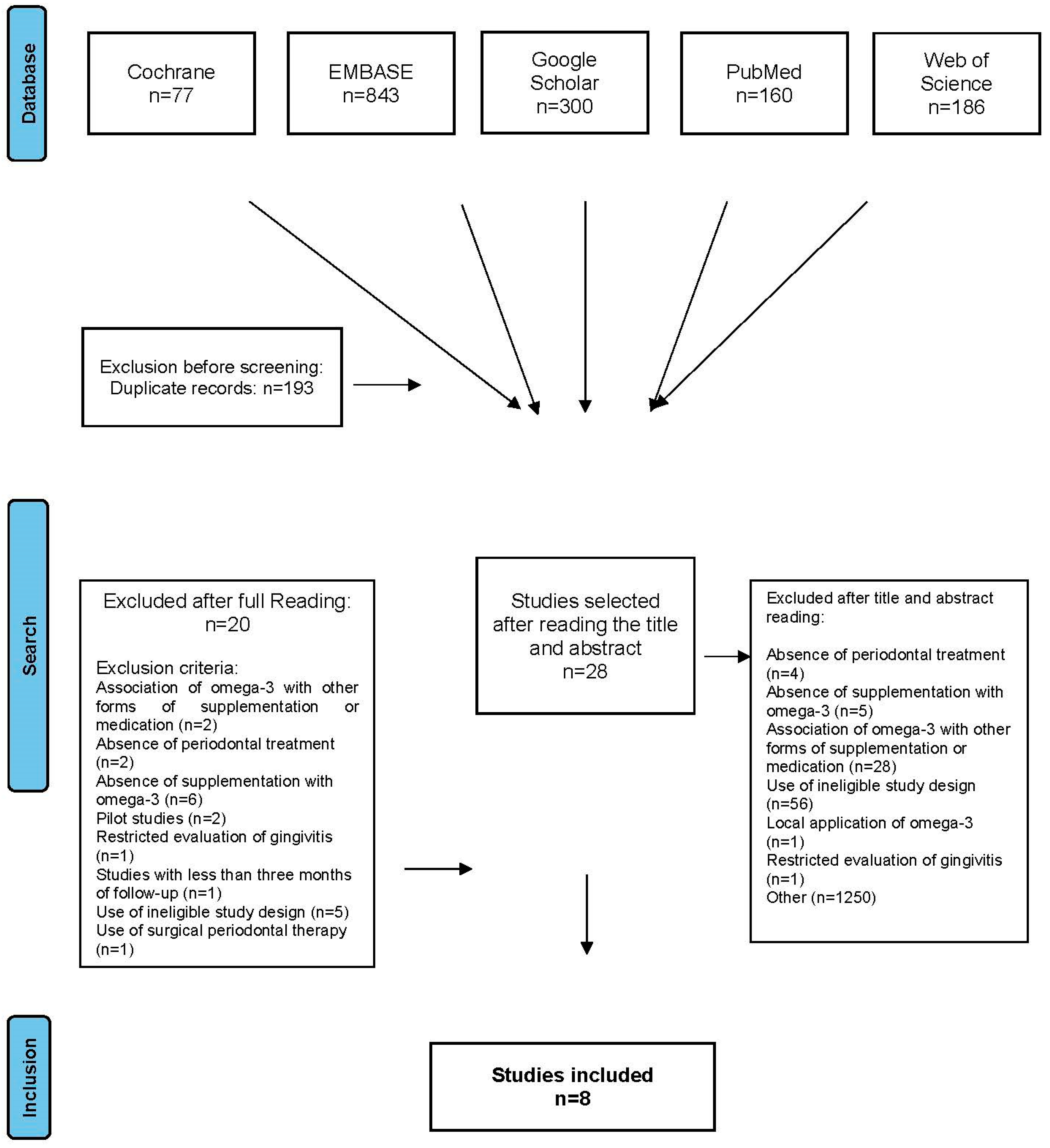
3. Results
- Absence of periodontal treatment (n = 4)
- Absence of supplementation with omega-3 (n = 5)
- Association of omega-3 with other forms of supplementation or medication (n = 28)
- Use of ineligible study design (n = 56)
- Local application of omega-3 (n = 1)
- Restricted evaluation of gingivitis (n = 1)
- Lack of connection with periodontal evaluation (n = 1250)
- Study length of <3 months (n = 1)
- Association of omega-3 with other forms of supplementation or medication (n = 2)
- Absence of periodontal treatment (n = 2)
- Absence of supplementation with omega-3 (n = 6)
- Pilot studies (n = 2)
- Restricted to evaluation of gingivitis (n = 1)
- Use of ineligible study design (n = 5)
- Use of surgical periodontal therapy (n = 1)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faust, K.; Sathirapongsasuti, F.; Izard, J.; Segata, N.; Gevers, D.; Raes, J.; Huttenhower, C. Microbial Co-occurrence Relationships in the Human Microbiome. PLoS Comput. Biol. 2012, 8, e1002606. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Head, D.A.; Devine, D.A. Ecological Approaches to Oral Biofilms: Control without Killing. Caries Res. 2015, 49, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontol. 2000 2020, 83, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lamont, R.J. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Gheewala, N.M.; O’Keefe, J.O. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J. Am. Coll. Cardiol. 2008, 51, 249–255. [Google Scholar] [CrossRef]
- Saito, T.; Shimazaki, Y. Metabolic disorders related to obesity and periodontal disease. Periodontol. 2000 2007, 43, 254–266. [Google Scholar] [CrossRef]
- Saito, T.; Yamaguchi, N.; Shimazaki, Y.; Hayashida, H.; Yonemoto, K.; Doi, Y.; Kiyohara, Y.; Iida, M.; Yamashita, Y. Serum Levels of Resistin and Adiponectin in Women with Periodontitis: The Hisayama Study. J. Dent. Res. 2008, 87, 319–322. [Google Scholar] [CrossRef]
- Dawson, D.R., 3rd; Branch-Mays, G.; Gonzalez, O.A.; Ebersole, J.L. Dietary modulation of the inflammatory cascade. Periodontol. 2000 2014, 64, 161–197. [Google Scholar] [CrossRef]
- Ball, D.W.; Hill, J.W.; Scott, R.J. The Basics of General, Organic, and Biological Chemistry, 1st ed.; Flatword: Boston, MA, USA, 2011; pp. 957–1003. [Google Scholar]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef]
- Alikhani, M.; MacLellan, C.M.; Raptis, M.; Vora, S.; Trackman, P.C.; Graves, D.T. Advanced glycation end products induce apoptosis in fibroblasts through activation of ROS, MAP kinases, and the FOXO1 transcription factor. American journal of physiology. Cell Physiol. 2007, 292, 2. [Google Scholar]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Plourde, M.; Cunnane, S.C. Extremely limited synthesis of long chain polyunsaturates in adults: Implications for their dietary essentiality and use as supplements. Appl. Physiol. Nutr. Metab. 2007, 32, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Wiktorowska-Owczarek, A.; Berezinska, M.; Nowak, J.Z. PUFAs: Structures, Metabolism and Functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Poorani, R.; Bhatt, A.N.; Dwarakanath, B.; Das, U.N. COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance. Eur. J. Pharmacol. 2016, 785, 116–132. [Google Scholar] [CrossRef]
- Nelson, J.R.; Budoff, M.J.; Wani, O.R.; Le, V.; Patel, D.K.; Nelson, A.; Nemiroff, R.L. EPA’s pleiotropic mechanisms of action: A narrative review. Postgrad. Med. 2021, 133, 651–664. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Nelson, J.R.; Raskin, S. The eicosapentaenoic acid:arachidonic acid ratio and its clinical utility in cardiovascular disease. Postgrad. Med. 2019, 131, 268–277. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- GISSI Prevezione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin e after myocardial infarction: Results of the gissi-prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar] [CrossRef]
- Raheja, B.S.; Sadikot, S.M.; Phatak, R.B.; Rao, M.B. Significance of the N-6/N-3 ratio for insulin action in diabetes. Ann. N. Y. Acad. Sci. 1993, 683, 258–271. [Google Scholar] [CrossRef] [PubMed]
- James, M.J.; Cleland, L.G. Dietary n-3 fatty acids and therapy for rheumatoid arthritis. Semin. Arthritis Rheum. 1997, 27, 85–97. [Google Scholar] [CrossRef]
- Broughton, K.S.; Johnson, C.S.; Pace, B.K.; Liebman, M.; Kleppinger, K.M. Reduced asthma symptoms with n-3 fatty acid ingestion are related to 5-series leukotriene production. Am. J. Clin. Nutr. 1997, 65, 1011–1017. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Belury, M.A.; Porter, K.; Beversdorf, D.; Lemeshow, S.; Glaser, R. Depressive Symptoms, omega-6:omega-3 Fatty Acids, and Inflammation in Older Adults. Psychosom. Med. 2007, 69, 217–224. [Google Scholar] [CrossRef]
- Maillard, V.; Bougnoux, P.; Ferrari, P.; Jourdan, M.-L.; Pinault, M.; Lavillonnière, F.; Body, G.; Le Floch, O.; Chajès, V. N-3 and N-6 fatty acids in breast adipose tissue and relative risk of breast cancer in a case-control study in Tours, France. Int. J. Cancer 2002, 98, 78–83. [Google Scholar] [CrossRef]
- Huang, C.; Ebersole, J. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol. Oral Microbiol. 2010, 25, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Vidal, H.; Sánchez, M.C.; Alonso-Español, A.; Figuero, E.; Ciudad, M.J.; Collado, L.; Herrera, D.; Sanz, M. Antimicrobial Activity of EPA and DHA against Oral Pathogenic Bacteria Using an In Vitro Multi-Species Subgingival Biofilm Model. Nutrients 2020, 12, 2812. [Google Scholar] [CrossRef]
- Kesavalu, L.; Vasudevan, B.; Raghu, B.; Browning, E.; Dawson, D.; Novak, J.M.; Correll, M.; Steffen, M.; Bhattacharya, A.; Fernandes, G.; et al. Omega-3 Fatty Acid Effect on Alveolar Bone Loss in Rats. J. Dent. Res. 2006, 85, 648–652. [Google Scholar] [CrossRef]
- Kesavalu, L.; Sathishkumar, S.; Bakthavatchalu, V.; Matthews, C.; Dawson, D.; Steffen, M.; Ebersole, J.L. Rat Model of Polymicrobial Infection, Immunity, and Alveolar Bone Resorption in Periodontal Disease. Infect. Immun. 2007, 75, 1704–1712. [Google Scholar] [CrossRef]
- Naqvi, A.Z.; Buettner, C.; Phillips, R.S.; Davis, R.B.; Mukamal, K.J. n-3 Fatty Acids and Periodontitis in US Adults. J. Am. Diet. Assoc. 2010, 110, 1669–1675. [Google Scholar] [CrossRef]
- Azzi, D.V.; Viafara, J.A.S.; Zangeronimo, M.G.; Lima, R.R.; Marques, L.S.; Pereira, L.J. n-3 Ingestion may modulate the severity of periodontal disease? Systematic review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Chatterjee, A.; Kalra, D.; Kapoor, A.; Vijay, S.; Jain, S. Role of adjunct use of omega 3 fatty acids in periodontal therapy of periodontitis. A systematic review and meta-analysis. J. Oral Biol. Craniofacial Res. 2022, 12, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.B.; Kowalski, C.D.; Leuthold, S.; Vach, K.; Ratka-Krüger, P.; Woelber, J.P. What is the impact of the adjunctive use of omega-3 fatty acids in the treatment of periodontitis? A systematic review and meta-analysis. Lipids Health Dis. 2020, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 5. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, 7. [Google Scholar] [CrossRef]
- Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group. 2019. Available online: http://www.gradeworkinggroup.org (accessed on 20 February 2022).
- Koduganti, R.R.; Rampally, P.; Ganapathi, S.N.; Panthula, V.R.; Surya, P.J. Comparison of effectiveness of low-dose aspirin versus omega-3 fatty acids as adjuvants to nonsurgical periodontal therapy in Type II diabetic patients with chronic periodontitis. J. Indian Soc. Periodontol. 2019, 23, 249–256. [Google Scholar] [CrossRef]
- Suramya, S.; Gujjari, S.K.; Ali, N. Evaluation of Efficacy of Omega 3 Fatty Acid Supplementation in Obese Patients: A Pilot Study. J. Dent. Med. Sci. 2014, 13, 49–60. [Google Scholar]
- Elgendy, E.A.; Kazem, H.H. Effect of Omega-3 Fatty Acids on Chronic Periodontitis Patients in Postmenopausal Women: A Randomised Controlled Clinical Study. Oral Heal. Prev. Dent. 2018, 16, 327–332. [Google Scholar]
- Deore, G.D.; Gurav, A.N.; Patil, R.; Shete, A.R.; NaikTari, R.S.; Inamdar, S.P. Omega 3 fatty acids as a host modulator in chronic periodontitis patients: A randomised, double-blind, palcebo-controlled, clinical trial. J. Periodontal Implant. Sci. 2014, 44, 25–32. [Google Scholar] [CrossRef]
- Keskiner, I.; Saygun, I.; Bal, V.; Serdar, M.; Kantarci, A. Dietary supplementation with low-dose omega-3 fatty acids reduces salivary tumor necrosis factor-α levels in patients with chronic periodontitis: A randomized controlled clinical study. J. Periodontal Res. 2017, 52, 695–703. [Google Scholar] [CrossRef]
- El-Sharkawy, H.M.; Elmeadawy, S.H. The impact of ômega-3 fatty acids combined with initial periodontal therapy on salivar visfatin and tnf-a levels in chronic periodontitis patients. Egypt. Dent. J. 2017, 63, 1437–1447. [Google Scholar] [CrossRef]
- Shalaby, H.K.; Morsy, S.M. Evaluation of adjunctive effect of daily dietary supplements with omega 3 and propolis to non-surgical periodontal therapy: Randomized Clinical Trial. Egypt. Dent. J. 2019, 65, 3543–3553. [Google Scholar] [CrossRef][Green Version]
- Stańdo, M.; Piatek, P.; Namiecinska, M.; Lewkowicz, P.; Lewkowicz, N. Omega-3 Polyunsaturated Fatty Acids EPA and DHA as an Adjunct to Non-Surgical Treatment of Periodontitis: A Randomized Clinical Trial. Nutrients 2020, 12, 2614. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and gradin g of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Löe, H.; Silness, J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.M.; Lamster, I.B.; Seiger, M.C. Efficacy of Listerine antiseptic in inhibiting the development of plaque and gingivitis. J. Clin. Periodontol. 1985, 12, 697–704. [Google Scholar] [CrossRef]
- Saxton, C.A.; Van Der Ouderaa, F.J. The effect of a dentifrice containing zinc citrate and Triclosan on developing gingivitis. J. Periodontal Res. 1989, 24, 75–80. [Google Scholar] [CrossRef]
- Browning, L.M.; Walker, C.G.; Mander, A.P.; West, A.L.; Madden, J.; Gambell, J.M.; Young, S.; Wang, L.; Jebb, S.A.A.; Calder, P.C. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 2012, 96, 748–758. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases; WHO Technical Report Series; no. 916; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- UK Scientific Advisory Committee on Nutrition. Advice on Fish Consumption: Benefits and Risks; UK Scientific Advisory Committee on Nutrition: London, UK, 2004.
- Cunnane, S.C. Recommendations for Intake of Polyunsaturated Fatty Acids in Healthy Adults. Int. Soc. Study Fat. Acids Lipids 2004, 11, 12–25. [Google Scholar]
- Cobb, C.M. Clinical significance of non-surgical periodontal therapy: An evidence-based perspective of scaling and root planing. J. Clin. Periodontol. 2002, 29 (Suppl. 2), 22–32. [Google Scholar] [CrossRef]
- Gomes, S.C.; Piccinin, F.B.; Susin, C.; Oppermann, R.V.; Marcantonio, R.A. Effect of Supragingival Plaque Control in Smokers and Never-Smokers: 6-Month Evaluation of Patients With Periodontitis. J. Periodontol. 2007, 78, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.C.; Romagna, R.; Rossi, V.; Corvello, P.C.; Angst, P.D.M. Supragingival treatment as an aid to reduce subgingival needs: A 450-day investigation. Braz. Oral Res. 2014, 28, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Angst, P.D.M.; Stadler, A.F.; Mendez, M.; Oppermann, R.V.; Van Der Velden, U.; Gomes, S.C. Supportive periodontal therapy in moderate-to-severe periodontitis patients: A two-year randomized clinical trial. J. Clin. Periodontol. 2019, 46, 1083–1093. [Google Scholar] [CrossRef] [PubMed]

| Database | Strategy |
|---|---|
| Cochrane | (Periodontitis OR Chronic periodontitis OR Periodontal disease OR probing pocket depth OR periodontal pocket) AND (Intervention OR Therapy OR Treatment OR Scaling and root planning OR SRP OR nonsurgical periodontal therapy OR non-surgical therapy OR Periodontal treatment OR Periodontal therapy) AND (fatty acids, omega-3 OR docosahexaenoic acids OR eicosapentaenoic acids OR fatty acids OR fish oils OR omega-3 OR ω-3 OR n-3 OR PUFA OR Long chain fatty acids) |
| Embase | #1 ‘fatty acids’ OR ‘omega-3’ OR ‘docosahexaenoic acids’ OR ‘eicosapentaenoic acids’ OR ‘fish oils’ OR ‘ω-3’ OR ‘n-3’ OR ‘pufa’ OR ‘long chain fatty acids’ #2 periodontitis OR ‘chronic periodontitis’ OR ‘periodontal disease’ OR ‘probing pocket depth’ OR ‘periodontal pocket’ #3 Intervention OR Therapy OR Treatment #4 ‘scaling and root planing’ OR ‘srp’ OR ‘nonsurgical periodontal therapy’ OR ‘non-surgical therapy’ OR ‘periodontal treatment’ OR ‘periodontal therapy’ #5 (#3 OR #4) #6 (#1 AND #2 AND #5) |
| Google Scholar | Periodontal diseases and non-surgical therapy and omega-3 |
| PubMed | #1 (fatty acids, ômega-3 [MeSH Terms]) OR (docosahexaenoic acids [Text Word]) OR (eicosapentaenoic acids [Text Word]) OR (fatty acids [Text Word]) OR (fish oils [Text Word]) OR (ômega-3 [Text Word]) OR (ω- 3 [Text Word]) OR (n-3 [Text Word]) OR (PUFA [Text Word]) OR (Long chain fatty acids [ Text Word]) #2 (periodontal diseases [ MeSH Terms]) OR (periodontitis [MeSH Terms]) OR (Chronic periodontitis [MeSH Terms]) OR (probing pocket depth [Text Word]) OR (periodontal pocket [Text Word]) #3 (Scaling and root planing [Text Word]) OR (SRP [Text Word]) OR (nonsurgical periodontal therapy [Text Word]) OR (non-surgical therapy [Text Word]) OR (Periodontal treatment [Text Word]) OR (Periodontal therapy [Text Word]) #4 (Therapy [MeSH Terms]) OR (Treatment [Text Word]) OR (Intervention [Text Word]) #5 #3 OR #4 #6 (#1) AND (#2)) AND (#5) |
| Web of Science | #1 (TS = (Fatty acids OR omega-3 OR docosahexaenoic acids OR eicosapentaenoic acids OR Fish oil OR ω-3 OR n-3 OR PUFA OR Long chain fatty acids)) #2 (TS = (Periodontitis OR Chronic periodontitis OR Periodontal disease OR probing pocket depth OR periodontal pocket)) #3 (TS = (Intervention OR Therapy OR Treatment)) #4 (TS = (Scaling and root planing OR SRP OR nonsurgical periodontal therapy OR non-surgical therapy OR Periodontal treatment OR Periodontal therapy)) #5 (#3 OR #4) #6 (#1 AND #2 AND #5) |
| Author/Year/ (Reference Number) | Sample Size | Age Years | Systemic Conditions | Periodontitis Diagnosis | Number of Teeth Present (and Mean Number of Teeth Examined) | Study Duration | Omega-3 | Capsules Intake Control | Side Effects Reported | Conflict of Interest | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | (n/Group) | Number of Sites Examined | EPA Dose per Day | DHA Dose per Day | |||||||
| Study Period | Periodontal Parameters Evaluated | ||||||||||
| Deore et al., 2014 [41] | 60 | T: 45.40 ± 40.90 | Healthy | Moderate/severe chronic periodontitis | NR (NR) | 180 mg | None | Not declared | |||
| India | (T:30/C:30) | 4 sites | 3 months | 120 mg | Yes | ||||||
| 3 months | C: 44.47 ± 5.20 | Plaque, GI, SBI, PPD, CAL | |||||||||
| Suramya et al., 2014 [39] | 40 | T + C > 30 | Obese | Generalized chronic periodontitis | At least 20 (NR) | 550 mg | None | Not declared | |||
| India | (T:20/C:20) | 6 sites | 3 months | 450 mg | Yes | ||||||
| 3 months | Plaque, GI, SBI, PPD, BOP, CAL | ||||||||||
| Keskiner et al., 2017 [42] | 30 | T: 40.87 ± 9.7 | Healthy | Chronic periodontitis | NR (T: 25.87 ± 1.19 C: 26.33 ± 1.23) | 12.5 mg | None | Not declared | |||
| Turkey | (T:15/C:15) | C: 42.54 ± 5.82 | 6 sites | 6 months | 38.38 mg | Yes | |||||
| 3 months | Plaque, GI, PPD, BOP, CAL | ||||||||||
| El-Sharkawy and Elmeadawy, 2017 [43] | 34 | T: 45.75 ± 2.05 | Healthy | Untreated advanced chronic periodontitis | At least 18 (T: 24.1 ± 3.2 C: 25.1 ± 2.1) | 2000 mg Omega-3 | None | Not declared | |||
| Egypt | (T:17/C:17) | C: 47.82 ± 2.21 | NI | 3 months | Yes | ||||||
| 3 months | Plaque, MGI, PPD, BOP, CAL | ||||||||||
| Elgendy and Kazem, 2018 [40] | 50 | T: 50.24 ± 3.04 | Post-menopause | Generalized chronic periodontitis | At least 6 (NR) | 600 mg | None | Not declared | |||
| Egypt | (T:25/C:25) | C: 51.44 ± 3.36 | 6 sites | 6 months | 400 mg | Yes | |||||
| 6 months | Plaque, GI, PPD, CAL | ||||||||||
| Rampally et al., 2019 [38] | 42 | T + C 30–65 | Diabetes II | Chronic periodontitis | At least 15 (NR) | 1000 mg | None | Not declared | |||
| India | (T:14) | NR | 3 months | No | |||||||
| 3 months | (C:14) | GI, PPD, CAL | |||||||||
| Shalaby and Morsy, 2019 [44] | 45 | T + C 35–55 | Healthy | Stage II and III, grade B periodontitis | NR (NR) | 3000 mg Omega-3 | None | Not declared | |||
| Egypt | (T1:15) (T2:15) | 6 sites | 6 months | Yes | |||||||
| 6 months | (C:15) | Plaque, GI II, PPD, CAL | |||||||||
| Stando et al., 2020 [45] | 40 | T: 45 ± 8 | Healthy | Stage III and IV periodontitis | At least 18 (NR) | Nausea and irritating fish- scented halitosis (6 subjects) | Not declared | ||||
| Poland | (T:16/C:14) | C: 54 ± 11 | 6 sites | 3 months | 2600 mg | 1800 mg | Yes | ||||
| 3 months | Plaque, PPD, BOP, CAL | ||||||||||
| Authors, Year, [Reference Number] | Groups | PPD (mm) | p-Value | Delta | CAL | p-Value | Delta | GI | p-Value | Delta | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Final | Inter-Group | PPD | Baseline | Final | Inter- Group | CAL | Baseline | Final | Inter-Group | GI | ||
| Deore et al., 2014 [41] | T | 4.26 ± 1.10 | 2.15 ± 0.53 | p < 0.05 | 2.11 | 5.53 ± 0.95 | 2.73 ± 0.98 | p < 0.05 | 2.80 | 1.93 ± 0.29 | 1.12 ± 0.14 | p < 0.01 | 0.81 |
| C | 4.05 ± 1.03 | 2.77 ± 0.47 | 1.28 | 5.20 ± 0.90 | 3.72 ± 0.62 | 1.48 | 2.04 ± 0.34 | 1.43 ± 0.33 | 0.61 | ||||
| Suramya et al., 2014 [39] | T | 5.45 ± 0.42 | 4.30 ± 0.79 | p > 0.05 | 1.15 | 5.55 ± 0.52 | 4.56 ± 0.80 | p > 0.05 | 0.99 | 2.38 ± 0.31 | 1.08 ± 0.0.16 | p > 0.05 | 1.30 |
| C | 5.50 ± 0.53 | 4.56 ± 0.80 | 0.94 | 5.42 ± 0.37 | 4.56 ± 0.80 | 0.86 | 2.31 ± 0.31 | 1.05 ± 0.12 | 1.26 | ||||
| Keskiner et al., 2017 [42] | T | 3.72 (2.23–4.75) 4 | 2.46 (1.83–3.32) | p > 0.05 | 1.26 | 4.59 (3.04–5.31) 4 | 3.53 (2.42–4.08) | p > 0.05 | 1.06 | 1.82(1.49–2.12) 4 | 1.23(0.67–1.44) | p > 0.05 | 0.59 |
| C | 3.73 (2.43–4.25) | 2.38 (2.04–3.23) | 1.35 | 4.20 (2.73–5.33) | 3.10 (2.68–4.16) | 1.10 | 1.68(1.45–1.92) | 1.18(1.06–1.34) | 0.50 | ||||
| El-Sharkawy and Elmeadawy, 2017 [43] | T | 4.76 ± 0.84 | 2.24 ± 0.27 | p < 0.001 | 2.52 | 5.16 ± 0.52 | 2.78 ± 0.38 | p < 0.001 | 2.38 | 2.32 ± 0.19 1 | 0.66 ± 0.16 | p > 0.05 | 1.66 |
| C | 4.46 ± 0.57 | 3.37 ± 0.64 | 1.09 | 5.08 ± 0.46 | 3.84 ± 0.49 | 1.24 | 2.27 ± 0.13 | 0.72 ± 0.14 | 1.55 | ||||
| Elgendy and Kazem, 2018 [40] | T | 6.00 ± 0.59 | 3.46 ± 0.49 | p < 0.05 | 2.54 | 5.96 ± 0.61 | 3.40 ± 0.50 | p < 0.05 | 2.56 | 1.98 ± 0.30 | 0.30 ± 0.23 | p < 0.05 | 1.68 |
| C | 5.84 ± 0.61 | 4.29 ± 0.75 | 1.55 | 5.79 ± 0.72 | 4.06 ± 0.59 | 1.73 | 2.06 ± 0.39 | 0.55 ± 0.32 | 1.51 | ||||
| Rampally et al., 2019 [38] | T | 6.71 ± 0.47 | 4.71 ± 0.47 | p > 0.05 | 2.00 | 5.71 ± 0.47 | 3.71 ± 0.47 | p > 0.05 | 2.00 | 2.03 ± 0.30 | 1.26 ± 0.44 | p > 0.05 | 0.77 |
| C | 6.43 ± 0.51 | 4.43 ± 0.51 | 2.00 | 5.43 ± 0.51 | 3.43 ± 0.51 | 2.00 | 1.96 ± 0.44 | 1.14 ± 0.57 | 0.82 | ||||
| Shalaby and Morsy, 2019 [44] | T | 5.47 ± 0.94 | 3.14 ± 0.64 | p < 0.01 | 2.33 | 4.08 ± 0.96 | 2.60 ± 0.52 | p < 0.01 | 1.48 | 1.95 ± 0.53 2 | 0.47 ± 0.25 | p > 0.05 | 1.48 |
| C | 5.82 ± 0.61 | 4.31 ± 0.84 | 1.51 | 4.28 ± 0.57 | 3.49 ± 0.85 | 0.79 | 2.03 ± 0.49 | 0.57 ± 0.30 | 1.46 | ||||
| Stando et al., 2020 [45] | T | 5.0 ± 0.5 | 3.7 ± 0.7 | p > 0.05 | 1.3 | 5.8 ± 0.8 | 4.4 ± 1.1 | p < 0.05 | 1.4 | 28 ± 16 3 | 14 ± 6 | p > 0.05 | 14 |
| C | 5.1 ± 0.8 | 4.0 ± 0.7 | 1.1 | 6.1 ± 1.1 | 5.3 ± 1.0 | 0.8 | 36 ± 19 | 21 ± 7 | 15 | ||||
| Participants (Studies) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Overall Certainty of Evidence | Impact |
|---|---|---|---|---|---|---|---|
| Probing depth (PPD) | |||||||
| 302 (8 RCTs) | very serious | serious | not serious | not serious | All plausible residual confounding would reduce the demonstrated effect dose response gradient | +++○ | Six studies have a high risk of bias. In general, studies report different results for PPD |
| Clinical attachment loss (CAL) | |||||||
| 302 (8 RCTs) | very serious | serious | not serious | not serious | All plausible residual confounding would reduce the demonstrated effect dose response gradient | +++○ | Six studies have a high risk of bias. In general, studies report different results for CAL |
| Gingival inflammation | |||||||
| 302 (8 RCTs) | very serious | serious | not serious | not serious | All plausible residual confounding would reduce the demonstrated effect dose response gradient | +++○ | Six studies have a high risk of bias. In general, studies report different results for gingival inflammation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, L.M.; Piccinin, F.B.; van der Velden, U.; Gomes, S.C. The Impact of Omega-3 Supplements on Non-Surgical Periodontal Therapy: A Systematic Review. Nutrients 2022, 14, 1838. https://doi.org/10.3390/nu14091838
Miller LM, Piccinin FB, van der Velden U, Gomes SC. The Impact of Omega-3 Supplements on Non-Surgical Periodontal Therapy: A Systematic Review. Nutrients. 2022; 14(9):1838. https://doi.org/10.3390/nu14091838
Chicago/Turabian StyleMiller, Luísa Martins, Flávia Benetti Piccinin, Ubele van der Velden, and Sabrina Carvalho Gomes. 2022. "The Impact of Omega-3 Supplements on Non-Surgical Periodontal Therapy: A Systematic Review" Nutrients 14, no. 9: 1838. https://doi.org/10.3390/nu14091838
APA StyleMiller, L. M., Piccinin, F. B., van der Velden, U., & Gomes, S. C. (2022). The Impact of Omega-3 Supplements on Non-Surgical Periodontal Therapy: A Systematic Review. Nutrients, 14(9), 1838. https://doi.org/10.3390/nu14091838