The Role of Dietary Intake in Type 2 Diabetes Mellitus: Importance of Macro and Micronutrients in Glucose Homeostasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Type of the Study
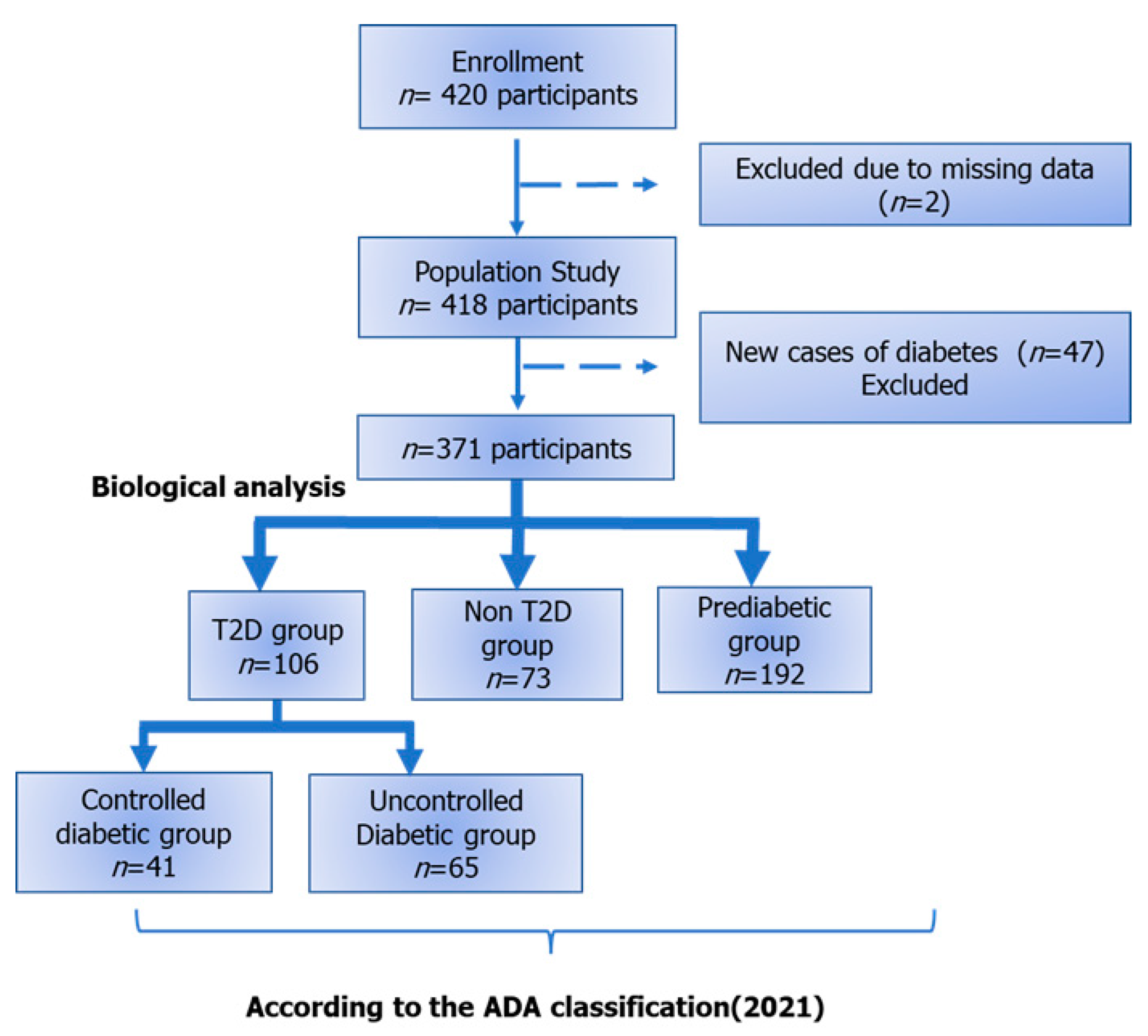
2.3. Participants
2.4. Measurements
2.4.1. Dietary Intake
2.4.2. Clinical Data and Biological Analyses
2.5. Ethics Approval and Consent to Participate
2.6. Statistical Analyses
3. Results
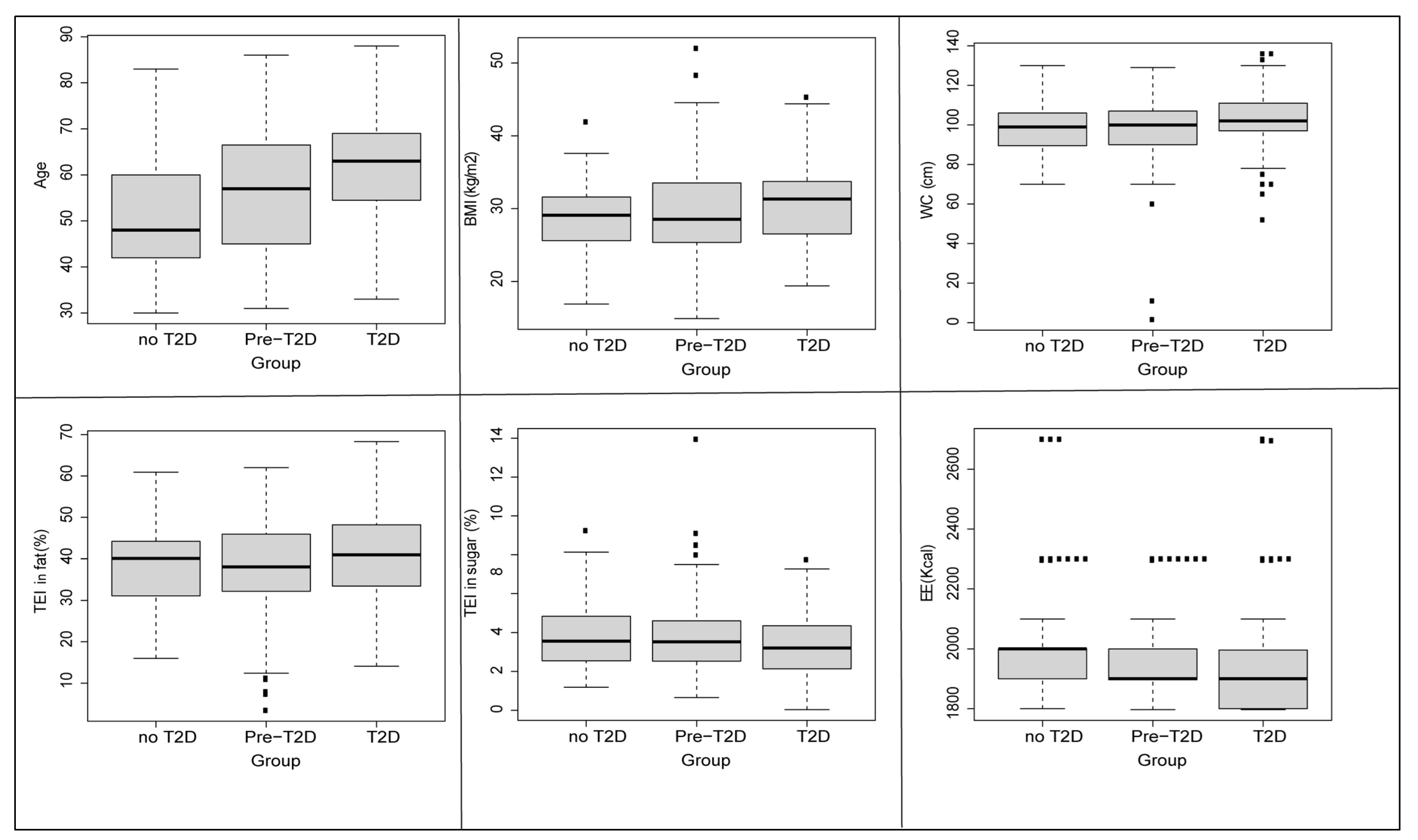
3.1. Baseline Characteristics
3.2. Differences in Macro and Micronutrients Intake between Studied Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Report on Diabetes. ISBN 2016, 978, 6–86. Available online: http://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1 (accessed on 1 March 2022).
- IDF Diabetes Atlas 10th Edition. Available online: https://www.diabetesatlas.org/data/en/ (accessed on 8 February 2022).
- Tunisian Health Examination. 2019, Ministry of Health, Tunis, Tunisia, February 2019. Available online: http://www.santetunisie.rns.tn/images/rapport-final-enquete2020.pdf (accessed on 1 March 2022).
- Jemaa, R.; Razgallah, R.; Ghorbel, I.B.; Rais, L.; Kallel, A. Prevalence of cardiovascular risk factors in the Tunisian population: The ATERA-survey. Arch. Cardiovasc. Dis. Suppl. 2020, 12, 159. [Google Scholar] [CrossRef]
- Saidi, O.; O’Flaherty, M.; Ben Mansour, N.; Aissi, W.; Lassoued, O.; Capewell, S.; A Critchley, J.; Malouche, D.; Ben Romdhane, H. Forecasting Tunisian type 2 diabetes prevalence to 2027: Validation of a simple model Biostatistics and methods. BMC Public Health 2015, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, H.; Kudoh, K. Diversity of pathophysiology in type 2 diabetes shown by islet pathology. J. Diabetes Investig. 2022, 13, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Davegårdh, C.; García-Calzón, S.; Bacos, K.; Ling, C. DNA methylation in the pathogenesis of type 2 diabetes in humans. Mol. Metab. 2018, 14, 12–25. [Google Scholar] [CrossRef]
- Ávila-Escalante, M.L.; Coop-Gamas, F.; Cervantes-Rodríguez, M.; Méndez-Iturbide, D.; Aranda-González, I.I. The effect of diet on oxidative stress and metabolic diseases—Clinically controlled trials. J. Food Biochem. 2020, 44, e13191. [Google Scholar] [CrossRef]
- Charles-Messance, H.; Mitchelson, K.A.J.; Castro, E.d.; Sheedy, F.J.; Roche, H.M. Regulating metabolic inflammation by nutritional modulation. J. Allergy Clin. Immunol. 2020, 146, 706–720. [Google Scholar] [CrossRef]
- Della Guardia, L.; Roggi, C.; Cena, H. Diet-induced acidosis and alkali supplementation. Int. J. Food Sci. Nutr. 2016, 67, 754–761. [Google Scholar] [CrossRef]
- Della Guardia, L.; Thomas, M.A.; Cena, H. Insulin sensitivity and glucose homeostasis can be influenced by metabolic acid load. Nutrients 2018, 10, 618. [Google Scholar] [CrossRef]
- Blanco-Rojo, R.; Alcala-Diaz, J.F.; Wopereis, S.; Perez-Martinez, P.; Quintana-Navarro, G.M.; Marin, C.; Ordovas, J.M.; Van Ommen, B.; Perez-Jimenez, F.; Delgado-Lista, J.; et al. The insulin resistance phenotype (muscle or liver) interacts with the type of diet to determine changes in disposition index after 2 years of intervention: The CORDIOPREV-DIAB randomised clinical trial. Diabetologia 2016, 59, 67–76. [Google Scholar] [CrossRef]
- Skytte, M.J.; Samkani, A.; Petersen, A.D.; Thomsen, M.N.; Astrup, A.; Chabanova, E.; Frystyk, J.; Holst, J.J.; Thomsen, H.S.; Madsbad, S.; et al. A carbohydrate-reduced high-protein diet improves HbA1c and liver fat content in weight stable participants with type 2 diabetes: A randomised controlled trial. Diabetologia 2019, 62, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Durán, A.M.; Beeson, W.L.; Firek, A.; Cordero-Macintyre, Z.; de León, M. Dietary Omega-3 Polyunsaturated Fatty-Acid Supplementation Upregulates Protective Cellular Pathways in Patients with Type 2 Diabetes Exhibiting Improvement in Painful Diabetic Neuropathy. Nutrients 2022, 14, 761. [Google Scholar] [CrossRef] [PubMed]
- 2021 Global Nutrition Report|The State of Global Nutrition—Global Nutrition Report. Available online: https://globalnutritionreport.org/reports/2021-global-nutrition-report/ (accessed on 31 March 2022).
- Doggui, R.; Aounallah-Skhiri, H.; Traissac, P.; el Ati, J. An overview on the nutrition transition and its health implications: Tunisia case. North Afr. J. Food Nutr. Res. 2021, 4, S75–S86. [Google Scholar] [CrossRef]
- Mahjoub, F.; Jemaa, H.b.; Sabeh, F.b.; Amor, N.B.; Gamoudi, A.; Jamoussi, H. Impact of nutrients and Mediterranean diet on the occurrence of gestational diabetes. Libyan J. Med. 2021, 16, 1930346. [Google Scholar] [CrossRef] [PubMed]
- ANSES. Les références nutritionnelles en vitamines et minéraux. 2021. Available online: https://www.anses.fr/fr/system/files/NUT2018SA0238Ra.pdf (accessed on 1 March 2022).
- ANSES. Actualisation des Références Elaboration des Repères Du PNNS: Elaboration des Références Nutritionnelles. 2016. Available online: https://www.anses.fr/fr/system/files/NUT2012SA0103Ra-1.pdf (accessed on 1 March 2022).
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body mass index: Obesity, BMI, and health: A critical review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Jacobsen, B.K.; Aars, N.A. Changes in waist circumference and the prevalence of abdominal obesity during 1994–2008—cross-sectional and longitudinal results from two surveys: The Tromsø Study. BMC Obes. 2016, 3, 41. [Google Scholar] [CrossRef]
- Li, S.J.; Wu, Y.Y.; Li, W.; Wang, S.J.; Fan, Y.M. Ultrastructural observation in a case of mucinous nevus. JDDG-J. Ger. Soc. Dermatol. 2018, 16, 778–780. [Google Scholar] [CrossRef]
- R Core Team. R A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.—References—Scientific Research Publishing. 2020. Available online: https://scirp.org/reference/referencespapers.aspx?referenceid=3064798 (accessed on 16 January 2022).
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic. Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- di Cesare, M.; Bentham, J.; Stevens, G.A.; Zhou, B.; Danaei, G.; Lu, Y.; Bixby, H.; Cowan, M.J.; Riley, L.M.; Hajifathalian, K.; et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Musaiger, A.O. Overweight and obesity in Eastern Mediterranean Region: Prevalence and possible causes. J. Obes. 2011, 2011, 407237. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef]
- Abassi, M.M.; Sassi, S.; El Ati, J.; Ben Gharbia, H.; Delpeuch, F.; Traissac, P. Gender inequalities in diet quality and their socioeconomic patterning in a nutrition transition context in the Middle East and North Africa: A cross-sectional study in Tunisia. Nutr. J. 2019, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Rock, E. Chronic diseases are first associated with the degradation and artificialization of food matrices rather than with food composition: Calorie quality matters more than calorie quantity. Eur. J. Nutr. 2022, 1–15. [Google Scholar] [CrossRef]
- Emadian, A.; Andrews, R.C.; England, C.Y.; Wallace, V.; Thompson, J.L. The effect of macronutrients on glycaemic control: A systematic review of dietary randomised controlled trials in overweight and obese adults with type 2 diabetes in which there was no difference in weight loss between treatment groups. Br. J. Nutr. 2015, 114, 1656–1666. [Google Scholar] [CrossRef]
- He, F.J.; Tan, M.; Ma, Y.; MacGregor, G.A. Salt Reduction to Prevent Hypertension and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol 2020, 75, 632–647. [Google Scholar] [CrossRef]
- Ruanpeng, D.; Thongprayoon, C.; Cheungpasitporn, W.; Harindhanavudhi, T. Sugar and artificially sweetened beverages linked to obesity: A systematic review and meta-analysis. QJM 2017, 110, 513–520. [Google Scholar] [CrossRef]
- Huang, C.; Huang, J.; Tian, Y.; Yang, X.; Gu, D. Sugar sweetened beverages consumption and risk of coronary heart disease: A meta-analysis of prospective studies. Atherosclerosis 2014, 234, 11–16. [Google Scholar] [CrossRef]
- Tucker, L.A. Macronutrient Intake and Insulin Resistance in 5665 Randomly Selected, Non-Diabetic U.S. Adults. Nutrients 2022, 14, 918. [Google Scholar] [CrossRef]
- Thakur, K.; Tomar, S.K.; Singh, A.K.; Mandal, S.; Arora, S. Riboflavin and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2017, 57, 3650–3660. [Google Scholar] [CrossRef]
- Valdés-Ramos, R.; Laura, G.-L.A.; Elina, M.-C.B.; Donají, B.-A.A. Vitamins and Type 2 Diabetes Mellitus. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. The role of cellular micronutrient analysis, nutraceuticals, vitamins, antioxidants and minerals in the prevention and treatment of hypertension and cardiovascular disease. Ther. Adv. Cardiovasc. Dis. 2010, 4, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Status, M.N.; Factors, R. Magnesium Nutritional Status, Risk Factors, and the Associations with Glucose Parameters of Childbearing Women in the China Adult Chronic Disease and Nutrition. Nutrients 2022, 14, 847. [Google Scholar]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Type 2 Diabetes Mellitus, Obesity, and Metabolic Syndrome. Nutrients 2022, 14, 714. [Google Scholar] [CrossRef]
- Kwak, J.H.; Choi, Y.H.; Paik, J.K. Vitamin D Status, Fiber Intake, and Type 2 Diabetes in U.S. Adults. J. Med. Food 2020, 23, 711–718. [Google Scholar] [CrossRef]
- Benetti, E.; Mastrocola, R.; Chiazza, F.; Nigro, D.; D’Antona, G.; Bordano, V.; Fantozzi, R.; Aragno, M.; Collino, M.; Minetto, M.A. Effects of Vitamin D on insulin resistance and myosteatosis in diet-induced obese mice. PLoS ONE 2018, 13, e0189707. [Google Scholar] [CrossRef]
- Muñoz-Garach, A.; García-Fontana, B.; Muñoz-Torres, M. Vitamin D Status, Calcium Intake and Risk of Developing Type 2 Diabetes: An Unresolved Issue. Nutrients 2019, 11, 642. [Google Scholar] [CrossRef]
- Wolden-Kirk, H.; Overbergh, L.; Christesen, H.T.; Brusgaard, K.; Mathieu, C. Vitamin D and diabetes: Its importance for beta cell and immune function. Mol. Cell. Endocrinol. 2011, 347, 106–120. [Google Scholar] [CrossRef]
- Fakhfakh, R.; Feki, S.; Elleuch, A.; Neifar, M.; Marzouk, S.; Elloumi, N.; Hachicha, H.; Abida, O.; Bahloul, Z.; Ayadi, F.; et al. Vitamin D status and CYP27B1-1260 promoter polymorphism in Tunisian patients with systemic lupus erythematosus. Mol. Genet. Genom. Med. 2021, 9, e1618. [Google Scholar] [CrossRef]
- Pfeiffer, A.F.H.; Pedersen, E.; Schwab, U.; Risérus, U.; Aas, A.-M.; Uusitupa, M.; Thanopoulou, A.; Kendall, C.; Sievenpiper, J.L.; Kahleová, H.; et al. The Effects of Different Quantities and Qualities of Protein Intake in People with Diabetes Mellitus. Nutrients 2020, 12, 365. [Google Scholar] [CrossRef] [PubMed]
- Schandelmaier, S.; Briel, M.; Saccilotto, R.; Olu, K.K.; Arpagaus, A.; Hemkens, L.G.; Nordmann, A.J. Niacin for primary and secondary prevention of cardiovascular events. Cochrane Database Syst. Rev. 2017, 6, CD009744. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.; Ward, M.; Hoey, L.; Hughes, C.F.; Pentieva, K. Addressing optimal folate and related B-vitamin status through the lifecycle: Health impacts and challenges. Proc. Nutr. Soc. 2019, 78, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, K.M.; Alam, M.M.; Iqbal, Z.; Naseem, I. Therapeutic effect of vitamin B3 on hyperglycemia, oxidative stress and DNA damage in alloxan induced diabetic rat model. Biomed. Pharmacother. 2018, 105, 1223–1231. [Google Scholar] [CrossRef]
- Gasperi, V.; Sibilano, M.; Savini, I.; Catani, M.V. Niacin in the Central Nervous System: An Update of Biological Aspects and Clinical Applications. Int. J. Mol. Sci. 2019, 20, 974. [Google Scholar] [CrossRef]
- Sharma, A.; Madan, N. Role of niacin in current clinical practice. Minerva Med. 2019, 110, 79–83. [Google Scholar] [CrossRef]
- Wang, P.P.; Dong, H.L.; Sun, H.; Pang, X.X.; Cai, C.J.; Bai, D.; Li, F.; Yang, M.Y.; Lan, X.; Zeng, G. Association between dietary vitamin A intake and gestational diabetes mellitus in the first trimester. Zhonghua Yu Fang Yi XueZaZhi 2021, 55, 1293–1298. [Google Scholar] [CrossRef]
- Plasma Vitamin A and E in Type 1 (Insulin-Dependent) and Type 2 (Non-Insulin-Dependent) Adult Diabetic Patients—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/1856044/ (accessed on 30 March 2022).
- Trasino, S.E.; Gudas, L.J. Vitamin A: A missing link in diabetes? Diabetes Manag. 2015, 5, 359. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, J.; Sun, B.; Xu, W.; Zhong, M.; Li, Y.; He, C.; Chen, Y.; Wang, X.; Jones, P.; et al. Vitamin A deficiency causes islet dysfunction by inducing islet stellate cell activation via cellular retinol binding protein 1. Int. J. Biol. Sci. 2020, 16, 947–956. [Google Scholar] [CrossRef]
- Blaner, W.S. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol. Ther. 2019, 197, 153–178. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Hu, X.; Chen, G. Vitamin A and Diabetes. J. Med. Food 2021, 24, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Xia, J.; Xue, X.; He, Q.; Ji, L.; Ding, S. Long-term treatment with nicotinamide induces glucose intolerance and skeletal muscle lipotoxicity in normal chow-fed mice: Compared to diet-induced obesity. J. Nutr. Biochem. 2016, 36, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Baudrand, R.; Lian, C.; Lian, B.; Ricchiuti, V.; Yao, T.; Li, J.; Williams, G.; Adler, G. Long-term dietary sodium restriction increases adiponectin expression and ameliorates the proinflammatory adipokine profile in obesity. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 34–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yatabe, M.S.; Yatabe, J.; Yoneda, M.; Watanabe, T.; Otsuki, M.; Felder, R.A.; A Jose, P.; Sanada, H. Salt sensitivity is associated with insulin resistance, sympathetic overactivity, and decreased suppression of circulating renin activity in lean patients with essential hypertension. Am. J. Clin. Nutr. 2010, 92, 77–82. [Google Scholar] [CrossRef]
- Baudrand, R.; Campino, C.; Carvajal, C.; Olivieri, O.; Guidi, G.; Faccini, G.; A Vöhringer, P.; Cerda, J.; Owen, G.; Kalergis, A.; et al. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin. Endocrinol. 2014, 80, 677–684. [Google Scholar] [CrossRef]
- Baqar, S.; Kong, Y.W.; Chen, A.X.; O’Callaghan, C.; MacIsaac, R.J.; Bouterakos, M.; Lambert, G.W.; Jerums, G.; E Lambert, E.; I Ekinci, E. Effect of Salt Supplementation on Sympathetic Activity and Endothelial Function in Salt-Sensitive Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2020, 105, E1187–E1200. [Google Scholar] [CrossRef]
- Baqar, S.; Michalopoulos, A.; Jerums, G.; Ekinci, E.I. Dietary sodium and potassium intake in people with diabetes: Are guidelines being met? Nutr. Diabetes 2020, 10, 23. [Google Scholar] [CrossRef]

| All n = 418 (100%) Mean ± SD | Women n = 317 (76%) Mean ± SD | MEN n = 101 (24%) Mean ± SD | p-Value | Reference Range (RDA) | |
|---|---|---|---|---|---|
| Anthropometric data | |||||
| Age | 57.14 ± 13.51 | 55.97 ± 13.05 | 60.89 ± 14.34 | 0.002 | - |
| BMI (kg/m2) | 30.03 ± 5.95 | 30.98 ± 5.93 | 27.02 ± 4.95 | <10−3 | 18.5–24.9 |
| WC (cm) | 100.80 ± 14.66 | 101.43 ± 14.72 | 98.77 ± 4.38 | 0.109 | F: 80 M: 94 |
| Life style data | |||||
| Physical activity, n (%) | Yes: 191 (45%) No: 227 (55%) | Yes:139 (33.25%) No: 178 (42.58%) | Yes: 52 (12.44%) No: 49 (11.72%) | 0.179 | - |
| Smoking habits, n (%) | Yes: 65 (16%) No: 353 (84%) | Yes: 11 (2.63%) No: 306 (73.17 | Yes: 54 (13%) No: 47 (11.2%) | <10−3 | - |
| Alcoholism status, n (%) | Yes: 2 (0.48%) No:416 (99.52%) | Yes: 2 (0.47%) No: 315 (75.35%) | Yes: 1 (0.24%) No: 100 (23.92%) | 0.709 | - |
| Energy Intake from macronutrients | |||||
| TEI (Kcal) | 1766.3 ± 729.45 | 1738.58 ± 744.19 | 1853.32 ± 677.30 | 0.149 | F: 2000 M: 2500 |
| TEI of Protein (g/Kg/day) | 0.97 ± 0.49 | 0.99 ± 0.51 | 0.92 ± 0.43 | 0.143 | 0.83–1 |
| TEI of Protein (%) | 12.450 ± 4.79 | 12.38 ± 4.91 | 12.66 ± 4.41 | 0.589 | 10–20 |
| TEI of Animal Protein (%) | 30.89 ± 7.616 | 33.16 ± 7.83 | 22.67 ± 6.95 | 0.793 | 25 |
| AP/PP | 0.7617 ± 0.99 | 0.76 ± 1.00 | 0.78 ± 0.98 | 0.873 | 0.33 |
| TEI of Fat (g/Kg/day) | 1.40 ± 0.69 | 1.42 ± 0.72 | 1.31 ± 0.60 | 0.139 | 0.7–1 |
| TEI of Fat (%) | 38.75 ± 10.82 | 38.61 ± 10.83 | 39.19 ± 10.86 | 0.640 | 33–35 |
| TEI of Saturated Fat (%) | 6.162 ± 2.65 | 6.29 ± 2.63 | 5.78 ± 2.69 | 0.148 | 12 |
| TEI of Monounsaturated Fat (%) | 17.00 ± 8.03 | 17.24 ± 8.14 | 16.25 ± 7.69 | 0.334 | 15–20 |
| TEI of Polyunsaturated Fat (%) | 7.66 ± 5.34 | 7.62 ± 5.75 | 7.778 ± 3.83 | 0.782 | 5 |
| TEI of Sugar (g/Kg/day) | 3.67 ± 0.72 | 3.79 ± 1.82 | 3.29 ± 1.30 | 0.002 | 4–5 |
| TEI of Simple Carbohydrates (%) | 11.29 ± 8.73 | 11.45 ± 7.90 | 3.29 ± 10.95 | 0.647 | <10 |
| TEI of Sugar (%) | 46.00 ± 10.00 | 46.00 ± 10.00 | 45.00 ± 10.00 | 0.467 | 50 to 55 |
| Dietary fiber (g) | 19.00 ± 13.00 | 18.00 ± 14.00 | 19.00 ± 16.00 | 0.823 | 25–30 |
| Cholesterol (mg) | 152 ± 150 | 150 ± 144 | 157 ± 168 | 0.711 | 300 |
| Energy Intake from Micronutrients (Vitamins and Minerals) | |||||
| Vitamin A (µg) | 89.00 ± 167.00 | 90.00 ± 73.00 | 87.00 ± 151.00 | 0.865 | - |
| Vitamin D (µg) | 9.00 ± 2.00 | 9.00 ± 2.94 | 9.54 ± 2.08 | 0.963 | 15 |
| Vitamin E (mg) | 18.00 ± 11.00 | 18.00 ± 10.00 | 20.00 ± 13.00 | 0.138 | F: 9 M: 10 |
| Vitamin C (mg) | 44.00 ± 37.00 | 42.00 ± 32.00 | 51.00 ± 50.00 | 0.152 | - |
| Vitamin B1 (mg) | 2.00 ± 22.00 | 2.00 ± 25.00 | 0.61 ± 0.28 | 0.315 | - |
| Vitamin B2 (Riboflavin) (mg) | 0.74 ± 0.46 | 0.75 ± 0.50 | 0.71 ± 0.33 | 0.476 | - |
| Vitamin B3 (Niacin) (mg) | 9.1 ± 6.87 | 9.20 ± 6.9 | 8.75 ± 6.58 | 0.604 | 10 |
| Vitamin B5 (mg) | 3.00 ± 1.63 | 3.00 ± 1.69 | 3.00 ± 1.42 | 0.917 | F: 5 M: 6 |
| Vitamin B6 (mg) | 0.98 ± 0.62 | 0.98 ± 0.64 | 0.97 ± 0.56 | 0.821 | - |
| Vitamin B9 (Folate) (µg) | 167 ± 167 | 166 ± 182 | 171 ± 110 | 0.758 | - |
| Vitamin B12(µg) | 2.00 ± 0.900 | 2.00 ± 1.00 | 2.00 ± 1.00 | 0.171 | 4 |
| Magnesium (mg) | 196 ± 10.00 | 194 ± 116 | 201 ± 90 | 0.615 | F:300–360 M:380–420 |
| Calcium (mg) | 296 ± 194 | 296 ± 200 | 293 ± 176 | 0.897 | - |
| Phosphorus (mg) | 701 ± 381 | 700 ± 398 | 705 ± 328 | 0.899 | 550–700 |
| Potassium (mg) | 1947 ± 1149 | 1927 ± 1182 | 2008 ± 1047 | 0.568 | 3500 |
| Sodium (mg) | 7568 ± 3871 | 7492 ± 4125 | 7805 ± 2964 | 0.465 | 1500 |
| Total Iron (mg) | 6.94 ± 4.67 | 6.92 ± 4.98 | 6.98 ± 3.56 | 0.908 | - |
| Zinc (mg) | 5.00 ± 3.00 | 4.00 ± 3.00 | 5.00 ± 2.00 | 0.327 | - |
| Copper (mg) | 4.00 ± 53.00 | 3.00 ± 35.00 | 10 ± 89 | 0.452 | F: 1.5 M: 1.9 |
| Manganese (mg) | 2.00 ± 1.00 | 2.00 ± 1.00 | 2.00 ± 1.00 | 0.189 | F: 2.5 M: 2.8 |
| Iodide (µg) | 209 ± 181 | 207.00 ± 191.00 | 215 ± 148 | 0.697 | 150 |
| Selenium (µg) | 85.00 ± 62.00 | 82.00 ± 57.00 | 97.00 ± 73.00 | 0.104 | - |
| Water intake (g) | 2123 ± 425 | 2100.8 ± 434 | 2192.01 ± 390 | 0.083 | 2500 |
| Diabetic Group n = 106 (28%) | Control Group n = 73 (20%) | Prediabetic Group n = 192 (52%) | F-Value | Adjusted p-Value | |
|---|---|---|---|---|---|
| F = 72(69%) M = 33(31%) | F = 53 (73%) M = 20 (27%) | F = 158(82%) M = 34 (17%) | |||
| Anthropometric data | |||||
| Age | 61.42 | 51.36 | 56.90 | 13.13 | <10−3 |
| BMI (kg/m2) | 30.84 | 28.61 | 29.75 | 3.28 | 0.03 |
| WC (cm) | F = 104.76 M = 100.42 | F = 98.44 M = 96.25 | F = 98.61 M = 95.14 | 5.62 | <10−3 |
| Energy Intake from macronutrients | |||||
| TEI (Kcal) | 1734.08 | 1896.26 | 1737.08 | 1.44 | 0.23 |
| TEI of Protein (g/Kg/day) | 0.96 | 1.05 | 0.94 | 1.21 | 0.29 |
| TEI of Protein (%) | 12.52 | 13.04 | 12.21 | 0.78 | 0.45 |
| TEI of Animal Protein (%) | 4.43 | 12.69 | 8.39 | 1.12 | 0.32 |
| AP/PP | 0.71 | 1.07 | 0.71 | 1.64 | 0.19 |
| TEI of Fat (g/Kg/day) | 1.39 | 1.40 | 1.39 | 10−3 | 0.99 |
| TEI of Fat (%) | 41.03 | 38.09 | 37.86 | 3.09 | <0.05 |
| TEI of Saturated Fat (%) | 6.19 | 5.98 | 6.18 | 0.16 | 0.84 |
| TEI of Monounsaturated Fat (%) | 18.29 | 16.15 | 16.93 | 1.35 | 0.26 |
| TEI of Polyunsaturated Fat (%) | 7.83 | 8.26 | 7.51 | 0.39 | 0.67 |
| TEI of Sugar (g/Kg/day) | 3.39 | 3.86 | 3.77 | 2.39 | 0.09 |
| TEI of Simple Carbohydrates (%) | 10.28 | 11.67 | 12.02 | 1.01 | 0.36 |
| TEI of Sugar (%) | 43.75 | 46.24 | 47.31 | 4.09 | <0.05 |
| Dietary fiber (g) | 20.20 | 20.60 | 17.96 | 1.02 | 0.36 |
| Cholesterol (mg) | 155.63 | 204.22 | 134.53 | 3.81 | 0.02 |
| Energy Intake from Micronutrients (Vitamins and Minerals) | |||||
| Vitamin A (µg) | 101.67 | 85.14 | 90.57 | 0.17 | 0.84 |
| Vitamin D (µg) | 9.46 | 10.58 | 9.22 | 5.34 | <10−3 |
| Vitamin E (mg) | 20.62 | 19.05 | 17.66 | 1.75 | 0.17 |
| Vitamin C (mg) | 46.69 | 53.33 | 40.67 | 2.51 | 0.08 |
| Vitamin B1 (mg) | 5.31 | 0.72 | 0.57 | 1.20 | 0.30 |
| Vitamin B2 (Riboflavin) (mg) | 0.82 | 0.88 | 0.65 | 5.74 | <10−3 |
| Vitamin B3 (Niacin) (mg) | 9.32 | 11.72 | 8.24 | 3.73 | 0.02 |
| Vitamin B5 (mg) | 3.31 | 3.61 | 2.93 | 4.23 | 0.01 |
| Vitamin B6 (mg) | 0.99 | 1.17 | 0.91 | 2.43 | 0.09 |
| Vitamin B9 (Folate) (µg) | 178.43 | 214.28 | 147.44 | 2.87 | 0.06 |
| Vitamin B12 (µg) | 3.95 | 2.58 | 1.50 | 1.47 | 0.23 |
| Magnesium (mg) | 206.44 | 235.37 | 179.36 | 4.30 | 0.01 |
| Calcium (mg) | 228.06 | 327.64 | 270.92 | 2.99 | 0.05 |
| Phosphorus (mg) | 719.51 | 841.70 | 650.75 | 3.60 | 0.03 |
| Potassium (mg) | 2059.51 | 2344.87 | 1777.97 | 4.17 | 0.01 |
| Sodium (mg) | 7928.18 | 7779.67 | 7338.97 | 0.70 | 0.49 |
| Total Iron (mg) | 7.36 | 8.25 | 6.29 | 3.37 | 0.03 |
| Zinc (mg) | 5.62 | 6.03 | 4.41 | 5.27 | <10−3 |
| Copper (mg) | 10.03 | 0.84 | 4.58 | 0.95 | 0.38 |
| Manganese (mg) | 2.34 | 2.42 | 2.06 | 1.60 | 0.20 |
| Iodide (µg) | 223.75 | 229.39 | 198.24 | 1.15 | 0.31 |
| Selenium (µg) | 86.91 | 99.30 | 80.96 | 1.76 | 0.17 |
| Water intake (g) | 2127.15 | 2282.41 | 2070.31 | 3.32 | 0.03 |
| For Controlled Diabetics (HbA1c ≤ 7%) n = 41 (38.67%) Mean ± SEM | For Uncontrolled Diabetics (HbA1c > 7%) n = 65 (61.32%) Mean ± SEM | Adjusted p-Value | |
|---|---|---|---|
| F = 30 (75%) M = 11 (25%) | F = 42 (65%) M = 23 (35%) | ||
| Anthropometric data | |||
| Age | 62.60 ± 1.96 | 60.65 ± 1.27 | 0.40 |
| BMI (kg/m2) | 30.21 ± 0.72 | 31.24 ± 0.71 | 0.31 |
| WC (cm) | 100.34 ± 2.49 | 105.27 ± 1.43 | 0.08 |
| Energy Intake from macronutrients | |||
| TEI (Kcal) | 1867.67 ± 11.56 | 1649.81 ± 80.92 | 0.11 |
| TEI of Protein (g/Kg/day) | 0.97 ± 0.07 | 0.96 ± 0.08 | 0.42 |
| TEI of Protein (%) | 12.57 ± 0.58 | 12.51 ± 0.68 | <0.05 |
| TEI of Animal Protein (%) | 3.63 ± 0.54 | 4.96 ± 0.61 | 0.15 |
| AP/PP | 0.61 ± 0.11 | 0.78 ± 0.14 | 0.42 |
| TEI of Fat (g/Kg/day) | 1.56 ± 0.15 | 1.29 ± 0.08 | 0.11 |
| TEI of Fat (%) | 41.37 ± 1.59 | 40.81 ± 1.31 | 0.78 |
| TEI of Saturated Fat (%) | 6.06 ± 0.43 | 6.27 ± 0.39 | 0.60 |
| TEI of Monounsaturated Fat (%) | 18.84 ± 1.54 | 17.93 ± 1.33 | 0.60 |
| TEI of Polyunsaturated Fat (%) | 8.78 ± 0.76 | 7.20 ± 0.55 | 0.06 |
| TEI of Sugar (g/Kg/day) | 3.64 ± 0.23 | 3.23 ± 0.20 | 0.19 |
| TEI of Simple Carbohydrates (%) | 10.26 ± 1.29 | 10.30 ± 0.87 | 0.97 |
| TEI of Sugar (%) | 44.92 ± 1.37 | 43.01 ± 1.23 | 0.30 |
| Dietary fiber (g) | 19.48 ± 1.81 | 20.68 ± 2.27 | 0.69 |
| Cholesterol (mg) | 124.88 ± 21.34 | 176.12 ± 21.36 | 0.14 |
| Energy Intake from micronutrients (Vitamins and Minerals) | |||
| Vitamin A (µg) | 101.67 ± 9.65 | 102.57 ± 34.03 | 0.14 |
| Vitamin D (µg) | 9.92 ± 0.72 | 9.15 ± 0.50 | 0.16 |
| Vitamin E (mg) | 23.54 ± 2.37 | 18.67 ± 1.74 | 0.10 |
| Vitamin C (mg) | 41.76 ± 4.64 | 49.98 ± 7.17 | 0.40 |
| Vitamin B1 (mg) | 5.31 ± 0.05 | 5.37 ± 6.12 | 0.31 |
| Vitamin B2 (Riboflavin) (mg) | 0.82 ± 0.06 | 0.83 ± 0.08 | 0.02 |
| Vitamin B3 (Niacin) (mg) | 9.32 ± 0.84 | 9.33 ± 1.12 | 0.03 |
| Vitamin B5 (mg) | 2.98 ± 0.24 | 3.53 ± 0.33 | 0.18 |
| Vitamin B6 (mg) | 0.85 ± 0.08 | 1.08 ± 0.10 | 0.08 |
| Vitamin B9 (Folate) (µg) | 164.28 ± 16.89 | 187.87 ± 19.47 | 0.38 |
| Vitamin B12 (µg) | 6.66 ± 4.23 | 2.14 ± 0.24 | 0.38 |
| Magnesium (mg) | 204.22 ± 18.43 | 207.93 ± 17.97 | 0.88 |
| Calcium (mg) | 299.26 ± 25.92 | 347.27 ± 33.16 | 0.26 |
| Phosphorus (mg) | 653.88 ± 59.13 | 763.27 ± 61.09 | 0.17 |
| Potassium (mg) | 2053.38 ± 166.19 | 2063.60 ± 186.51 | 0.96 |
| Sodium (mg) | 7928.00 ± 1183.43 | 7928.18 ± 529.97 | 0.13 |
| Total Iron (mg) | 6.63 ± 0.58 | 7.85 ± 0.85 | 0.26 |
| Zinc (mg) | 4.89 ± 0.46 | 6.11 ± 0.67 | 0.16 |
| Copper (mg) | 0.77 ± 0.06 | 16.20 ± 12.11 | 0.32 |
| Manganese (mg) | 2.36 ± 0.22 | 2.33 ± 0.26 | 0.91 |
| Iodide (µg) | 280.46 ± 53.41 | 185.95 ± 17.50 | 0.14 |
| Selenium (µg) | 101.54 ± 11.63 | 77.17 ± 7.35 | 0.09 |
| Water intake (g) | 2126.17 ± 132.80 | 2127.80 ± 126.57 | 0.98 |
| Covariates | OR (CI) 95% | p-Value |
|---|---|---|
| Energy Intake from Macronutrients | ||
| TEI (Kcal) | 1.00 (1.00–1.00) | 0.14 |
| TEI of Protein (g/Kg/day) | 0.88 (0.43–1.78) | 0.7 |
| TEI of protein (%) | 0.77 (0.52–1.10) | 0.2 |
| TEI of Animal Protein (%) | 0.97 (0.90–1.00) | 0.5 |
| AP/PP | 0.79 (0.58–1.04) | 0.11 |
| TEI of Fat (g/Kg/day) | 1.34 (0.80–2.32) | 0.3 |
| TEI of Fat (%) | 0.80 (0.55–1.13) | 0.2 |
| TEI of Saturated Fat (%) | 1.01 (0.86–1.19) | >0.9 |
| TEI of Monounsaturated Fat (%) | 1.03 (0.98–1.08) | 0.3 |
| TEI of Polyunsaturated Fat (%) | 0.99 (0.93–1.04) | 0.6 |
| TEI of Sugar (g/Kg/day) | 0.81 (0.63–1.02) | 0.07 |
| TEI of Sugar (%) | 0.77 (0.52–1.10) | 0.2 |
| TEI of Simple Carbohydrates (%) | 0.99 (0.94–1.03) | 0.6 |
| Dietary fiber (g) | 1.00 (0.98–1.02) | >0.9 |
| Cholesterol (mg) | 1.00 (1.00–1.00) | 0.05 |
| Energy Intake from Micronutrients (Vitamins and Minerals) | ||
| Vitamin A (µg) | 1.00 (1.00–1.00) | 0.5 |
| Vitamin D (µg) | 0.87 (0.73–0.98) | 0.05 |
| Vitamin E (mg) | 1.02 (0.99–1.05) | 0.3 |
| Vitamin C (mg) | 1.00 (0.99–1.01) | 0.6 |
| Vitamin B1 (mg) | 1.01 (0.99-NA) | 0.8 |
| Vitamin B2 (Riboflavin) (mg) | 0.92 (0.44–1.74) | 0.8 |
| Vitamin B3 (Niacin) (mg) | 0.95 (0.86–1.04) | 0.3 |
| Vitamin B5 (mg) | 1.09 (0.81–1.49) | 0.6 |
| Vitamin B6 (mg) | 1.04 (0.32–3.25) | 0.9 |
| Vitamin B9 (Folate) (µg) | 1.00 (1.00–1.00) | 0.9 |
| Vitamin B12 (µg) | 1.01 (0.98–1.09) | 0.6 |
| Magnesium (mg) | 1.00 (0.99–1.00) | 0.4 |
| Calcium (mg) | 1.00 (1.00–1.00) | 0.09 |
| Phosphorus (mg) | 1.00 (1.00–1.00) | 0.2 |
| Potassium (mg) | 1.00 (1.00–1.00) | 0.4 |
| Sodium (mg) | 1.00 (1.00–1.00) | 0.7 |
| Total Iron (mg) | 0.95 (0.83–1.07) | 0.4 |
| Zinc (mg) | 1.04 (0.89–1.22) | 0.7 |
| Copper (mg) | 1.00 (1.00-NA) | 0.8 |
| Manganese (mg) | 0.97 (0.76–1.20) | 0.8 |
| Iodide (µg) | 1.00 (1.00–1.00) | >0.9 |
| Selenium (µg) | 1.00 (0.99–1.00) | 0.2 |
| Water intake (g) | 1.00 (1.00–1.00) | 0.2 |
| Covariates | OR (IC) 95% | p-Value |
|---|---|---|
| Energy Intake from Macronutrients | ||
| TEI (Kcal) | 1.00 (1.00–1.00) | 0.11 |
| TEI of Protein (g/Kg/day) | 0.28 (0.08–0.78) | 0.02 |
| TEI of Protein (%) | 0.96 (0.92–1.01) | 0.9 |
| TEI of Animal Protein (%) | 0.99 (0.97–1.02) | 0.6 |
| AP/PP | 0.83 (0.46–1.30) | 0.5 |
| TEI of Fat (g/Kg/day) | 1.84 (0.95–3.75) | 0.07 |
| TEI of Fat (%) | 1.08 (0.65–1.85) | 0.8 |
| TEI of Saturated Fat (%) | 0.96(0.89–1.04) | 0.4 |
| TEI of Monounsaturated Fat (%) | 1.00 (0.96–1.03) | >0.9 |
| TEI of Polyunsaturated Fat (%) | 1.12 (1.00–1.27) | 0.06 |
| TEI of Sugar (g/Kg/day) | 1.32 (0.92–1.93) | 0.14 |
| TEI of Sugar (%) | 1.10 (0.65–1.93) | 0.7 |
| TEI of Simple Carbohydrates (%) | 1.00 (0.93–1.06) | >0.9 |
| Dietary fiber (g) | 1.04 (0.98–1.10) | 0.8 |
| Cholesterol (mg) | 1.00 (0.99–1.00) | 0.09 |
| Energy Intake from Micronutrients (Vitamins and Minerals) | ||
| Vitamin A (µg) | 0.99 (0.98–1.00) | 0.03 |
| Vitamin D (µg) | 1.27 (0.99–1.77) | 0.10 |
| Vitamin E (mg) | 1.03 (0.99–1.08) | 0.12 |
| Vitamin C (mg) | 1.00 (0.99–1.02) | >0.9 |
| Vitamin B1 (mg) | 0.99 (0.99–1.04) | >0.7 |
| Vitamin B2 (Riboflavin) (mg) | 0.28 (0.07–0.93) | 0.06 |
| Vitamin B3 (Niacin) (mg) | 0.90 (0.77–1.04) | 0.2 |
| Vitamin B5 (mg) | 1.10 (0.70–1.72) | 0.7 |
| Vitamin B6 (mg) | 1.12 (0.18–6.87) | 0.9 |
| Vitamin B9 (Folate) (µg) | 1.00 (0.99–1.00) | 0.2 |
| Vitamin B12 (µg) | 1.02 (0.99-NA) | 0.4 |
| Magnesium (mg) | 1.00 (1.00–1.01) | 0.3 |
| Calcium (mg) | 1.00 (1.00–1.00) | 0.7 |
| Phosphorus (mg) | 1.00 (1.00–1.00) | 0.09 |
| Potassium (mg) | 1.00 (1.00–1.00) | 0.09 |
| Sodium (mg) | 1.00 (1.00–1.00) | 0.03 |
| Total Iron (mg) | 0.98 (0.79–1.17) | 0.8 |
| Zinc (mg) | 0.87 (0.67–1.12) | 0.3 |
| Copper (mg) | 0.73 (0.08–0.90) | 0.8 |
| Manganese (mg) | 1.01 (1.00–1.72) | >0.9 |
| Iodide (µg) | 1.00 (1.00–1.01) | 0.2 |
| Selenium (µg) | 1.01 (1.00–1.01) | 0.12 |
| Water intake (g) | 1.00 (1.00–1.00) | >0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kheriji, N.; Boukhalfa, W.; Mahjoub, F.; Hechmi, M.; Dakhlaoui, T.; Mrad, M.; Hadj Salah Bahlous, A.; Ben Amor, N.; Jamoussi, H.; Kefi, R. The Role of Dietary Intake in Type 2 Diabetes Mellitus: Importance of Macro and Micronutrients in Glucose Homeostasis. Nutrients 2022, 14, 2132. https://doi.org/10.3390/nu14102132
Kheriji N, Boukhalfa W, Mahjoub F, Hechmi M, Dakhlaoui T, Mrad M, Hadj Salah Bahlous A, Ben Amor N, Jamoussi H, Kefi R. The Role of Dietary Intake in Type 2 Diabetes Mellitus: Importance of Macro and Micronutrients in Glucose Homeostasis. Nutrients. 2022; 14(10):2132. https://doi.org/10.3390/nu14102132
Chicago/Turabian StyleKheriji, Nadia, Wided Boukhalfa, Faten Mahjoub, Meriem Hechmi, Thouraya Dakhlaoui, Mehdi Mrad, Afef Hadj Salah Bahlous, Nadia Ben Amor, Henda Jamoussi, and Rym Kefi. 2022. "The Role of Dietary Intake in Type 2 Diabetes Mellitus: Importance of Macro and Micronutrients in Glucose Homeostasis" Nutrients 14, no. 10: 2132. https://doi.org/10.3390/nu14102132
APA StyleKheriji, N., Boukhalfa, W., Mahjoub, F., Hechmi, M., Dakhlaoui, T., Mrad, M., Hadj Salah Bahlous, A., Ben Amor, N., Jamoussi, H., & Kefi, R. (2022). The Role of Dietary Intake in Type 2 Diabetes Mellitus: Importance of Macro and Micronutrients in Glucose Homeostasis. Nutrients, 14(10), 2132. https://doi.org/10.3390/nu14102132







