Human Milk Metabolomics Are Related to Maternal Adiposity, Infant Growth Rate and Allergies: The Chinese Human Milk Project
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Chinese Human Milk Project
2.2. Sample Collection Protocol
2.3. Maternal and Infant Characteristics
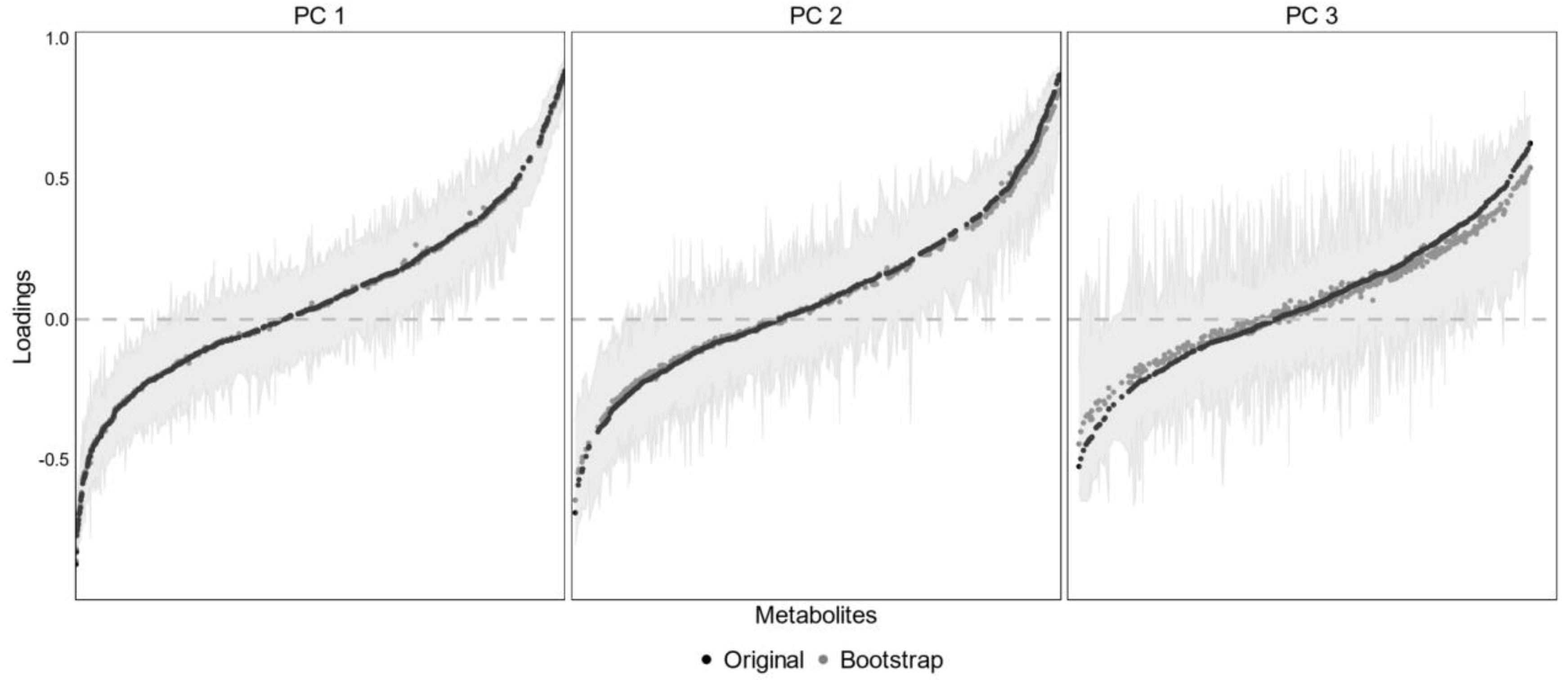
2.4. Feature Selection and Principal Component Analysis (PCA)
2.5. Statistical Analysis
3. Results
3.1. Characteristics of HM Metabotypes
3.2. Regression and Mediation Analysis
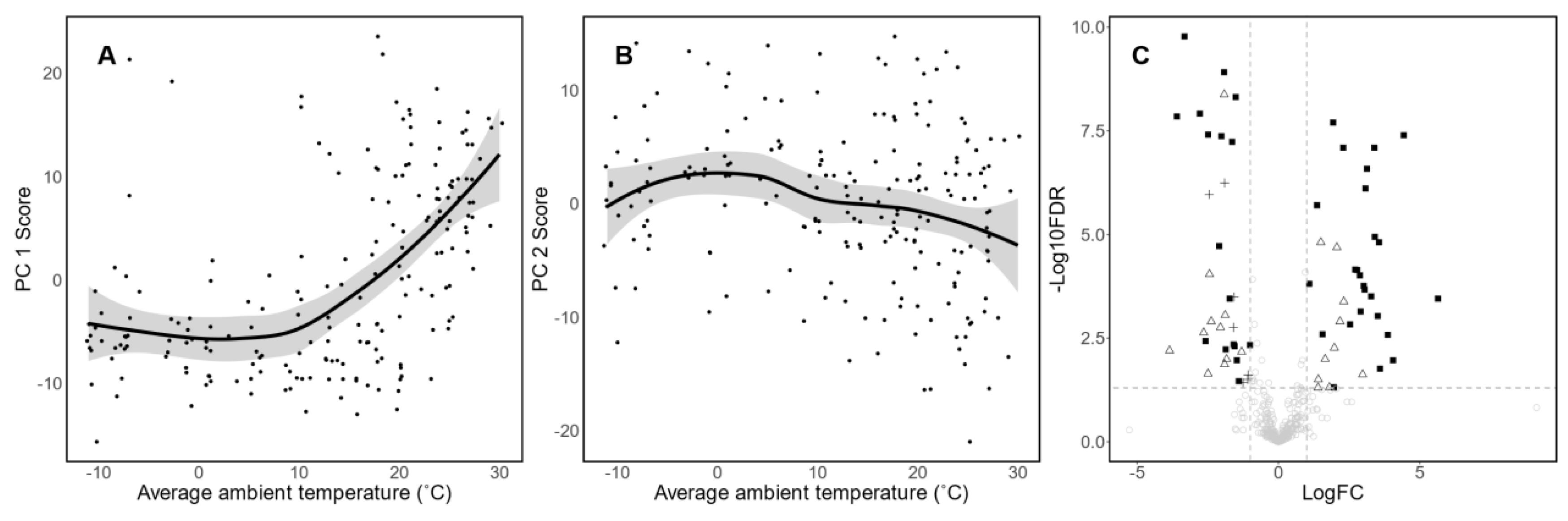
3.3. Association between Ambient Temperature, Season and Human Milk Metabolomic Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; Franca, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Martin, P.E.; Eckert, J.K.; Koplin, J.J.; Lowe, A.J.; Gurrin, L.C.; Dharmage, S.C.; Vuillermin, P.; Tang, M.L.K.; Ponsonby, A.-L.; Matheson, M.; et al. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clin. Exp. Allergy 2015, 45, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.F.; Redsell, S.A.; Swift, J.A.; Yang, M.; Glazebrook, C.P. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch. Dis. Child. 2012, 97, 1019–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.W.; Tsai, C.L.; Lu, C.Y. Exclusive breastfeeding and incident atopic dermatitis in childhood: A systematic review and meta-analysis of prospective cohort studies. Br. J. Dermatol. 2009, 161, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Isganaitis, E. Milky ways: Effects of maternal obesity on human milk composition and childhood obesity risk. Am. J. Clin. Nutr. 2021, 113, 772–774. [Google Scholar] [CrossRef]
- Zeisel, S.H. Precision (Personalized) Nutrition: Understanding Metabolic Heterogeneity. Annu. Rev. Food Sci. Technol. 2020, 11, 71–92. [Google Scholar] [CrossRef] [Green Version]
- Holmes, E.; Loo, R.L.; Stamler, J.; Bictash, M.; Elliott, P. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature 2008, 453, 396–400. [Google Scholar] [CrossRef]
- Jiang, S.; Pan, J.; Li, Y.; Ju, M.; Zhang, W.; Lu, J.; Lv, J.; Li, K. Comprehensive Human Milk Patterns Are Related To Infant Growth and Allergy in the CHMP Study. Mol. Nutr. Food Res. 2021, 65, 2170046. [Google Scholar] [CrossRef]
- Saben, J.L.; Sims, C.R.; Piccolo, B.D.; Andres, A. Maternal adiposity alters the human milk metabolome: Associations between nonglucose monosaccharides and infant adiposity. Am. J. Clin. Nutr. 2020, 112, 1228–1239. [Google Scholar] [CrossRef]
- SteinLê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- WHO. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age. Available online: https://apps.who.int/iris/handle/10665/43413 (accessed on 24 March 2022).
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 24 March 2022).
- Muthén, B.; Muthén, L.; Asparouhov, T. Regression and Mediation Analysis Using Mplus; Muthén & Muthén: Los Angeles, CA, USA, 2016. [Google Scholar]
- Worthmann, A.; John, C.; Rühlemann, M.C.; Baguhl, M.; Heinsen, F.-A.; Schaltenberg, N.; Heine, M.; Schlein, C.; Evangelakos, I.; Mineo, C.; et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat. Med. 2017, 23, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Broeders, E.P.; Nascimento, E.B.; Havekes, B.; Brans, B.; Roumans, K.H.; Tailleux, A.; Schaart, G.; Kouach, M.; Charton, J.; Deprez, B.; et al. The Bile Acid Chenodeoxycholic Acid Increases Human Brown Adipose Tissue Activity. Cell Metab. 2015, 22, 418–426. [Google Scholar] [CrossRef] [Green Version]
- Gu, Z.; Li, L.; Tang, S.; Liu, C.; Fu, X.; Shi, Z.; Mao, H. Metabolomics Reveals that Crossbred Dairy Buffaloes Are More Thermotolerant than Holstein Cows under Chronic Heat Stress. J. Agric. Food Chem. 2018, 66, 12889–12897. [Google Scholar] [CrossRef] [PubMed]
- Lynes, M.D.; Leiria, L.O.; Lundh, M.; Bartelt, A.; Shamsi, F.; Huang, T.L.; Takahashi, H.; Hirshman, M.F.; Schlein, C.; Lee, A.; et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 2017, 23, 631–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouchani, E.T.; Kajimura, S. Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab. 2019, 1, 189–200. [Google Scholar] [CrossRef]
- Bast-Habersbrunner, A.; Fromme, T. Purine Nucleotides in the Regulation of Brown Adipose Tissue Activity. Front. Endocrinol. 2020, 11, 118. [Google Scholar] [CrossRef]
- Fromme, T.; Kleigrewe, K.; Dunkel, A.; Retzler, A.; Li, Y.; Maurer, S.; Fischer, N.; Diezko, R.; Kanzleiter, T.; Hirschberg, V. Degradation of brown adipocyte purine nucleotides regulates uncoupling protein 1 activity. Mol. Metab. 2018, 8, 77–85. [Google Scholar] [CrossRef]
- Speakman, J.R.; Król, E. Maximal heat dissipation capacity and hyperthermia risk: Neglected key factors in the ecology of endotherms. J. Anim. Ecol. 2010, 79, 726–746. [Google Scholar] [CrossRef]
- Król, E.; Speakman, J.R. Limits to sustained energy intake. VII. Milk energy output in laboratory mice at thermoneutrality. J. Exp. Biol. 2003, 206, 4267–4281. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, B.P.V.B.; Lanferdini, E.; Palencia, J.Y.P.; Lemes, M.A.G.; de Abreu, M.L.T.; de Souza Cantarelli, V.; Ferreira, R.A. Heat negatively affects lactating swine: A meta-analysis. J. Therm. Biol. 2018, 74, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Król, E.; Speakman, J.R. Switching off the furnace: Brown adipose tissue and lactation. Mol. Asp. Med. 2019, 68, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.-J.; Enerbäck, S.; et al. Functional Brown Adipose Tissue in Healthy Adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef]
- Landgraf, K.; Rockstroh, D.; Wagner, I.V.; Weise, S.; Tauscher, R.; Schwartze, J.T.; Löffler, D.; Bühligen, U.; Wojan, M.; Till, H.; et al. Evidence of Early Alterations in Adipose Tissue Biology and Function and Its Association with Obesity-Related Inflammation and Insulin Resistance in Children. Diabetes 2015, 64, 1249–1261. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Dilbaz, S.; Coßmann, J.; Hoang, A.C.; Diedrich, V.; Herwig, A.; Harauma, A.; Hoshi, Y.; Moriguchi, T.; Landgraf, K.; et al. Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. J. Clin. Investig. 2019, 129, 2485–2499. [Google Scholar] [CrossRef] [Green Version]
- Bublin, M.; Eiwegger, T.; Breiteneder, H. Do lipids influence the allergic sensitization process? J. Allergy Clin. Immunol. 2014, 134, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Moreno, F.J.; Mackie, A.R.; Mills, E.N.C. Phospholipid Interactions Protect the Milk Allergen α-Lactalbumin from Proteolysis during in Vitro Digestion. J. Agric. Food Chem. 2005, 53, 9810–9816. [Google Scholar] [CrossRef]
- Bossios, A.; Theodoropoulou, M.; Mondoulet, L.; Rigby, N.M.; Papadopoulos, N.G.; Bernard, H.; Adel-Patient, K.; Wal, J.-M.; Mills, C.E.; Papageorgiou, P. Effect of simulated gastro-duodenal digestion on the allergenic reactivity of beta-lactoglobulin. Clin. Transl. Allergy 2011, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Tse, S.M.; Rifas-Shiman, S.L.; Coull, B.A.; Litonjua, A.A.; Oken, E.; Gold, D.R. Sex-Specific Risk Factors for Childhood Wheeze and Longitudinal Phenotypes of Wheeze. J. Allergy Clin. Immunol. 2016, 138, 1561–1568.e6. [Google Scholar] [CrossRef] [Green Version]
- Ballardini, N.; Kull, I.; Lind, T.; Hallner, E.; Almqvist, C.; Östblom, E.; Melén, E.; Pershagen, G.; Lilja, G.; Bergström, A.; et al. Development and Comorbidity of Eczema, Asthma and Rhinitis to Age 12—Data from the BAMSE Birth Cohort. Allergy 2012, 67, 537–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauschert, S.; Uhl, O.; Koletzko, B.; Kirchberg, F.; Mori, T.A.; Huang, R.-C.; Beilin, L.J.; Hellmuth, C.; Oddy, W.H. Lipidomics Reveals Associations of Phospholipids with Obesity and Insulin Resistance in Young Adults. J. Clin. Endocrinol. Metab. 2016, 101, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Anjos, S.; Feiteira, E.; Cerveira, F.; Melo, T.; Reboredo, A.; Colombo, S.; Dantas, R.; Costa, E.; Moreira, A.; Santos, S.; et al. Lipidomics Reveals Similar Changes in Serum Phospholipid Signatures of Overweight and Obese Pediatric Subjects. J. Proteome Res. 2019, 18, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Gerl, M.J.; Klose, C.; Surma, M.A.; Fernandez, C.; Melander, O.; Männistö, S.; Borodulin, K.; Havulinna, A.S.; Salomaa, V.; Ikonen, E.; et al. Machine learning of human plasma lipidomes for obesity estimation in a large population cohort. PLoS Biol. 2019, 17, e3000443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhu, J.; Lyu, J.; Xia, Y.; Ying, Y.; Hu, Y.; Qu, J.; Tong, S.; Li, S. Association of Maternal Prepregnancy Weight and Gestational Weight Gain with Children’s Allergic Diseases. JAMA Netw. Open 2020, 3, e2015643. [Google Scholar] [CrossRef]
- Drucker, A.M.; Pope, E.I.; Field, A.E.; Qureshi, A.A.; Dumas, O.; Camargo, C.A. Association Between Maternal Pre-Pregnancy Body Mass Index, Gestational Weight Gain, and Offspring Atopic Dermatitis: A Prospective Cohort Study. J. Allergy Clin. Immunol. Pract. 2019, 7, 96–102.e102. [Google Scholar] [CrossRef]
- Srugo, S.A.; Gaudet, L.; Corsi, D.; Fakhraei, R.; Guo, Y.; Fell, D.B. Examining the effects of pre-pregnancy weight and gestational weight gain on allergic disease development in offspring: A protocol for a population-based study using health administrative databases in Ontario, Canada. BMJ Paediatr. Open 2021, 5, e000893. [Google Scholar] [CrossRef]
| - | Total (n = 200) | |
|---|---|---|
| Maternal Characteristics | Mean | SD |
| Age (years) | 30.0 | 5.4 |
| BMI (kg/m2) | 22.8 | 2.8 |
| Gestational weight gain (kg) | 14.2 | 5.4 |
| Parity | ||
| 1 | 196 (98.5%) | |
| 2 | 3 (1.5%) | |
| City | ||
| Beijing | 27 (13.5%) | |
| Chengdu | 29 (14.5%) | |
| Guangzhou | 25 (12.5%) | |
| Jinhua | 30 (15.0%) | |
| Lanzhou | 36 (18.0%) | |
| Weihai | 20 (10.0%) | |
| Zhengzhou | 33 (16.5%) | |
| Infant characteristics | ||
| Sex (n) | ||
| Male | 114 (57.0%) | |
| Female | 86 (43.0%) | |
| Birth weight (gram) | 3361.0 | 659.1 |
| Birth length (cm) | 50.1 | 2.4 |
| Age | ||
| 2 mo | 106 (53.0%) | |
| 6 mo | 94 (47.0%) | |
| Self-reported eczema, Yes | 63 (31.5%) | |
| z Score | ||
| WAZ | 0.57 | 1.73 |
| LAZ | 0.14 | 2.09 |
| BAZ | 0.74 | 2.27 |
| WFL | 0.90 | 2.50 |
| PC 1 | PC 2 | ||||
|---|---|---|---|---|---|
| - | - | Estimates | p Value | Estimates | p Value |
| Maternal BMI (kg/m2) | Model 1 | −0.03 | 0.471 | 0.08 | 0.018 |
| Model 2 | −0.03 | 0.497 | 0.08 | 0.018 | |
| BAZ | Model 1 | −0.02 | 0.472 | 0.00 | 0.996 |
| Model 2 | −0.03 | 0.344 | −0.01 | 0.666 | |
| WAZ | Model 1 | −0.06 | 0.007 | 0.01 | 0.605 |
| Model 2 | −0.06 | 0.004 | 4.9 × 10−3 | 0.795 | |
| LAZ | Model 1 | −0.09 | 0.001 | 0.014 | 0.554 |
| Model 2 | −0.08 | 0.004 | 0.019 | 0.412 | |
| Eczema | Model 1 | 0.96 | 0.241 | 1.06 | 0.032 |
| Model 2 | 0.97 | 0.306 | 1.07 | 0.019 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Li, K.; Zheng, C.; Sun, H.; Pan, J.; Li, Y.; Liu, Y.; Wang, W.; Ju, M.; Xu, Y.; et al. Human Milk Metabolomics Are Related to Maternal Adiposity, Infant Growth Rate and Allergies: The Chinese Human Milk Project. Nutrients 2022, 14, 2097. https://doi.org/10.3390/nu14102097
Zhang W, Li K, Zheng C, Sun H, Pan J, Li Y, Liu Y, Wang W, Ju M, Xu Y, et al. Human Milk Metabolomics Are Related to Maternal Adiposity, Infant Growth Rate and Allergies: The Chinese Human Milk Project. Nutrients. 2022; 14(10):2097. https://doi.org/10.3390/nu14102097
Chicago/Turabian StyleZhang, Wei, Kaifeng Li, Chengdong Zheng, Han Sun, Jiancun Pan, Yuanyuan Li, Ying Liu, Wenqing Wang, Mengnan Ju, Yajun Xu, and et al. 2022. "Human Milk Metabolomics Are Related to Maternal Adiposity, Infant Growth Rate and Allergies: The Chinese Human Milk Project" Nutrients 14, no. 10: 2097. https://doi.org/10.3390/nu14102097
APA StyleZhang, W., Li, K., Zheng, C., Sun, H., Pan, J., Li, Y., Liu, Y., Wang, W., Ju, M., Xu, Y., & Jiang, S. (2022). Human Milk Metabolomics Are Related to Maternal Adiposity, Infant Growth Rate and Allergies: The Chinese Human Milk Project. Nutrients, 14(10), 2097. https://doi.org/10.3390/nu14102097







