Effects of Baru Almond Oil (Dipteryx alata Vog.) Treatment on Thrombotic Processes, Platelet Aggregation, and Vascular Function in Aorta Arteries
Abstract
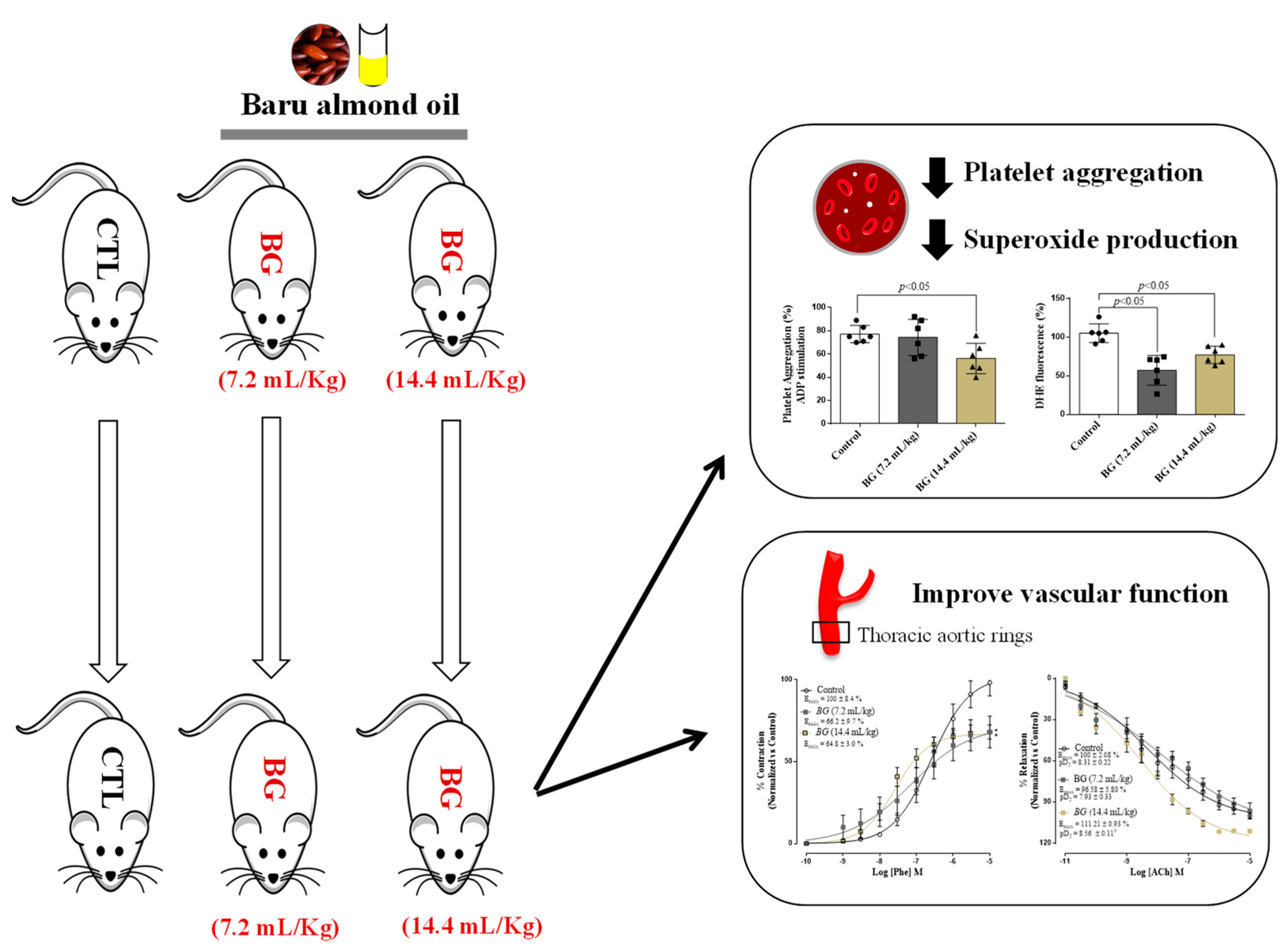
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Determination of the Serum Tocopherol Concentration
2.3. Induction of Thrombosis and Monitoring of Blood Flow
2.4. Obtaining Whole Blood and the Preparation of Platelets
2.5. Measurement of Platelet Aggregation and Superoxide Anions (O2•−) Production
2.6. Vascular Reactivity
2.7. Statistical Analysis
3. Results
3.1. Tocopherol Measurements in Baru Oil
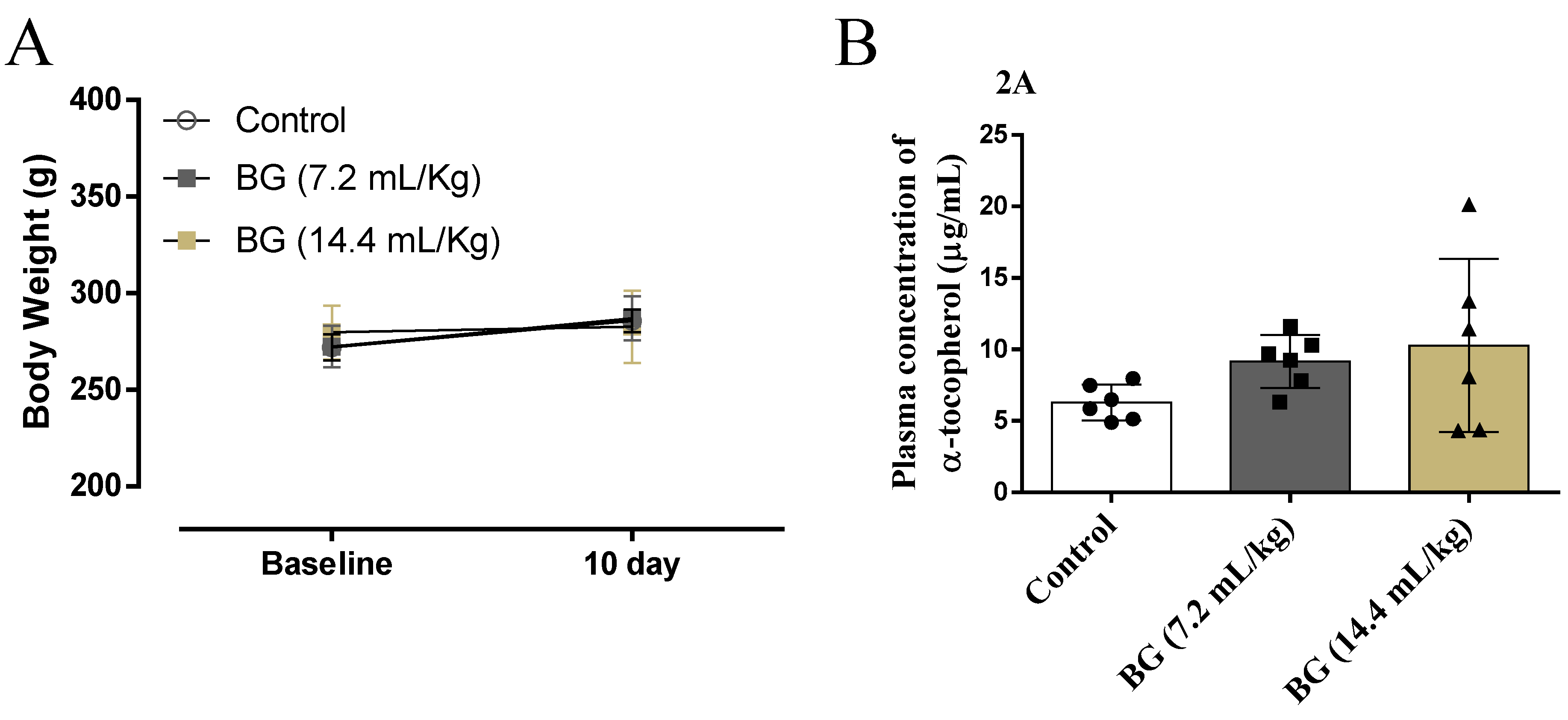
3.2. Effects of Baru Oil Treatment Plasma Body Weight and α-Tocopherol Concentration
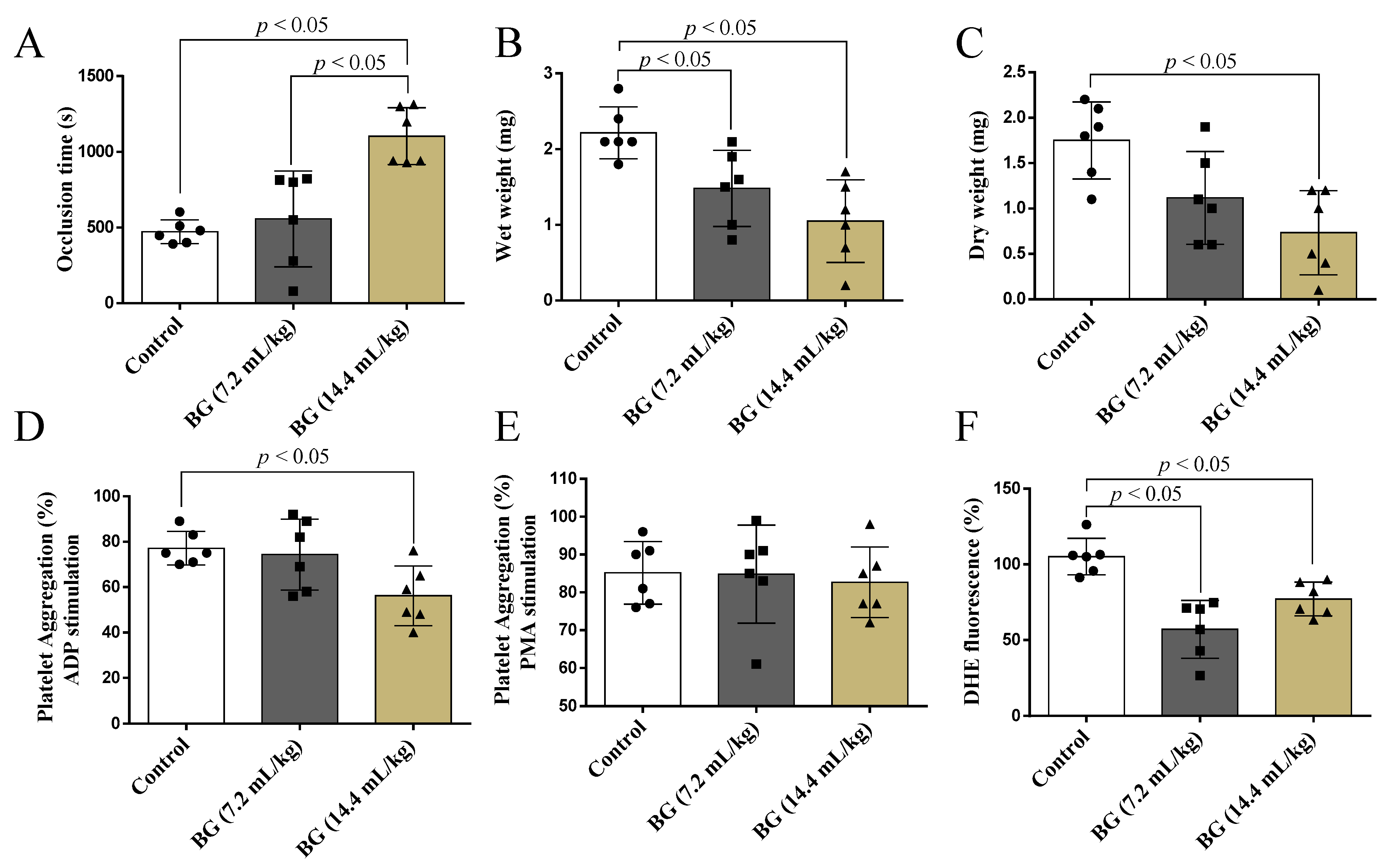
3.3. Effects of Baru Oil Treatment on Thrombus Formation, Platelets Aggregation, and Superoxide Anion Scavenging
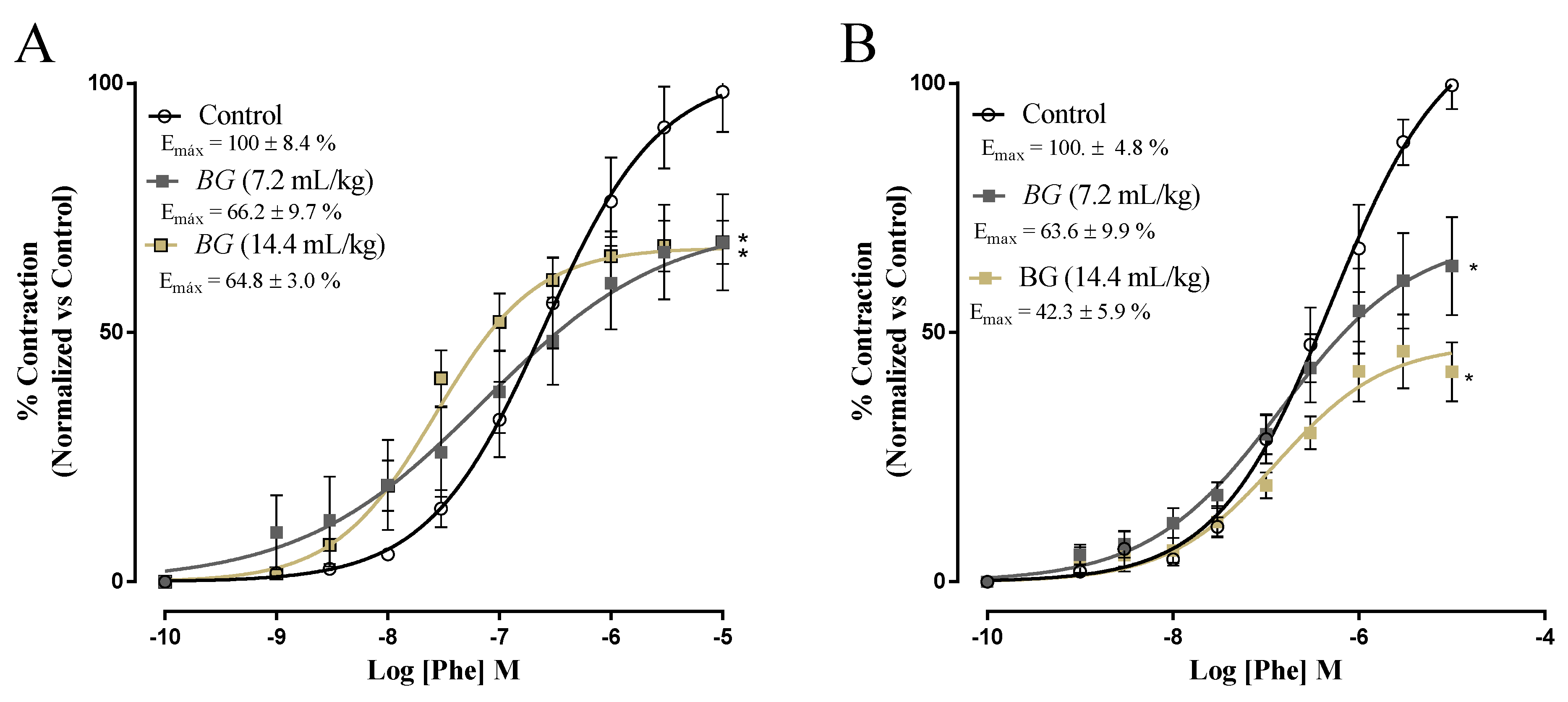
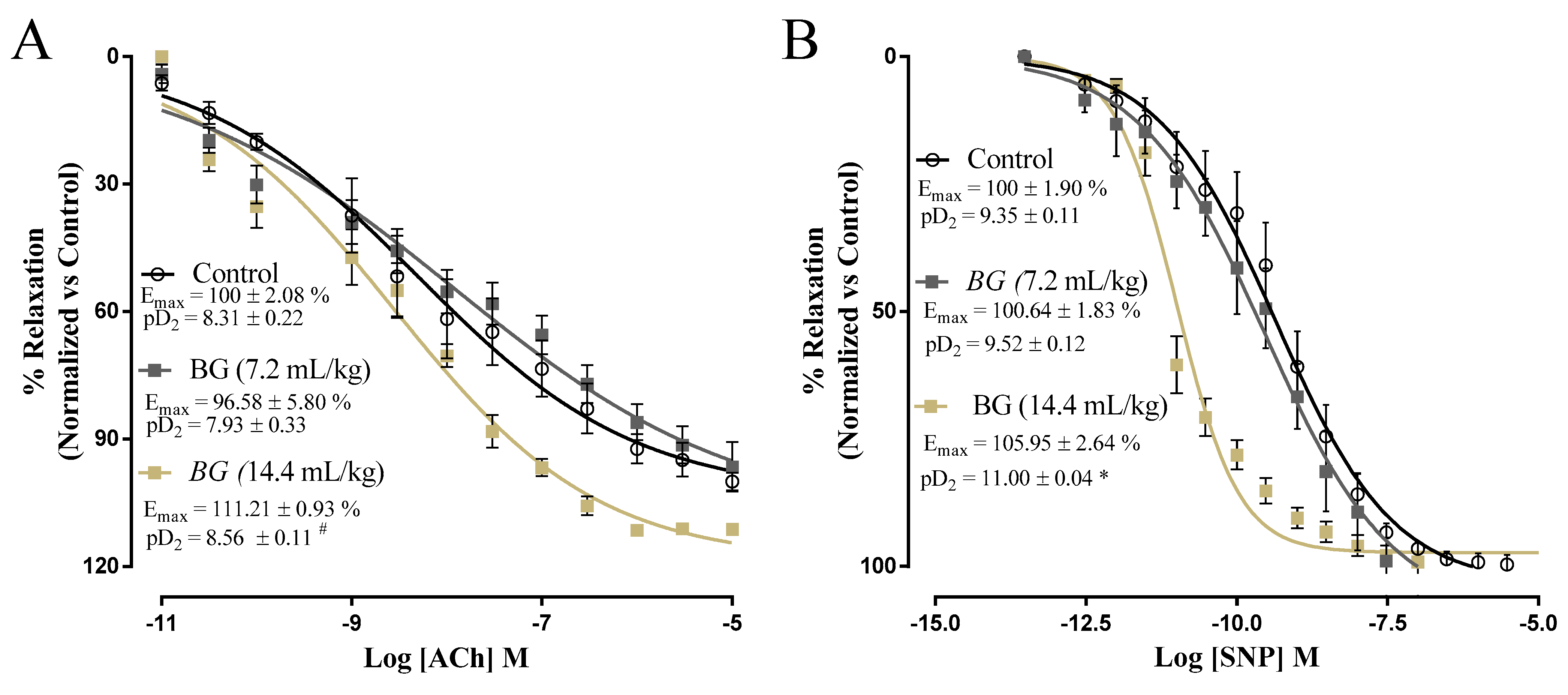
3.4. Effects of Baru Oil Treatment on Vascular Reactivity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Fernandes, D.C.; Freitas, J.B.; Czeder, L.P.; Naves, M.M. Nutritional composition and protein value of the baru (Dipteryx alata Vog.) almond from the Brazilian Savanna. J. Sci. Food Agric. 2010, 90, 1650–1655. [Google Scholar] [CrossRef]
- Bailao, E.F.; Devilla, I.A.; da Conceicao, E.C.; Borges, L.L. Bioactive Compounds Found in Brazilian Cerrado Fruits. Int. J. Mol. Sci. 2015, 16, 23760–23783. [Google Scholar] [CrossRef]
- Lemos, M.R.B.; Siqueira, E.M.d.A.; Arruda, S.F.; Zambiazi, R.C. The effect of roasting on the phenolic compounds and antioxidant potential of baru nuts [Dipteryx alata Vog.]. Food Res. Int. 2012, 48, 592–597. [Google Scholar] [CrossRef] [Green Version]
- Schincaglia, R.M.; Cuppari, L.; Neri, H.F.S.; Cintra, D.E.; Sant’Ana, M.R.; Mota, J.F. Effects of baru almond oil (Dipteryx alata Vog.) supplementation on body composition, inflammation, oxidative stress, lipid profile, and plasma fatty acids of hemodialysis patients: A randomized, double-blind, placebo-controlled clinical trial. Complement Ther. Med. 2020, 52, 102479. [Google Scholar] [CrossRef]
- Reis, M.Á.; Novaes, R.D.; Baggio, S.R.; Viana, A.L.; Salles, B.C.; Duarte, S.M.; Rodrigues, M.R.; Paula, F.B. Hepatoprotective and Antioxidant Activities of Oil from Baru Almonds (Dipteryx alata Vog.) in a Preclinical Model of Lipotoxicity and Dyslipidemia. Evid.-Based Complementary Altern. Med. 2018, 2018, 8376081. [Google Scholar] [CrossRef] [Green Version]
- de Souza, R.G.M.; Gomes, A.C.; de Castro, I.A.; Mota, J.F. A baru almond-enriched diet reduces abdominal adiposity and improves high-density lipoprotein concentrations: A randomized, placebo-controlled trial. Nutrition 2018, 55–56, 154–160. [Google Scholar] [CrossRef]
- de Souza, R.G.; Gomes, A.C.; Navarro, A.M.; Cunha, L.C.; Silva, M.A.; Junior, F.B.; Mota, J.F. Baru Almonds Increase the Activity of Glutathione Peroxidase in Overweight and Obese Women: A Randomized, Placebo-Controlled Trial. Nutrients 2019, 11, 1750. [Google Scholar] [CrossRef] [Green Version]
- Bento, A.P.; Cominetti, C.; Simoes Filho, A.; Naves, M.M. Baru almond improves lipid profile in mildly hypercholesterolemic subjects: A randomized, controlled, crossover study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1330–1336. [Google Scholar] [CrossRef]
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Gallus, H.B.A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.V.; McCumber, M.; Ozaki, Y. Thrombosis: A major contributor to the global disease burden. J. Thromb. Haemost. 2014, 12, 1580–1590. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, F.; Palomo, I.; Fuentes, E. Platelet oxidative stress as a novel target of cardiovascular risk in frail older people. Vascul. Pharmacol. 2017, 93–95, 14–19. [Google Scholar] [CrossRef]
- Qiao, J.; Arthur, J.F.; Gardiner, E.E.; Andrews, R.K.; Zeng, L.; Xu, K. Regulation of platelet activation and thrombus formation by reactive oxygen species. Redox Biol. 2018, 14, 126–130. [Google Scholar] [CrossRef]
- Williams, J.C.; Forster, L.A.; Tull, S.P.; Wong, M.; Bevan, R.J.; Ferns, G.A. Dietary vitamin E supplementation inhibits thrombin-induced platelet aggregation, but not monocyte adhesiveness, in patients with hypercholesterolaemia. Int. J. Exp. Pathol. 1997, 78, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Colette, C.; Pares-Herbute, N.; Monnier, L.H.; Cartry, E. Platelet function in type I diabetes: Effects of supplementation with large doses of vitamin E. Am. J. Clin. Nutr. 1988, 47, 256–261. [Google Scholar] [CrossRef]
- Kim, J.E.; Han, M.; Hanl, K.S.; Kim, H.K. Vitamin E inhibition on platelet procoagulant activity: Involvement of aminophospholipid translocase activity. Thromb. Res. 2011, 127, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Cimadevilla, H.M.; Hevia, D.; Miar, A.; Mayo, J.C.; Lombo, F.; Sainz, R.M. Development and validation of a single HPLC method for determination of α-tocopherol in cell culture and in human or mouse biological samples. Biomed. Chromatogr. BMC 2015, 29, 843–852. [Google Scholar] [CrossRef]
- Kurz, K.D.; Main, B.W.; Sandusky, G.E. Rat model of arterial thrombosis induced by ferric chloride. Thromb. Res. 1990, 60, 269–280. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, T.S.; Cai, J.; Kim, J.; Kim, Y.; Shin, K.; Kim, K.S.; Lee, S.P.; Kang, M.H.; Choi, E.K.; et al. Perilla oil improves blood flow through inhibition of platelet aggregation and thrombus formation. Lab. Anim. Res. 2014, 30, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Morishima, Y.; Kamisato, C.; Honda, Y. Treatment of venous thrombosis with an oral direct factor Xa inhibitor edoxaban by single and multiple administrations in rats. Eur. J. Pharmacol. 2014, 742, 15–21. [Google Scholar] [CrossRef]
- Radomski, A.; Jurasz, P.; Alonso-Escolano, D.; Drews, M.; Morandi, M.; Malinski, T.; Radomski, M.W. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br. J. Pharmacol. 2005, 146, 882–893. [Google Scholar] [CrossRef] [Green Version]
- Owens, A.P.; 3rd Lu, Y.; Whinna, H.C.; Gachet, C.; Fay, W.P.; Mackman, N. Towards a standardization of the murine ferric chloride-induced carotid arterial thrombosis model. J. Thromb. Haemost. 2011, 9, 1862–1863. [Google Scholar] [CrossRef]
- Shattil, S.J.; Cunningham, M.; Hoxie, J.A. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood 1987, 70, 307–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Cuyper, I.M.; Meinders, M.; Van De Vijver, E.; De Korte, D.; Porcelijn, L.; De Haas, M.; Eble, J.A.; Seeger, K.; Rutella, S.; Pagliara, D.; et al. A novel flow cytometry-based platelet aggregation assay. Blood 2013, 121, e70–e80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Wang, S.; Luo, X.; Xie, Y.; Shi, X. Cinnamaldehyde reduction of platelet aggregation and thrombosis in rodents. Thromb. Res. 2007, 119, 337–342. [Google Scholar] [CrossRef]
- Bezerra, L.S.; Magnani, M.; Castro-Gomez, R.J.H.; Cavalcante, H.C.; da Silva, T.A.F.; Vieira, R.L.P.; de Medeiros, I.A.; Veras, R.C. Modulation of vascular function and anti-aggregation effect induced by (1→3) (1→6)-β-d-glucan of Saccharomyces cerevisiae and its carboxymethylated derivative in rats. Pharmacol. Rep. 2017, 69, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Melhaoui, R.; Kodad, S.; Houmy, N.; Belhaj, K.; Mansouri, F.; Abid, M.; Addi, M.; Mihamou, A.; Sindic, M.; Serghini-Caid, H.; et al. Characterization of Sweet Almond Oil Content of Four European Cultivars (Ferragnes, Ferraduel, Fournat, and Marcona) Recently Introduced in Morocco. Scientifica 2021, 2021, 9141695. [Google Scholar] [CrossRef]
- Peixoto, V.O.D.; Silva, L.O.; Castelo-Branco, V.N.; Torres, A.G. Baru (Dipteryx alata Vogel) Oil Extraction by Supercritical-CO2: Improved Composition by Using Water as Cosolvent. J. Oleo. Sci. 2022, 71, 201–213. [Google Scholar] [CrossRef]
- Siqueira, A.P.; Castro, C.F.; Silveira, E.V.; Lourenço, M.F. Chemical quality of Baru almond (Dipteryx alata oil). Food Technol. 2016, 46, 1865–1867. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, E.; Palomo, I. Role of oxidative stress on platelet hyperreactivity during aging. Life Sci. 2016, 148, 17–23. [Google Scholar] [CrossRef]
- Violi, F.; Pignatelli, P. Platelet oxidative stress and thrombosis. Thromb. Res. 2012, 129, 378–381. [Google Scholar] [CrossRef]
- Gachet, C. ADP receptors of platelets and their inhibition. Thromb. Haemost. 2001, 86, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, G.; Badimon, L. Antiplatelet properties of natural products. Vascul. Pharmacol. 2013, 59, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Perri, L.; Nocella, C.; Violi, F. Antioxidant and antiplatelet activity by polyphenol-rich nutrients: Focus on extra virgin olive oil and cocoa. Br. J. Clin. Pharmacol. 2017, 83, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, R.; Kaur, M.; Singh, J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: Molecular insights and therapeutic strategies. Cardiovasc. Diabetol. 2018, 17, 121. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Luis, C.C.; de Brito Alves, J.L.; de Oliveira, J.C.P.L.; de Sousa Luis, J.A.; Araújo, I.G.A.; Tavares, J.F.; do Nascimento, Y.M.; Bezerra, L.S.; Araújo de Azevedo, F.d.L.A.; Sobral, M.V.; et al. Effects of Baru Almond Oil (Dipteryx alata Vog.) Treatment on Thrombotic Processes, Platelet Aggregation, and Vascular Function in Aorta Arteries. Nutrients 2022, 14, 2098. https://doi.org/10.3390/nu14102098
Silva-Luis CC, de Brito Alves JL, de Oliveira JCPL, de Sousa Luis JA, Araújo IGA, Tavares JF, do Nascimento YM, Bezerra LS, Araújo de Azevedo FdLA, Sobral MV, et al. Effects of Baru Almond Oil (Dipteryx alata Vog.) Treatment on Thrombotic Processes, Platelet Aggregation, and Vascular Function in Aorta Arteries. Nutrients. 2022; 14(10):2098. https://doi.org/10.3390/nu14102098
Chicago/Turabian StyleSilva-Luis, Cristiane Cosmo, José Luiz de Brito Alves, Júlio César Pinheiro Lúcio de Oliveira, José Alixandre de Sousa Luis, Islania Giselia Albuquerque Araújo, Josean Fechine Tavares, Yuri Mangueira do Nascimento, Lorena Soares Bezerra, Fátima de Lourdes Assunção Araújo de Azevedo, Marianna Vieira Sobral, and et al. 2022. "Effects of Baru Almond Oil (Dipteryx alata Vog.) Treatment on Thrombotic Processes, Platelet Aggregation, and Vascular Function in Aorta Arteries" Nutrients 14, no. 10: 2098. https://doi.org/10.3390/nu14102098
APA StyleSilva-Luis, C. C., de Brito Alves, J. L., de Oliveira, J. C. P. L., de Sousa Luis, J. A., Araújo, I. G. A., Tavares, J. F., do Nascimento, Y. M., Bezerra, L. S., Araújo de Azevedo, F. d. L. A., Sobral, M. V., Mangueira, V. M., de Medeiros, I. A., & Veras, R. C. (2022). Effects of Baru Almond Oil (Dipteryx alata Vog.) Treatment on Thrombotic Processes, Platelet Aggregation, and Vascular Function in Aorta Arteries. Nutrients, 14(10), 2098. https://doi.org/10.3390/nu14102098





