The Association of Formula Protein Content and Growth in Early Infancy: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
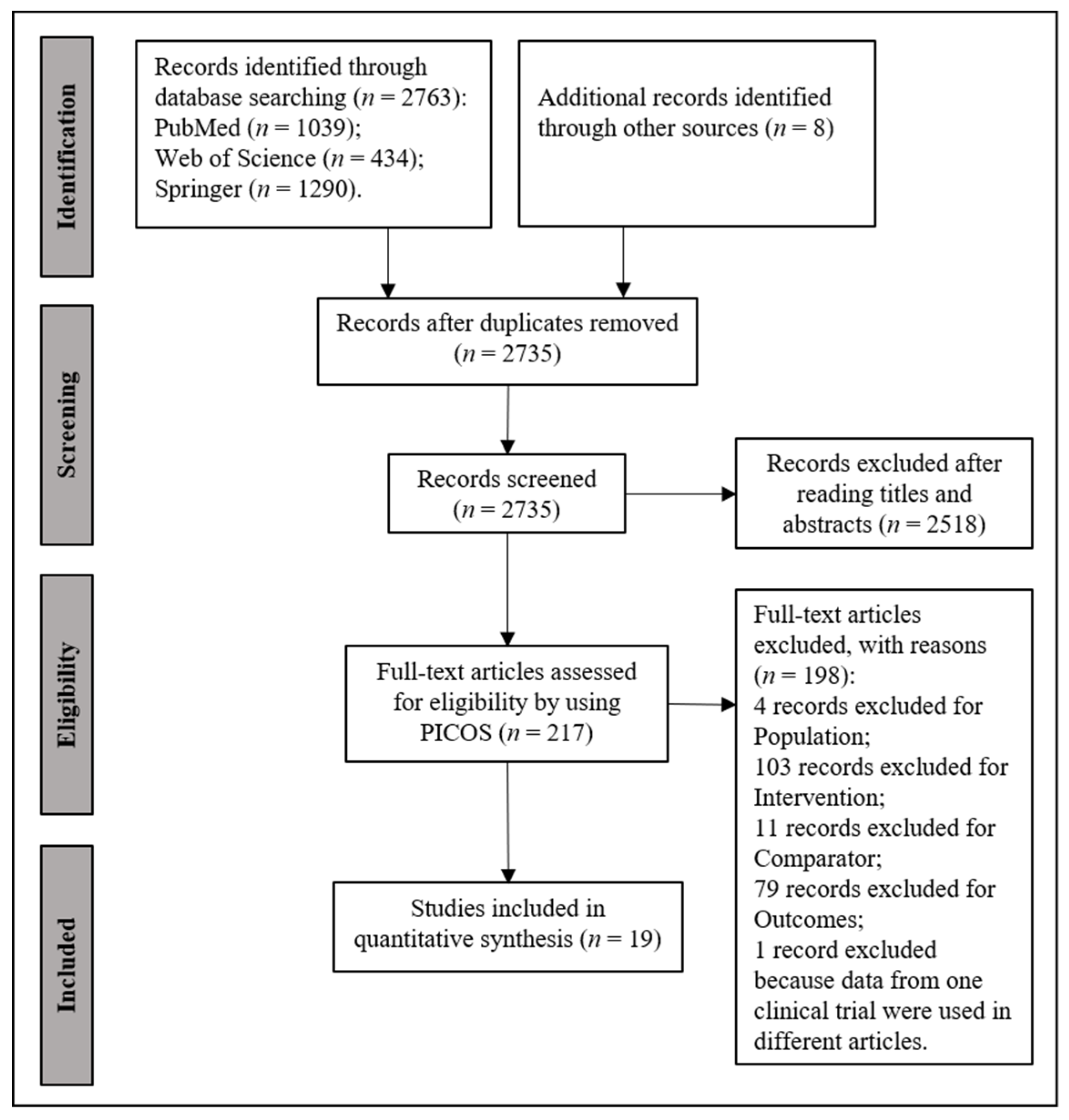
2.1. Literature Screening
2.2. Data Extraction and Analysis
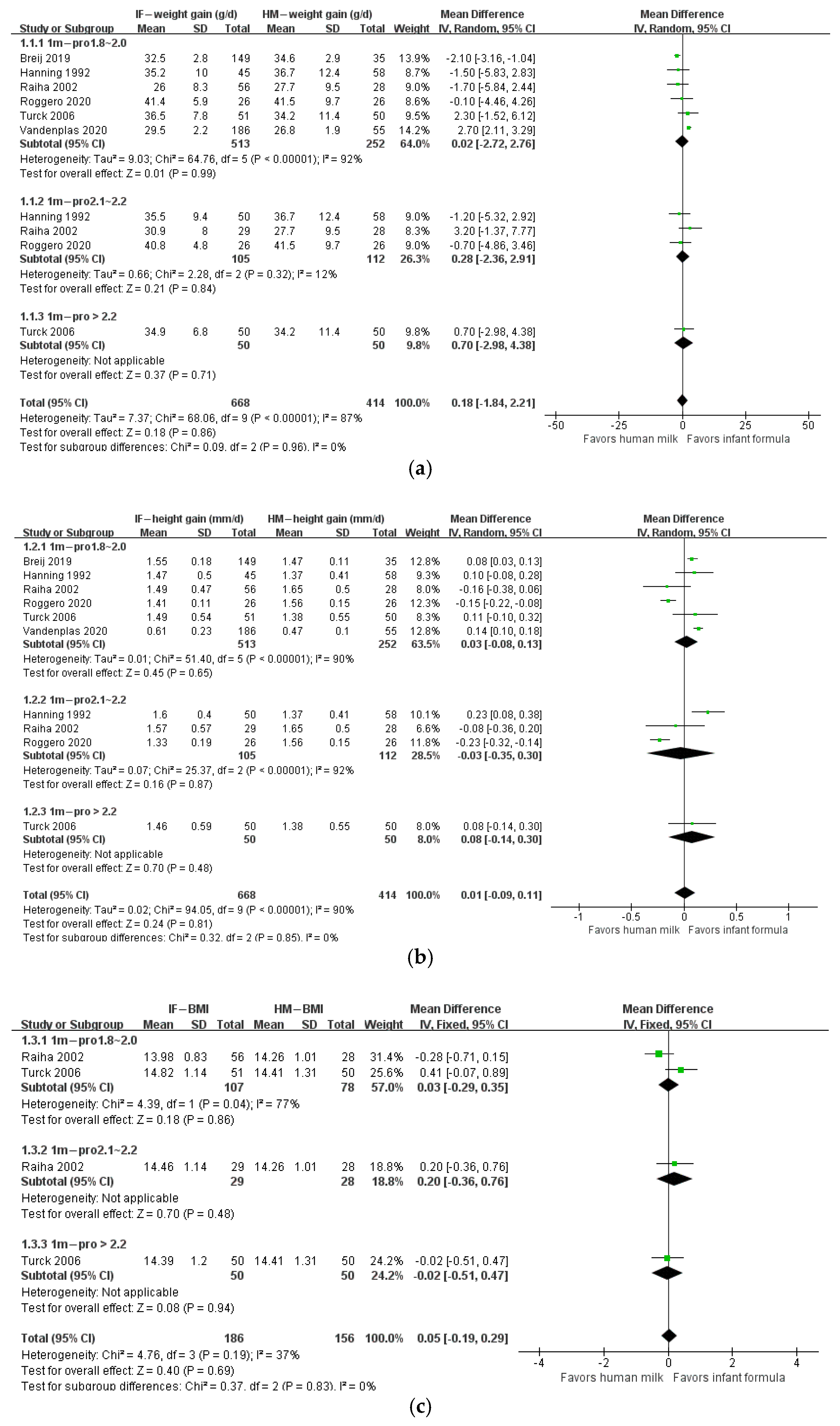
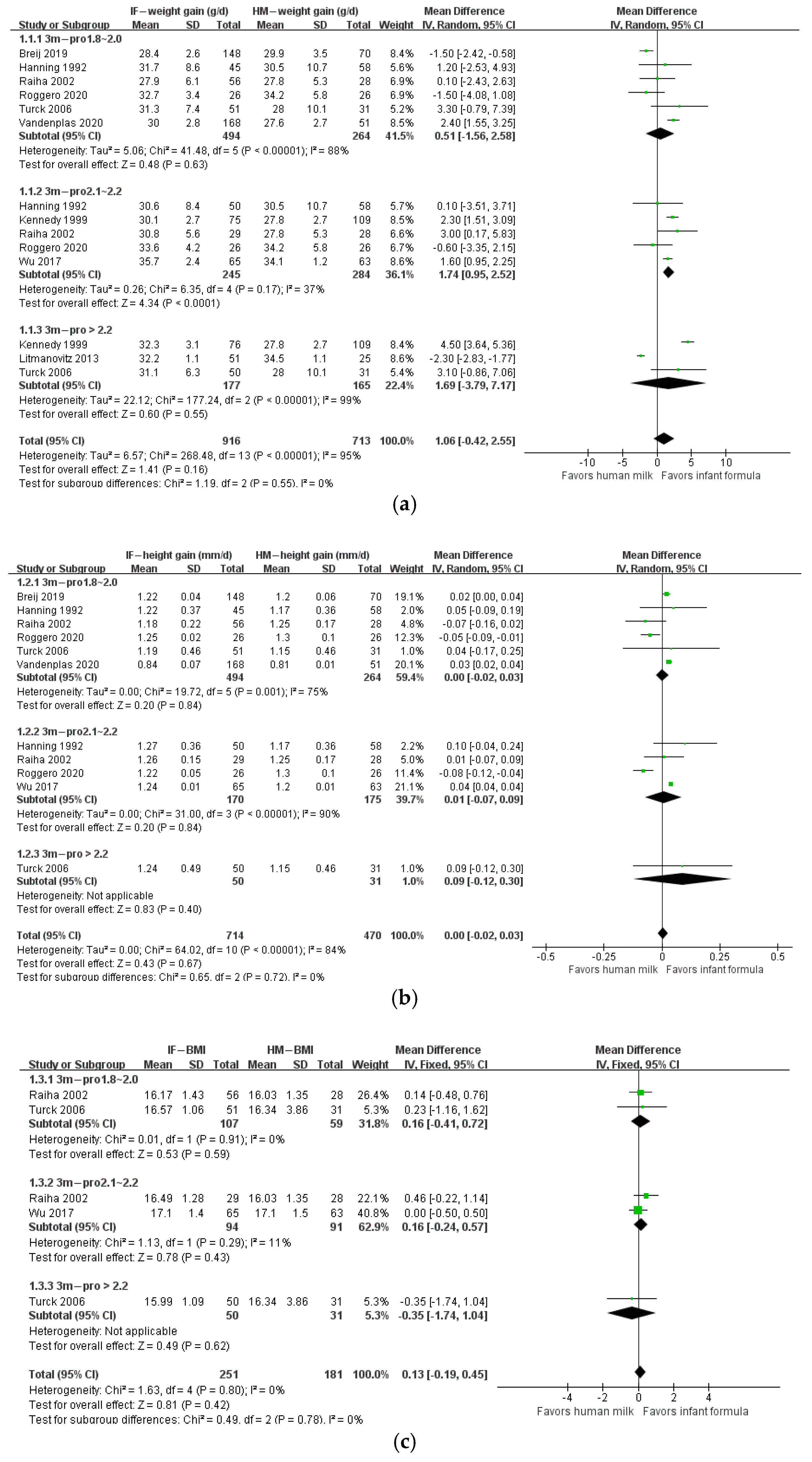
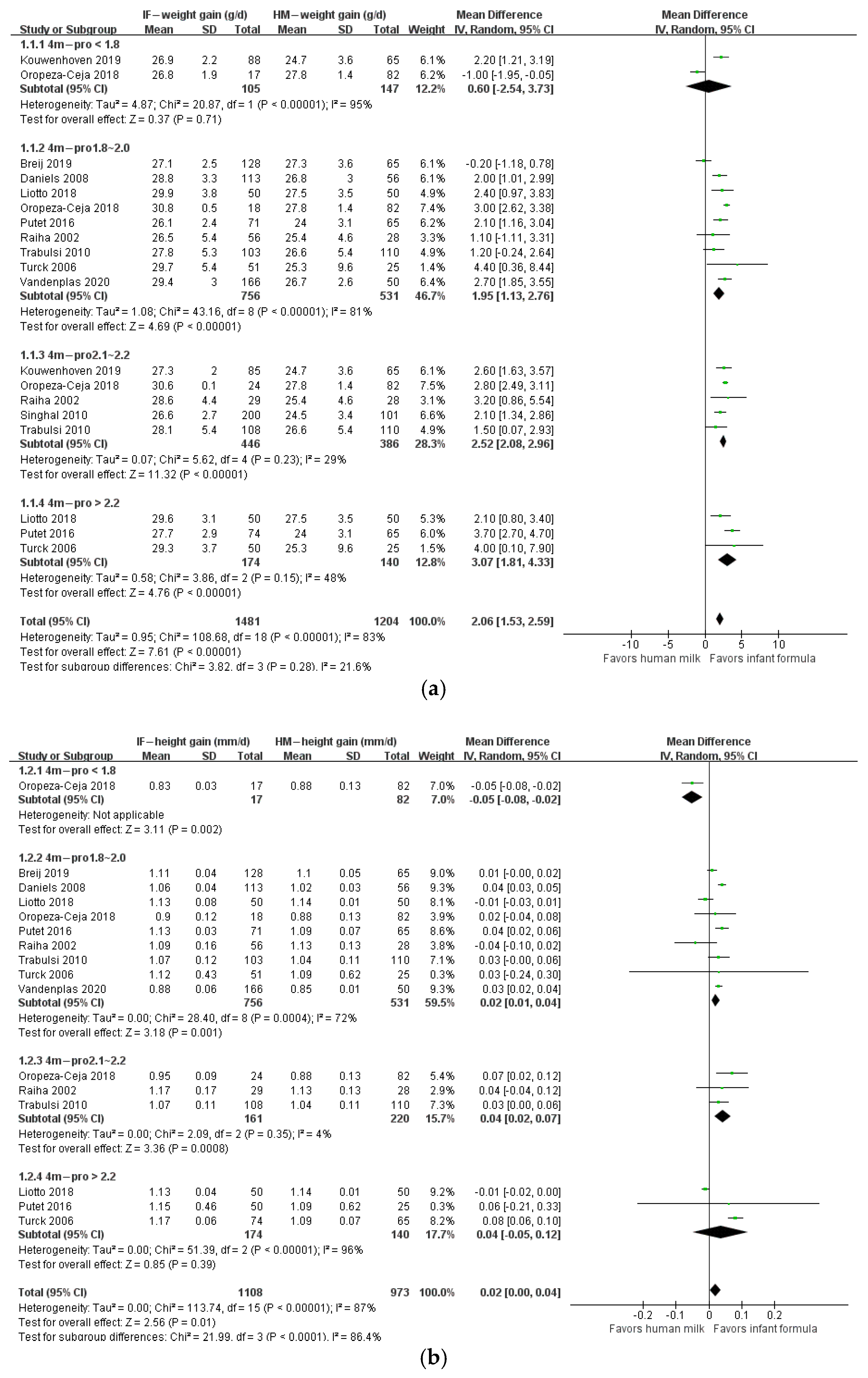
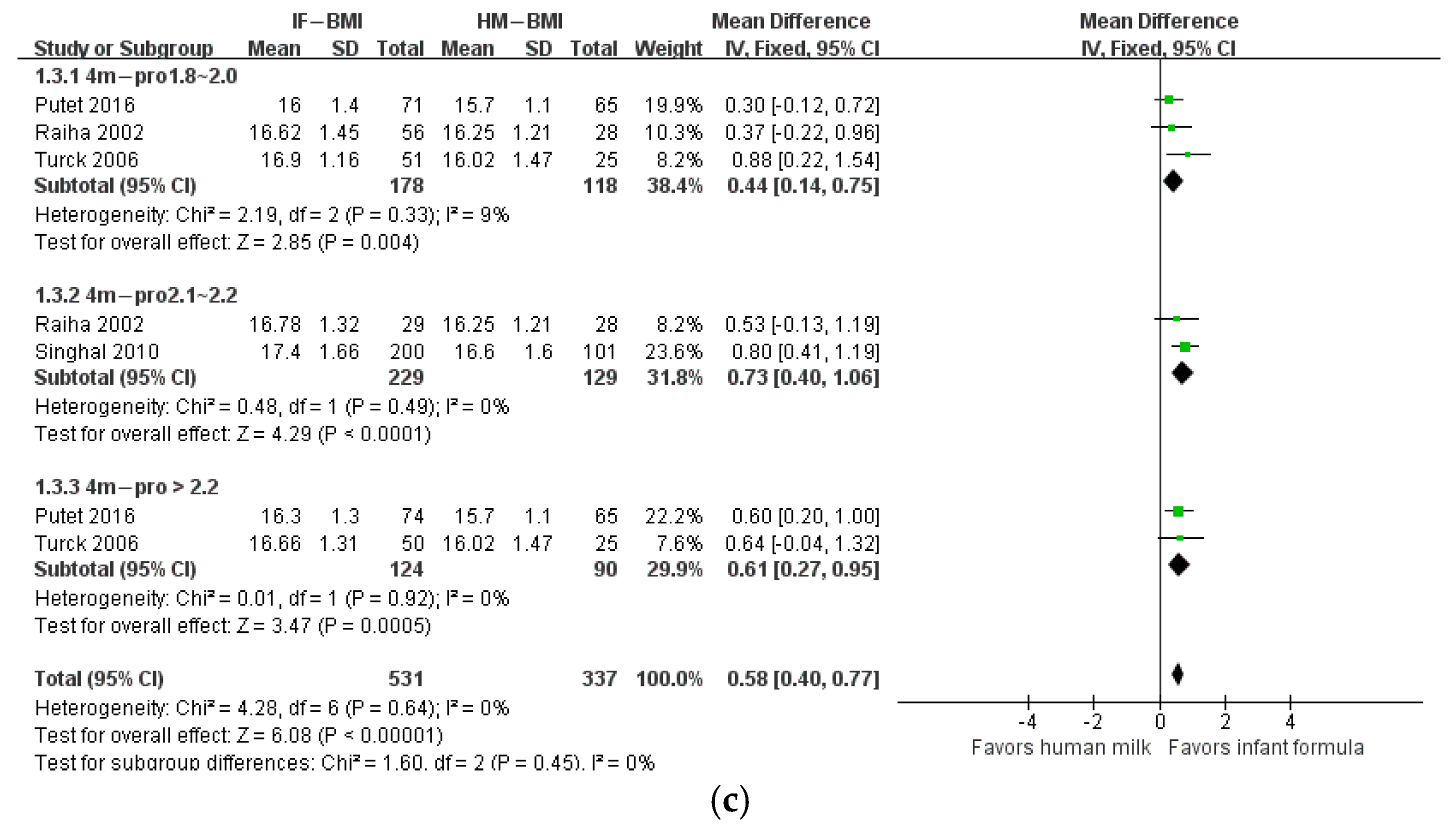
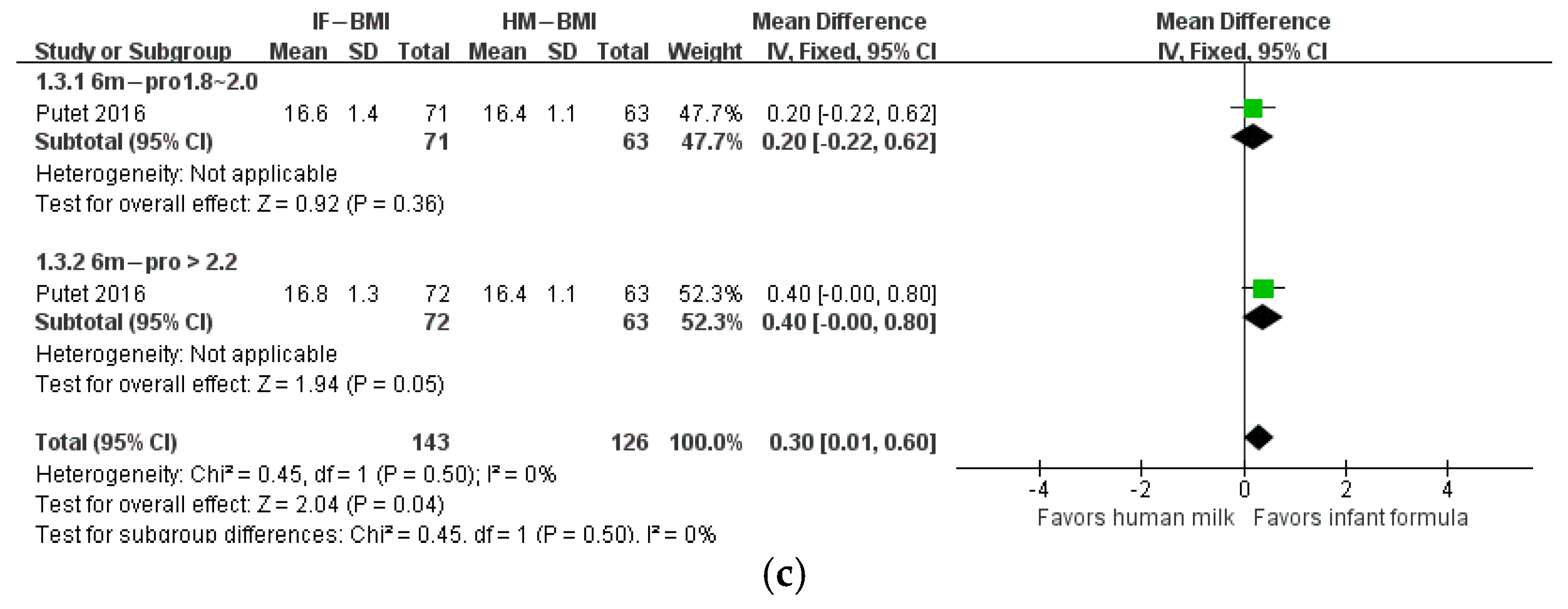
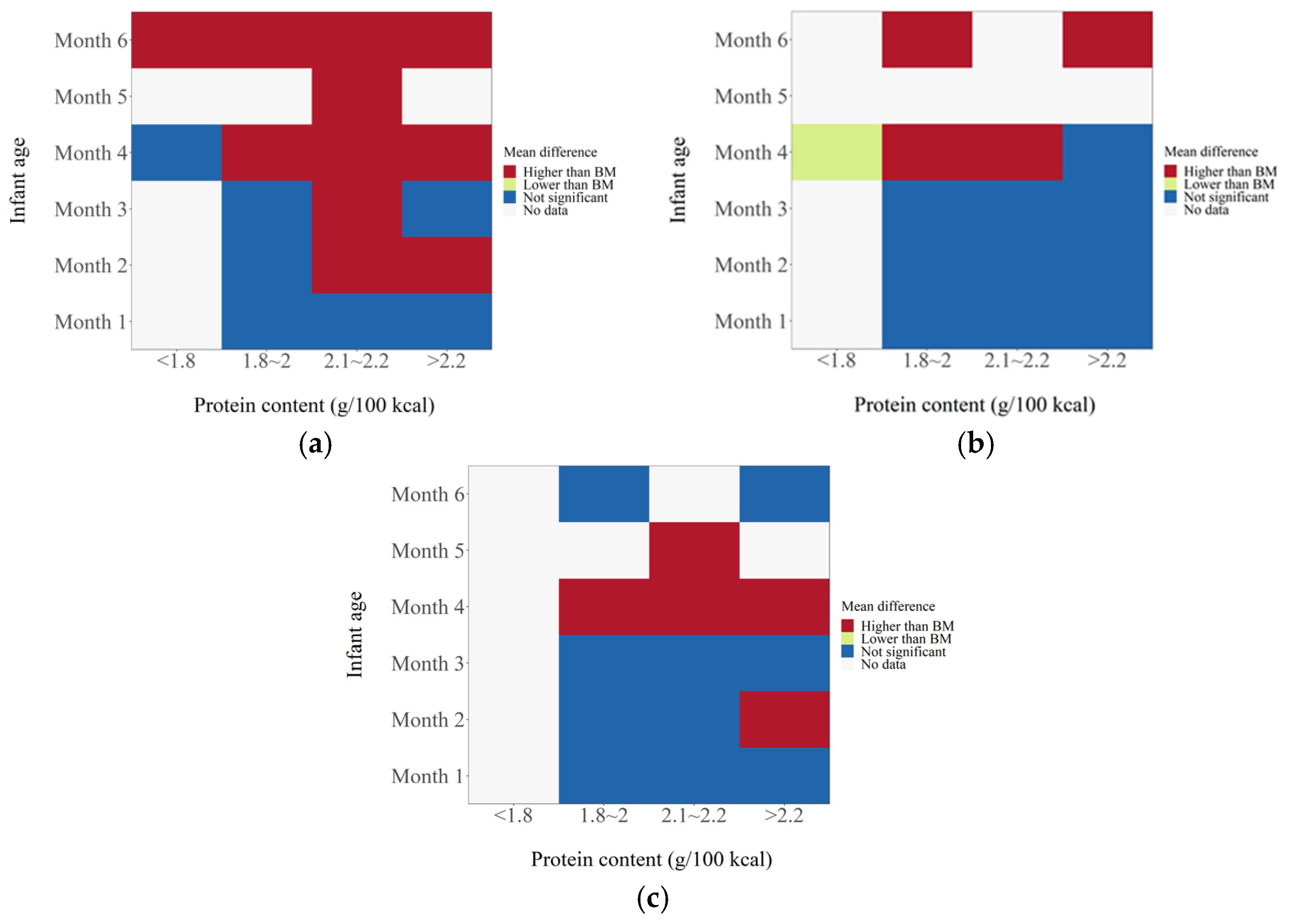
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duale, A.; Singh, P.; Al Khodor, S. Breast Milk: A Meal Worth Having. Front. Nutr. 2022, 8, 800927. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Protecting, Promoting, and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services: The Revised Baby-Friendly Hospital Initiative; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization; UNICEF. Global Nutrition Targets 2025: Breastfeeding Policy Brief; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- North, K.; Gao, M.; Allen, G.; Lee, A.C. Breastfeeding in a Global Context: Epidemiology, Impact, and Future Directions. Clin. Ther. 2022, 44, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Beggs, B.; Koshy, L.; Neiterman, E. Women’s Perceptions and Experiences of Breastfeeding: A scoping review of the literature. BMC Public Health 2021, 21, 2169. [Google Scholar] [CrossRef] [PubMed]
- Tomori, C. Overcoming barriers to breastfeeding. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, in press. [Google Scholar] [CrossRef]
- Dewey, K.G.; Heinig, M.J.; Nommsen-Rivers, L.A. Differences in morbidity between breast-fed and formula-fed infants. J. Pediatr. 1995, 126, 696–702. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.; França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Lancet Breastfeeding Series Group. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Gillman, M.W. Developmental origins of health and disease. N. Engl. J. Med. 2005, 353, 1848–1850. [Google Scholar] [CrossRef] [Green Version]
- Ong, K.K.; Loos, R.J. Rapid infancy weight gain and subsequent obesity: Systematic reviews and hopeful suggestions. Acta Paediatr. 2006, 95, 904–908. [Google Scholar] [CrossRef]
- Clifton, E.A.D.; Ahern, A.L.; Day, F.R.; Sharp, S.J.; Griffin, S.J.; Ong, K.K.; Lakshman, R. Positive maternal attitudes to following healthy infant feeding guidelines attenuate the associations between infant appetitive traits and both infant milk intake and weight. Appetite 2021, 161, 105124. [Google Scholar] [CrossRef]
- Giugliani, E.R.J. Growth in exclusively breastfed infants. J. Pediatr. 2019, 95, 79–84. [Google Scholar] [CrossRef]
- Dewey, K.G. Growth characteristics of breast-fed compared to formula-fed infants. Biol. Neonate 1998, 74, 94–105. [Google Scholar] [CrossRef] [PubMed]
- WHO; FAO; United Nations University. Protein and Amino Acid Requirements in Human Nutrition. Report of a Joint FAO/WHO/UNU Expert Consultation; WHO Technical Report Series 935; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Lönnerdal, B.; Erdmann, P.; Thakkar, S.K.; Sauser, J.; Destaillats, F. Longitudinal evolution of true protein, amino acids and bioactive proteins in breast milk: A developmental perspective. J. Nutr. Biochem. 2017, 41, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrams, S.A.; Hawthorne, K.M.; Pammi, M. A systematic review of controlled trials of lower-protein or energy-containing infant formulas for use by healthy full-term infants. Adv. Nutr. 2015, 6, 178–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patro-Gołąb, B.; Zalewski, B.M.; Kouwenhoven, S.M.; Karaś, J.; Koletzko, B.; Bernard van Goudoever, J.; Szajewska, H. Protein Concentration in Milk Formula, Growth, and Later Risk of Obesity: A Systematic Review. J. Nutr. 2016, 146, 551–564. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Davis, A.M.; Harris, B.J.; Lien, E.L.; Pramuk, K.; Trabulsi, J. Alpha-lactalbumin-rich infant formula fed to healthy term infants in a multicenter study: Plasma essential amino acids and gastrointestinal tolerance. Eur J. Clin. Nutr. 2008, 62, 1294–1301. [Google Scholar] [CrossRef] [Green Version]
- Singhal, A.; Kennedy, K.; Lanigan, J.; Clough, H.; Jenkins, W.; Elias-Jones, A.; Stephenson, T.; Dudek, P.; Lucas, A. Dietary nucleotides and early growth in formula-fed infants: A randomized controlled trial. Pediatrics 2010, 126, 946–953. [Google Scholar] [CrossRef]
- Turck, D.; Grillon, C.; Lachambre, E.; Robiliard, P.; Beck, L.; Maurin, J.L.; Kempf, C.; Bernet, J.P.; Marx, J.; Lebrun, F.; et al. Adequacy and safety of an infant formula with a protein/energy ratio of 1.8 g/100 kcal and enhanced protein efficiency for term infants during the first 4 months of life. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 364–371. [Google Scholar] [CrossRef]
- Meli, F.; Puccio, G.; Cajozzo, C.; Ricottone, G.L.; Pecquet, S.; Sprenger, N.; Steenhout, P. Growth and safety evaluation of infant formulae containing oligosaccharides derived from bovine milk: A randomized, double-blind, noninferiority trial. BMC Pediatr. 2014, 14, 306. [Google Scholar] [CrossRef] [Green Version]
- Putet, G.; Labaune, J.M.; Mace, K.; Steenhout, P.; Grathwohl, D.; Raverot, V.; Morel, Y.; Picaud, J.C. Effect of dietary protein on plasma insulin-like growth factor-1, growth, and body composition in healthy term infants: A randomised, double-blind, controlled trial (Early Protein and Obesity in Childhood (EPOCH) study). Br. J. Nutr. 2016, 115, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Litmanovitz, I.; Davidson, K.; Eliakim, A.; Regev, R.H.; Dolfin, T.; Arnon, S.; Bar-Yoseph, F.; Goren, A.; Lifshitz, Y.; Nemet, D. High Beta-palmitate formula and bone strength in term infants: A randomized, double-blind, controlled trial. Calcif. Tissue Int. 2013, 92, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trabulsi, J.; Capeding, R.; Lebumfacil, J.; Ramanujam, K.; Feng, P.; McSweeney, S.; Harris, B.; DeRusso, P. Effect of an α-lactalbumin-enriched infant formula with lower protein on growth. Eur. J. Clin. Nutr. 2011, 65, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, K.; Fewtrell, M.S.; Morley, R.; Abbott, R.; Quinlan, P.T.; Wells, J.C.; Bindels, J.G.; Lucas, A. Double-blind, randomized trial of a synthetic triacylglycerol in formula-fed term infants: Effects on stool biochemistry, stool characteristics, and bone mineralization. Am. J. Clin. Nutr. 1999, 70, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Breij, L.M.; Abrahamse-Berkeveld, M.; Vandenplas, Y.; Jespers, S.N.J.; de Mol, A.C.; Khoo, P.C.; Kalenga, M.; Peeters, S.; van Beek, R.H.T.; Norbruis, O.F.; et al. An infant formula with large, milk phospholipid-coated lipid droplets containing a mixture of dairy and vegetable lipids supports adequate growth and is well tolerated in healthy, term infants. Am. J. Clin. Nutr. 2019, 109, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Liotto, N.; Orsi, A.; Menis, C.; Piemontese, P.; Morlacchi, L.; Condello, C.C.; Giannì, M.L.; Roggero, P.; Mosca, F. Clinical evaluation of two different protein content formulas fed to full-term healthy infants: A randomized controlled trial. BMC Pediatr. 2018, 18, 59, Erratum in: BMC Pediatr. 2018, 18, 135. [Google Scholar] [CrossRef] [Green Version]
- Oropeza-Ceja, L.G.; Rosado, J.L.; Ronquillo, D.; García, O.P.; Caamaño, M.D.C.; García-Ugalde, C.; Viveros-Contreras, R.; Duarte-Vázquez, M.Á. Lower Protein Intake Supports Normal Growth of Full-Term Infants Fed Formula: A Randomized Controlled Trial. Nutrients 2018, 10, 886. [Google Scholar] [CrossRef] [Green Version]
- Daniels, L.; Gibson, R.A.; Simmer, K.; van Dael, P.; Makrides, M. Selenium status of term infants fed selenium-supplemented formula in a randomized dose-response trial. Am. J. Clin. Nutr. 2008, 88, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Räihä, N.C.; Fazzolari-Nesci, A.; Cajozzo, C.; Puccio, G.; Monestier, A.; Moro, G.; Minoli, I.; Haschke-Becher, E.; Bachmann, C.; Van’t Hof, M.; et al. Whey predominant, whey modified infant formula with protein/energy ratio of 1.8 g/100 kcal: Adequate and safe for term infants from birth to four months. J. Pediatr. Gastroenterol. Nutr. 2002, 35, 275–281. [Google Scholar] [CrossRef]
- Timby, N.; Domellöf, M.; Holgerson, P.L.; West, C.E.; Lönnerdal, B.; Hernell, O.; Johansson, I. Oral Microbiota in Infants Fed a Formula Supplemented with Bovine Milk Fat Globule Membranes—A Randomized Controlled Trial. PLoS ONE. 2017, 12, e0169831. [Google Scholar] [CrossRef]
- Roggero, P.; Liotto, N.; Pozzi, C.; Braga, D.; Troisi, J.; Menis, C.; Giannì, M.L.; Berni Canani, R.; Paparo, L.; Nocerino, R.; et al. Analysis of immune, microbiota and metabolome maturation in infants in a clinical trial of Lactobacillus paracasei CBA L74-fermented formula. Nat. Commun. 2020, 11, 2703. [Google Scholar] [CrossRef]
- Hanning, R.M.; Paes, B.; Atkinson, S.A. Protein metabolism and growth of term infants in response to a reduced-protein, 40:60 whey: Casein formula with added tryptophan. Am. J. Clin. Nutr. 1992, 56, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.L.; Ding, D.; Fang, A.P.; Chen, P.Y.; Chen, S.; Jing, L.P.; Chen, Y.M.; Zhu, H.L. Growth, Gastrointestinal Tolerance and Stool Characteristics of Healthy Term Infants Fed an Infant Formula Containing Hydrolyzed Whey Protein (63%) and Intact Casein (37%): A Randomized Clinical Trial. Nutrients 2017, 9, 1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouwenhoven, S.M.P.; Antl, N.; Finken, M.J.J.; Twisk, J.W.R.; van der Beek, E.M.; Abrahamse-Berkeveld, M.; van de Heijning, B.J.M.; Schierbeek, H.; Holdt, L.M.; van Goudoever, J.B.; et al. A modified low-protein infant formula supports adequate growth in healthy, term infants: A randomized, double-blind, equivalence trial. Am. J. Clin. Nutr. 2020, 111, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; de Halleux, V.; Arciszewska, M.; Lach, P.; Pokhylko, V.; Klymenko, V.; Schoen, S.; Abrahamse-Berkeveld, M.; Mulder, K.A.; Porcel Rubio, R.; et al. A Partly Fermented Infant Formula with Postbiotics Including 3′-GL, Specific Oligosaccharides, 2′-FL, and Milk Fat Supports Adequate Growth, Is Safe and Well-Tolerated in Healthy Term Infants: A Double-Blind, Randomised, Controlled, Multi-Country Trial. Nutrients 2020, 12, 3560. [Google Scholar] [CrossRef] [PubMed]
- Karlsland Akeson, P.K.; Axelsson, I.E.; Räihä, N.C.; Warm, A.; Minoli, I.; Moro, G. Protein intake and metabolism in formula-fed infants given Swedish or Italian weaning foods. Acta Paediatr. 2000, 89, 158–164. [Google Scholar] [CrossRef]
- Akeson, P.M.; Axelsson, I.E.; Räihä, N.C. Growth and nutrient intake in three- to twelve-month-old infants fed human milk or formulas with varying protein concentrations. J. Pediatr. Gastroenterol. Nutr. 1998, 26, 1–8. [Google Scholar] [CrossRef]
- Weber, M.; Grote, V.; Closa-Monasterolo, R.; Escribano, J.; Langhendries, J.P.; Dain, E.; Giovannini, M.; Verduci, E.; Gruszfeld, D.; Socha, P.; et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: Follow-up of a randomized trial. Am. J. Clin. Nutr. 2014, 99, 1041–1051. [Google Scholar] [CrossRef]
- Ren, Q.; Sun, H.; Zhao, M.; Xu, Y.; Xie, Q.; Jiang, S.; Zhao, X.; Zhang, W. Longitudinal Changes in Crude Protein and Amino Acids in Human Milk in Chinese Population: A Systematic Review. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 555–561. [Google Scholar] [CrossRef]
- Camier, A.; Davisse-Paturet, C.; Scherdel, P.; Lioret, S.; Heude, B.; Charles, M.A.; de Lauzon-Guillain, B. Early growth according to protein content of infant formula: Results from the EDEN and ELFE birth cohorts. Pediatr. Obes. 2021, 16, e12803. [Google Scholar] [CrossRef]
- Hörnell, A.; Lagström, H.; Lande, B.; Thorsdottir, I. Protein intake from 0 to 18 years of age and its relation to health: A systematic literature review for the 5th Nordic Nutrition Recommendations. Food Nutr. Res. 2013, 57, 21083. [Google Scholar] [CrossRef] [Green Version]
- Fenton, T.R.; Al-Wassia, H.; Premji, S.S.; Sauve, R.S. Higher versus lower protein intake in formula-fed low birth weight infants. Cochrane Database Syst. Rev. 2020, 6, CD003959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liotto, N. Protein content of infant formula for the healthy full-term infant. Am. J. Clin. Nutr. 2020, 111, 946–947. [Google Scholar] [CrossRef] [PubMed]
- Monge-Montero, C.; van der Merwe, L.F.; Papadimitropoulou, K.; Agostoni, C.; Vitaglione, P. Mixed milk feeding: A systematic review and meta-analysis of its prevalence and drivers. Nutr. Rev. 2020, 78, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Huang, X.; Wang, H.; Xu, Y.; Pan, X.; Jin, X. Determinants of growth for 4 months old infants in rural areas. Chin. J. Child Health Care 2017, 25, 18–20. [Google Scholar]
- Lakshman, R.; Sharp, S.J.; Whittle, F.; Schiff, A.; Hardeman, W.; Irvine, L.; Wilson, E.; Griffin, S.J.; Ong, K.K. Randomised controlled trial of a theory-based behavioural intervention to reduce formula milk intake. Arch. Dis. Child 2018, 103, 1054–1060. [Google Scholar] [CrossRef]
- Ambrożej, D.; Dumycz, K.; Dziechciarz, P.; Ruszczyński, M. Milk Fat Globule Membrane Supplementation in Children: Systematic Review with Meta-Analysis. Nutrients 2021, 13, 714. [Google Scholar] [CrossRef]
- Bronsky, J.; Campoy, C.; Embleton, N.; Fewtrell, M.; Mis, N.F.; Gerasimidis, K.; Hojsak, I.; Hulst, J.; Indrio, F.; Lapillonne, A.; et al. Palm Oil and Beta-palmitate in Infant Formula: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 742–760. [Google Scholar] [CrossRef]
- Skórka, A.; Pieścik-Lech, M.; Kołodziej, M.; Szajewska, H. Infant formulae supplemented with prebiotics: Are they better than unsupplemented formulae? An updated systematic review. Br. J. Nutr. 2018, 119, 810–825. [Google Scholar] [CrossRef]
- Bazzano, A.N.; Kaji, A.; Felker-Kantor, E.; Bazzano, L.A.; Potts, K.S. Qualitative Studies of Infant and Young Child Feeding in Lower-Income Countries: A Systematic Review and Synthesis of Dietary Patterns. Nutrients 2017, 9, 1140. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Mu, S.; Xu, X.; Shi, Z.; Shen, L. Effects of dietary nucleotide supplementation on growth in infants: A meta-analysis of randomized controlled trials. Eur. J. Nutr. 2019, 58, 1213–1221. [Google Scholar] [CrossRef]
- Sun, J.; Marwah, G.; Westgarth, M.; Buys, N.; Ellwood, D.; Gray, P.H. Effects of Probiotics on Necrotizing Enterocolitis, Sepsis, Intraventricular Hemorrhage, Mortality, Length of Hospital Stay, and Weight Gain in Very Preterm Infants: A Meta-Analysis. Adv. Nutr. 2017, 8, 749–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Q.; Li, K.; Sun, H.; Zheng, C.; Zhou, Y.; Lyu, Y.; Ye, W.; Shi, H.; Zhang, W.; Xu, Y.; et al. The Association of Formula Protein Content and Growth in Early Infancy: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2255. https://doi.org/10.3390/nu14112255
Ren Q, Li K, Sun H, Zheng C, Zhou Y, Lyu Y, Ye W, Shi H, Zhang W, Xu Y, et al. The Association of Formula Protein Content and Growth in Early Infancy: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(11):2255. https://doi.org/10.3390/nu14112255
Chicago/Turabian StyleRen, Qiqi, Kaifeng Li, Han Sun, Chengdong Zheng, Yalin Zhou, Ying Lyu, Wanyun Ye, Hanxu Shi, Wei Zhang, Yajun Xu, and et al. 2022. "The Association of Formula Protein Content and Growth in Early Infancy: A Systematic Review and Meta-Analysis" Nutrients 14, no. 11: 2255. https://doi.org/10.3390/nu14112255
APA StyleRen, Q., Li, K., Sun, H., Zheng, C., Zhou, Y., Lyu, Y., Ye, W., Shi, H., Zhang, W., Xu, Y., & Jiang, S. (2022). The Association of Formula Protein Content and Growth in Early Infancy: A Systematic Review and Meta-Analysis. Nutrients, 14(11), 2255. https://doi.org/10.3390/nu14112255






