High Maternal Triglyceride Levels Mediate the Association between Pre-Pregnancy Overweight/Obesity and Macrosomia among Singleton Term Non-Diabetic Pregnancies: A Prospective Cohort Study in Central China
Abstract
:1. Introduction
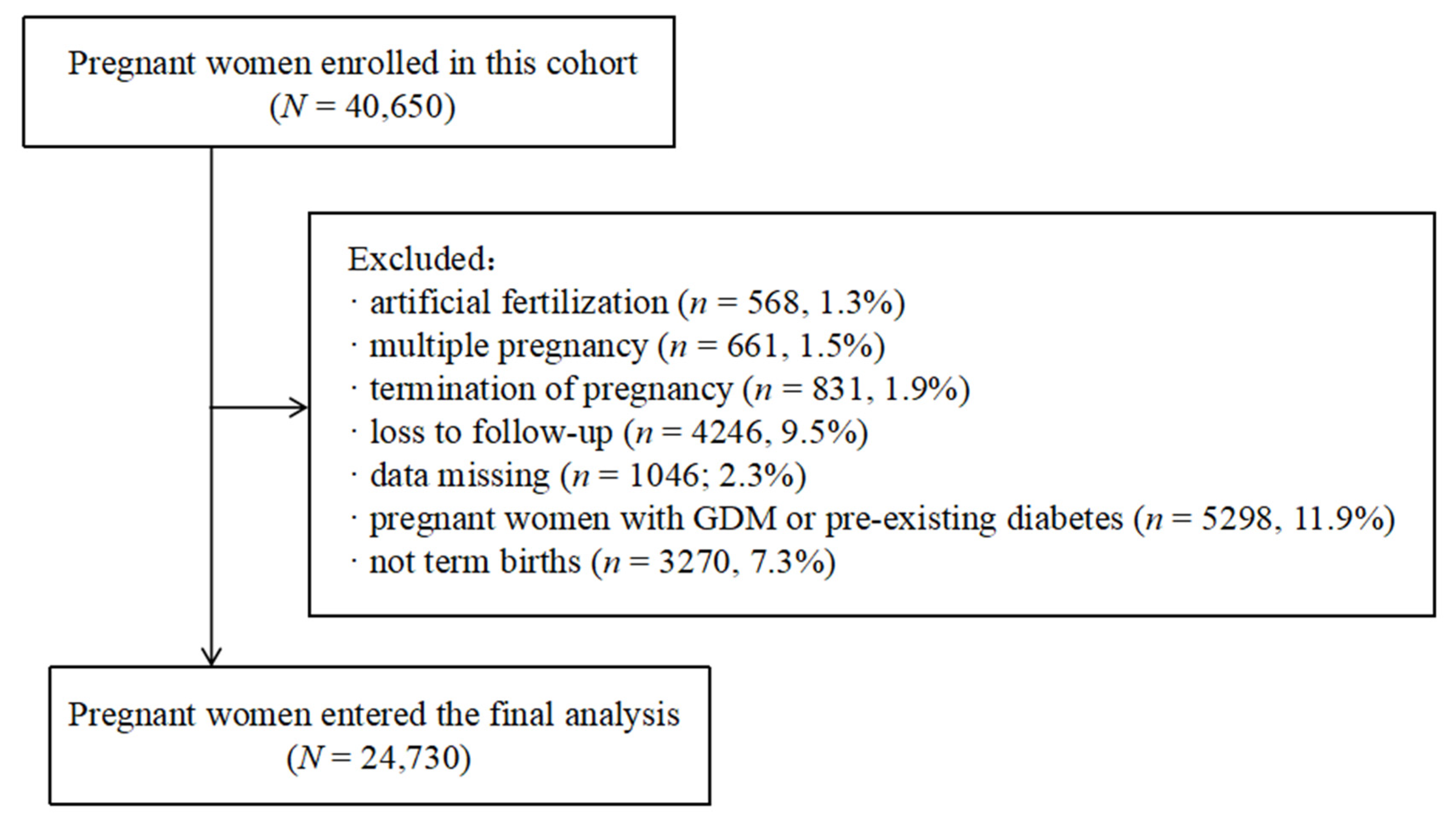
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Statistical Analyses
3. Result
3.1. Baseline Characteristics of the Participants
3.2. Prevalence of High mTG Levels and Fetal Macrosomia across Pre-Pregnancy BMI Categories
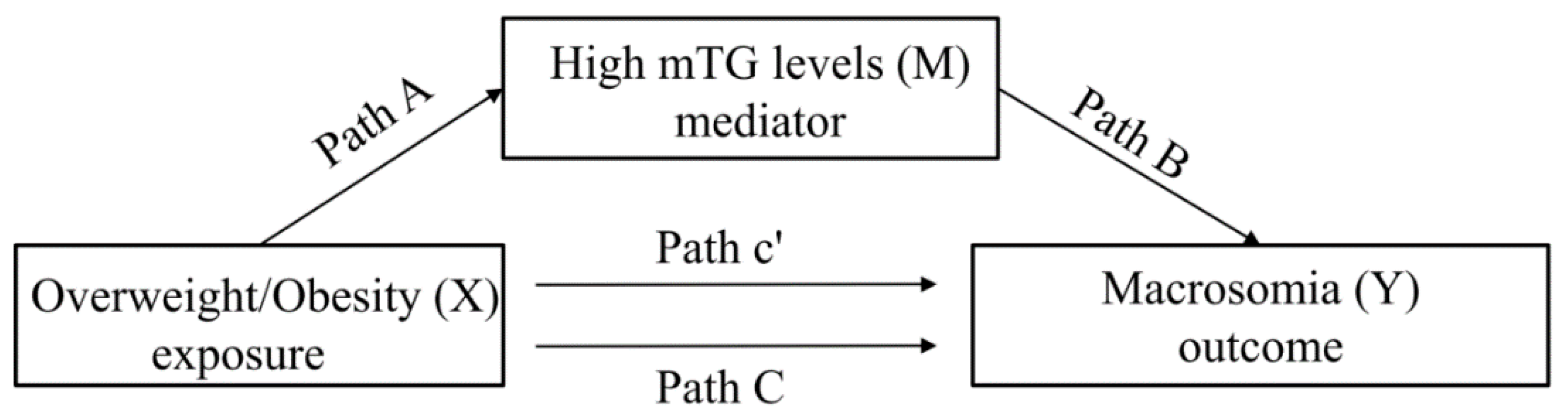
3.3. Testing for Significance of Path A, B, and C
3.4. Mediation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Category | Total Effect (95% CI) | Direct Effect (95% CI) | Indirect Effect (95% CI) | Mediated Proportion, % |
|---|---|---|---|---|
| Overweight | 0.006 (0.001–0.010) ** | 0.005 (0.001–0.009) * | 0.001 (0.000–0.001) *** | 11.1 |
| Obese | 0.026 (0.018–0.036) *** | 0.025 (0.018–0.036) *** | 0.001 (0.000–0.001) * | 3.8 |
References
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 549: Obesity in pregnancy. Obs. Gynecol. 2013, 121, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Araujo Júnior, E.; Peixoto, A.B.; Zamarian, A.C.; Elito Júnior, J.; Tonni, G. Macrosomia. Best Pr. Res. Clin. Obs. Gynaecol. 2017, 38, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Ouzounian, J.G. Evaluation and Management of Fetal Macrosomia. Obs. Gynecol. Clin. N. Am. 2021, 48, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Amadou, C.; Nabi, O.; Serfaty, L. Association between birth weight, preterm birth and non-alcoholic fatty liver disease in a community-based cohort. Hepatology 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Shi, X.; Jia, X.; Wang, Y.; Zhao, Y.; Bao, J.; Zhang, H.; Yang, Y. Birth weight, childhood obesity and risk of hypertension: A Mendelian randomization study. J. Hypertens. 2021, 39, 1876–1883. [Google Scholar] [CrossRef]
- Cui, D.; Yang, W.; Shao, P.; Li, J.; Wang, P.; Leng, J.; Wang, S.; Liu, E.; Chan, J.C.N.; Yu, Z.; et al. Interactions between Prepregnancy Overweight and Passive Smoking for Macrosomia and Large for Gestational Age in Chinese Pregnant Women. Obes. Facts 2021, 14, 520–530. [Google Scholar] [CrossRef]
- Chen, S.; Wang, S.; Li, T.; Zhu, H.; Liang, S.; Xu, K.; Zhang, Y.; Yuan, X.; Yang, Y.; Pan, H. Effect of PM2.5 on macrosomia in China: A nationwide prospective cohort study. Pediatr. Obes. 2020, 15, e12584. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, B.; Zhao, Z.; Yang, L.; Zhang, M.; Jiang, Y.; Li, Y.; Zhou, M.; Wang, L.; Huang, Z.; et al. Body-mass index and obesity in urban and rural China: Findings from consecutive nationally representative surveys during 2004–18. Lancet 2021, 398, 53–63. [Google Scholar] [CrossRef]
- Vidakovic, A.J.; Jaddoe, V.W.; Gishti, O.; Felix, J.F.; Williams, M.A.; Hofman, A.; Demmelmair, H.; Koletzko, B.; Tiemeier, H.; Gaillard, R. Body mass index, gestational weight gain and fatty acid concentrations during pregnancy: The Generation R Study. Eur. J. Epidemiol. 2015, 30, 1175–1185. [Google Scholar] [CrossRef] [Green Version]
- Kc, K.; Shakya, S.; Zhang, H. Gestational diabetes mellitus and macrosomia: A literature review. Ann. Nutr. Metab. 2015, 66 (Suppl. S2), 14–20. [Google Scholar] [CrossRef]
- Geraghty, A.A.; Alberdi, G.; O’Sullivan, E.J.; O’Brien, E.C.; Crosbie, B.; Twomey, P.J.; McAuliffe, F.M. Maternal Blood Lipid Profile during Pregnancy and Associations with Child Adiposity: Findings from the ROLO Study. PLoS ONE 2016, 11, e0161206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol. 1954, 16, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Crume, T.L.; Shapiro, A.L.; Brinton, J.T.; Glueck, D.H.; Martinez, M.; Kohn, M.; Harrod, C.; Friedman, J.E.; Dabelea, D. Maternal fuels and metabolic measures during pregnancy and neonatal body composition: The healthy start study. J. Clin. Endocrinol. Metab. 2015, 100, 1672–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Retnakaran, R.; Ye, C.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B.; Hamilton, J.K. Effect of maternal weight, adipokines, glucose intolerance and lipids on infant birth weight among women without gestational diabetes mellitus. CMAJ 2012, 184, 1353–1360. [Google Scholar] [CrossRef] [Green Version]
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; McIntyre, H.D.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar]
- Adank, M.C.; Benschop, L.; Kors, A.W.; Peterbroers, K.R.; Smak Gregoor, A.M.; Mulder, M.T.; Schalekamp-Timmermans, S.; Roeters Van Lennep, J.E.; Steegers, E.A.P. Maternal lipid profile in early pregnancy is associated with foetal growth and the risk of a child born large-for-gestational age: A population-based prospective cohort study: Maternal lipid profile in early pregnancy and foetal growth. BMC Med. 2020, 18, 276. [Google Scholar] [CrossRef]
- Xi, F.; Chen, H.; Chen, Q.; Chen, D.; Chen, Y.; Sagnelli, M.; Chen, G.; Zhao, B.; Luo, Q. Second-trimester and third-trimester maternal lipid profiles significantly correlated to LGA and macrosomia. Arch. Gynecol. Obstet. 2021, 304, 885–894. [Google Scholar] [CrossRef]
- Nasioudis, D.; Doulaveris, G.; Kanninen, T.T. Dyslipidemia in pregnancy and maternal-fetal outcome. Minerva Ginecol. 2019, 71, 155–162. [Google Scholar] [CrossRef]
- Harmon, K.A.; Gerard, L.; Jensen, D.R.; Kealey, E.H.; Hernandez, T.L.; Reece, M.S.; Barbour, L.A.; Bessesen, D.H. Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: Metabolic determinants of fetal growth. Diabetes Care 2011, 34, 2198–2204. [Google Scholar] [CrossRef] [Green Version]
- Barbour, L.A.; Hernandez, T.L. Maternal Lipids and Fetal Overgrowth: Making Fat from Fat. Clin. Ther. 2018, 40, 1638–1647. [Google Scholar] [CrossRef] [Green Version]
- Szabo, A.J. Transferred maternal fatty acids stimulate fetal adipogenesis and lead to neonatal and adult obesity. Med. Hypotheses 2019, 122, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Fu, Y.; Wu, Y.Y.; Mao, A.F.; Xu, M.Y.; Zheng, G.; Cai, F.C.; Wang, X.H.; Shi, M.Q.; Hu, W.S. Mediating Effects of Maternal Blood Triglycerides on the Relationship between Prepregnancy Body Mass Index and Fetal Macrosomia. J. Pediatr. 2020, 226, 118–122.e111. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Desoye, G. Maternal and fetal lipid metabolism under normal and gestational diabetic conditions. Horm. Mol. Biol. Clin. Investig. 2016, 26, 109–127. [Google Scholar] [CrossRef] [PubMed]
- National Health Commission of the People’s Republic of China. Criteria of Weight for Adults. 2013. Available online: http://www.nhc.gov.cn/ewebeditor/uploadfile/2013/08/20130808135715967 (accessed on 1 February 2022).
- Jiang, X.F.; Wang, H.; Wu, D.D.; Zhang, J.L.; Gao, L.; Chen, L.; Zhang, J.; Fan, J.X. The Impact of Gestational Weight Gain on the Risks of Adverse Maternal and Infant Outcomes among Normal BMI Women with High Triglyceride Levels during Early Pregnancy. Nutrients 2021, 13, 3454. [Google Scholar] [CrossRef]
- Lin, X.H.; Wu, D.D.; Li, C.; Xu, Y.J.; Gao, L.; Lass, G.; Zhang, J.; Tian, S.; Ivanova, D.; Tang, L.; et al. Maternal High Triglyceride Levels During Early Pregnancy and Risk of Preterm Delivery: A Retrospective Cohort Study. J. Clin. Endocrinol. Metab. 2019, 104, 1249–1258. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z. Monte Carlo based statistical power analysis for mediation models: Methods and software. Behav. Res. Methods 2014, 46, 1184–1198. [Google Scholar] [CrossRef]
- Furse, S.; White, S.L.; Meek, C.L.; Jenkins, B.; Petry, C.J.; Vieira, M.C.; Ozanne, S.E.; Dunger, D.B.; Poston, L.; Koulman, A. Altered triglyceride and phospholipid metabolism predates the diagnosis of gestational diabetes in obese pregnancy. Mol. Omics 2019, 15, 420–430. [Google Scholar] [CrossRef]
- Steinhauser, C.B.; Askelson, K.; Lambo, C.A.; Hobbs, K.C.; Bazer, F.W.; Satterfield, M.C. Lipid metabolism is altered in maternal, placental, and fetal tissues of ewes with small for gestational age fetuses. Biol. Reprod. 2021, 104, 170–180. [Google Scholar] [CrossRef]
- Wang, J.; Moore, D.; Subramanian, A.; Cheng, K.K.; Toulis, K.A.; Qiu, X.; Saravanan, P.; Price, M.J.; Nirantharakumar, K. Gestational dyslipidaemia and adverse birthweight outcomes: A systematic review and meta-analysis. Obes. Rev. 2018, 19, 1256–1268. [Google Scholar] [CrossRef]
- Di Cianni, G.; Miccoli, R.; Volpe, L.; Lencioni, C.; Ghio, A.; Giovannitti, M.G.; Cuccuru, I.; Pellegrini, G.; Chatzianagnostou, K.; Boldrini, A.; et al. Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet. Med. 2005, 22, 21–25. [Google Scholar] [CrossRef]
- Kitajima, M.; Oka, S.; Yasuhi, I.; Fukuda, M.; Rii, Y.; Ishimaru, T. Maternal serum triglyceride at 24--32 weeks’ gestation and newborn weight in nondiabetic women with positive diabetic screens. Obstet. Gynecol. 2001, 97, 776–780. [Google Scholar] [CrossRef]
- Kulkarni, S.R.; Kumaran, K.; Rao, S.R.; Chougule, S.D.; Deokar, T.M.; Bhalerao, A.J.; Solat, V.A.; Bhat, D.S.; Fall, C.H.; Yajnik, C.S. Maternal lipids are as important as glucose for fetal growth: Findings from the Pune Maternal Nutrition Study. Diabetes Care 2013, 36, 2706–2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoury, J.; Henriksen, T.; Christophersen, B.; Tonstad, S. Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: A randomized clinical trial. Am. J. Obstet. Gynecol. 2005, 193, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Fard, N.M.; Mehrabian, F.; Sarraf-Zadegan, N.; Sajadi, F. Fat-modified diets during pregnancy and lactation and serum lipids after birth. Indian J. Pediatr. 2004, 71, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Amirani, E.; Asemi, Z.; Asbaghi, O.; Milajerdi, A.; Reiner, Ž.; Mansournia, M.A.; Hallajzadeh, J.; Moazzami, B.; Chaichian, S. The effects of omega-3 fatty acids supplementation on metabolic status in pregnant women: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Metab. Disord. 2020, 19, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Larqué, E.; Gil-Sánchez, A.; Prieto-Sánchez, M.T.; Koletzko, B. Omega 3 fatty acids, gestation and pregnancy outcomes. Br. J. Nutr. 2012, 107 (Suppl. S2), S77–S84. [Google Scholar] [CrossRef] [Green Version]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA 2010, 304, 1675–1683. [Google Scholar] [CrossRef] [Green Version]
- Thangaratinam, S.; Rogozinska, E.; Jolly, K.; Glinkowski, S.; Roseboom, T.; Tomlinson, J.W.; Kunz, R.; Mol, B.W.; Coomarasamy, A.; Khan, K.S. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: Meta-analysis of randomised evidence. BMJ 2012, 344, e2088. [Google Scholar] [CrossRef] [Green Version]
- Meyer, B.J.; Stewart, F.M.; Brown, E.A.; Cooney, J.; Nilsson, S.; Olivecrona, G.; Ramsay, J.E.; Griffin, B.A.; Caslake, M.J.; Freeman, D.J. Maternal obesity is associated with the formation of small dense LDL and hypoadiponectinemia in the third trimester. J. Clin. Endocrinol. Metab. 2013, 98, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Farias, D.R.; Franco-Sena, A.B.; Vilela, A.; Lepsch, J.; Mendes, R.H.; Kac, G. Lipid changes throughout pregnancy according to pre-pregnancy BMI: Results from a prospective cohort. BJOG 2016, 123, 570–578. [Google Scholar] [CrossRef]
- Grivell, R.M.; Yelland, L.N.; Deussen, A.; Crowther, C.A.; Dodd, J.M. Antenatal dietary and lifestyle advice for women who are overweight or obese and the effect on fetal growth and adiposity: The LIMIT randomised trial. BJOG 2016, 123, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Zhang, H.P.; Yang, J.; Huang, Z.Q.; Xu, H.X.; Jin, J.; Xu, K.; Tong, Y.; Dong, Q.Q.; Zheng, J.Q. The relationship between maternal vitamin D deficiency and glycolipid metabolism and adverse pregnancy outcome. Clin. Endocrinol. 2020, 93, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Heerwagen, M.J.R.; Gumina, D.L.; Hernandez, T.L.; Van Pelt, R.E.; Kramer, A.W.; Janssen, R.C.; Jensen, D.R.; Powell, T.L.; Friedman, J.E.; Winn, V.D.; et al. Placental lipoprotein lipase activity is positively associated with newborn adiposity. Placenta 2018, 64, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wang, X.; Chen, T.; Xu, W.; Feng, F.; Zhao, S.; Wang, Z.; Hu, Y.; Xie, B. Maternal lipids, BMI and IL-17/IL-35 imbalance in concurrent gestational diabetes mellitus and preeclampsia. Exp. Ther. Med. 2018, 16, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Xu, X.X.; Sun, H.; Han, Y.; Lei, Z.F.; Wang, Y.C.; Yan, H.T.; Yang, X.J. Cord blood leptin DNA methylation levels are associated with macrosomia during normal pregnancy. Pediatr. Res. 2019, 86, 305–310. [Google Scholar] [CrossRef]
- Nahavandi, S.; Seah, J.M.; Shub, A.; Houlihan, C.; Ekinci, E.I. Biomarkers for Macrosomia Prediction in Pregnancies Affected by Diabetes. Front. Endocrinol. 2018, 9, 407. [Google Scholar] [CrossRef] [Green Version]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef]
| Maternal and Infant Characteristics | Total Births n (%) | High mTG n (%) | Macrosomia n (%) |
|---|---|---|---|
| N = 24,730 | 2487 (10.1) | 959 (3.9) | |
| Pre-pregnancy BMI (kg/m2) | |||
| Underweight (<18.5) | 3781 (15.3) | 301 (12.1) | 111 (11.6) |
| Normal (18.5–23.9) | 17,593 (71.1) | 1739 (69.9) | 646 (67.4) |
| Overweight (24.0–27.9) | 2822 (11.4) | 370 (14.9) | 140 (14.6) |
| Obese (≥28.0) | 534 (2.2) | 77 (3.1) | 62 (6.5) |
| Age at pregnancy onset (year) | |||
| <25 | 1351 (5.5) | 150 (6.0) | 57 (5.9) |
| 25–29 | 9103 (36.8) | 732 (29.4) | 367 (38.3) |
| 30–34 | 9248 (37.4) | 955 (38.4) | 359 (37.4) |
| ≥35 | 5028 (20.3) | 650 (26.1) | 176 (18.4) |
| Education | |||
| High school or less | 8625 (34.9) | 928 (52.0) | 321 (33.5) |
| Some college | 12,687 (51.3) | 1293 (37.3) | 530 (55.3) |
| Bachelor’s or higher | 3418 (13.8) | 266 (10.7) | 108 (11.3) |
| Smoke | |||
| No | 24,476 (99.0) | 2459 (98.9) | 946 (98.6) |
| Yes | 254 (1.0) | 28 (1.1) | 13 (1.4) |
| Drink | |||
| No | 24,353 (98.5) | 2440 (98.1) | 943(98.3) |
| Yes | 377 (1.5) | 47 (1.9) | 16(1.7) |
| Parity | |||
| Primipara | 12,088 (48.9) | 1157 (46.5) | 484 (50.5) |
| Multipara | 12,642 (51.1) | 1330 (53.5) | 475 (49.5) |
| Infant sex | |||
| Male | 13,184 (53.3) | 1318 (53.0) | 645 (67.3) |
| Female | 11,546 (46.7) | 1169 (47.0) | 314 (32.7) |
| Gestational weight gain (kg) | |||
| <10 | 18,353 (13.6) | 1798 (13.4) | 684 (6.6) |
| 10–20 | 3373 (74.2) | 333 (72.3) | 63 (71.3) |
| ≥20 | 3004 (12.1) | 356 (14.3) | 212 (22.1) |
| Gestational hypertension | |||
| No | 23,950 (96.8) | 2414 (97.1) | 935 (97.5) |
| Yes | 780 (3.2) | 73 (2.9) | 24 (2.5) |
| Category | High mTG % (95% CI) | Macrosomia % (95% CI) |
|---|---|---|
| Normal (18.5–23.9) | 9.9 (9.4–10.3) | 3.7 (3.4–3.9) |
| Overweight (24.0–27.9) | 13.1 (11.9–14.4) | 5.0 (4.2–5.8) |
| Obese (≥28.0) | 14.4 (11.4–17.4) | 11.6 (8.9–14.3) |
| Category | Path A aRR (95%CI) a | Path B aRR (95%CI) b | Path C aRR (95%CI) c |
|---|---|---|---|
| Overweight | 1.35 (1.20–1.53) | 2.26 (1.89–2.72) | 1.45 (1.20–1.76) |
| Obese | 1.48 (1.15–1.91) | 1.86 (1.52–2.28) | 4.26 (3.20–5.68) |
| Category | Total Effect (95% CI) | Direct Effect (95% CI) | Indirect Effect (95% CI) | Mediated Proportion, % |
|---|---|---|---|---|
| Overweight | 0.006 (0.001–0.010) ** | 0.005 (0.001–0.009) * | 0.001 (0.000–0.001) *** | 11.1 |
| Obese | 0.026 (0.018–0.036) *** | 0.025 (0.017–0.036) *** | 0.001 (0.000–0.001) * | 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Chen, L.; Zhang, S.; Liu, Y.; Wei, J.; Sun, M.; Shu, J.; Wang, T.; Qin, J. High Maternal Triglyceride Levels Mediate the Association between Pre-Pregnancy Overweight/Obesity and Macrosomia among Singleton Term Non-Diabetic Pregnancies: A Prospective Cohort Study in Central China. Nutrients 2022, 14, 2075. https://doi.org/10.3390/nu14102075
Song X, Chen L, Zhang S, Liu Y, Wei J, Sun M, Shu J, Wang T, Qin J. High Maternal Triglyceride Levels Mediate the Association between Pre-Pregnancy Overweight/Obesity and Macrosomia among Singleton Term Non-Diabetic Pregnancies: A Prospective Cohort Study in Central China. Nutrients. 2022; 14(10):2075. https://doi.org/10.3390/nu14102075
Chicago/Turabian StyleSong, Xinli, Letao Chen, Senmao Zhang, Yiping Liu, Jianhui Wei, Mengting Sun, Jing Shu, Tingting Wang, and Jiabi Qin. 2022. "High Maternal Triglyceride Levels Mediate the Association between Pre-Pregnancy Overweight/Obesity and Macrosomia among Singleton Term Non-Diabetic Pregnancies: A Prospective Cohort Study in Central China" Nutrients 14, no. 10: 2075. https://doi.org/10.3390/nu14102075
APA StyleSong, X., Chen, L., Zhang, S., Liu, Y., Wei, J., Sun, M., Shu, J., Wang, T., & Qin, J. (2022). High Maternal Triglyceride Levels Mediate the Association between Pre-Pregnancy Overweight/Obesity and Macrosomia among Singleton Term Non-Diabetic Pregnancies: A Prospective Cohort Study in Central China. Nutrients, 14(10), 2075. https://doi.org/10.3390/nu14102075






