Dairy Animal Ownership and Household Milk Production Associated with Better Child and Family Diet in Rural Nepal during the COVID-19 Pandemic
Abstract
1. Introduction
2. Methods
2.1. Ethics
2.2. Study Design
2.3. Field Procedures
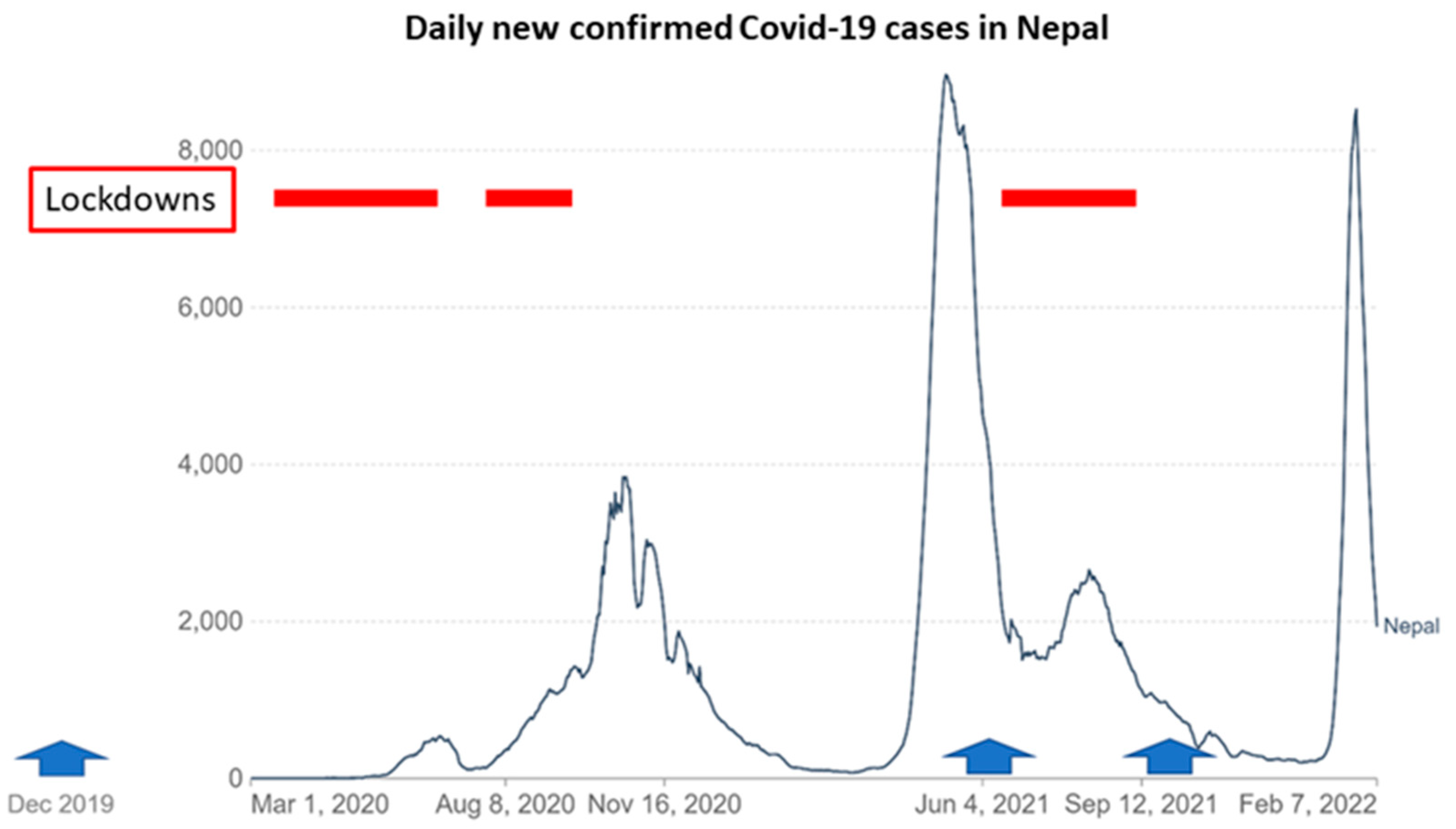
2.4. Impact of COVID-19 on Field Activities
2.5. Participants
3. Diet
3.1. Household Demographic Characteristics
3.2. Statistical Analysis
4. Results
4.1. Participants
4.2. Child Diet
4.3. Family Diet
4.4. Household Dairy Animal Ownership and Milk Production
4.5. Dairy Animal Ownership and Child and Family Diet
4.6. Milk Production and Child and Family Diet
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Covid Pandemic Data Wikipedia 2022. Available online: https://en.wikipedia.org/wiki/Template:COVID-19_pandemic_data (accessed on 10 April 2022).
- Raut, N.K. A Review of the Economic Impacts of the COVID-19 Pandemic and Economic Policies in Nepal; MPRA: Munich, Germany, 2020. [Google Scholar]
- Meyer, M.A. The role of resilience in food system studies in low- and middle-income countries. Glob. Food Secur. 2020, 24, 100356. [Google Scholar] [CrossRef]
- Chackalackal, D.J.; Al-Aghbari, A.A.; Jang, S.Y.; Ramirez, T.R.; Vincent, J.; Joshi, A.; Banjara, M.R.; Asaga, P.; Sanchez, R.C.; Carrillo, M.A.; et al. The Covid-19 pandemic in low- and middle-income countries, who carries the burden? Review of mass media and publications from six countries. Pathog. Glob. Health 2021, 115, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, S.N. Impact of COVID-19 on Nepali Businesses: Highlights of the NRB Second Follow-Up Survey: Nepal Economic Forum. 2021. Available online: https://nepaleconomicforum.org/impact-of-covid-19-on-nepali-businesses-highlights-of-the-nrb-second-follow-up-survey/ (accessed on 10 April 2022).
- Picchioni, F.; Goulao, L.F.; Roberfroid, D. The impact of COVID-19 on diet quality, food security and nutrition in low and middle income countries: A systematic review of the evidence. Clin. Nutr. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Mainali, R.P.; Marasini, S.; Acharya, K.P.; Adhikari, S. Nepal at the edge of sword with two edges: The COVID-19 pandemics and sustainable development goals. J. Agric. Food Res. 2021, 4, 100138. [Google Scholar] [CrossRef]
- Middendorf, B.J.; Faye, A.; Middendorf, G.; Stewart, Z.P.; Jha, P.K.; Prasad, P.V. Smallholder farmer perceptions about the impact of COVID-19 on agriculture and livelihoods in Senegal. Agric. Syst. 2021, 190, 103108. [Google Scholar] [CrossRef] [PubMed]
- Béné, C. Resilience of local food systems and links to food security–A review of some important concepts in the context of COVID-19 and other shocks. Food Secur. 2020, 12, 805–822. [Google Scholar] [CrossRef]
- Adhikari, J.; Timsina, J.; Khadka, S.R.; Ghale, Y.; Ojha, H. COVID-19 impacts on agriculture and food systems in Nepal: Implications for SDGs. Agric. Syst. 2020, 186, 102990. [Google Scholar] [CrossRef]
- Headey, D.; Heidkamp, R.; Osendarp, S.; Ruel, M.; Scott, N.; Black, R.; Shekar, M.; Bouis, H.; Flory, A.; Haddad, L. Impacts of COVID-19 on childhood malnutrition and nutrition-related mortality. Lancet 2020, 396, 519–521. [Google Scholar] [CrossRef]
- Kabir, M.; Saqib, M.A.N.; Zaid, M.; Ahmed, H.; Afzal, M.S. COVID-19, economic impact and child mortality: A global concern. Clin. Nutr. 2020, 39, 2322. [Google Scholar] [CrossRef]
- Akseer, N.; Kandru, G.; Keats, E.C.; A Bhutta, Z. COVID-19 pandemic and mitigation strategies: Implications for maternal and child health and nutrition. Am. J. Clin. Nutr. 2020, 112, 251–256. [Google Scholar] [CrossRef]
- World Bank. COVID-19 Impact on Nepal’s Economy Hits Hardest Informal Sector; World Bank: Washington, DC, USA, 2020. [Google Scholar]
- Birthal, P.S.; Negi, D.S. Livestock for higher, sustainable and inclusive agricultural growth. Econ. Political Wkly. 2012, 47, 89–99. [Google Scholar]
- Jumrani, J.; Birthal, P. Livestock, women, and child Nutrition in rural India. Agric. Econ. Res. Rev. 2015, 28, 223–246. [Google Scholar] [CrossRef]
- Jodlowski, M.; Winter-Nelson, A.; Baylis, K.; Goldsmith, P.D. Milk in the Data: Food Security Impacts from a Livestock Field Experiment in Zambia. World Dev. 2016, 77, 99–114. [Google Scholar] [CrossRef]
- Nkomoki, W.; Bavorová, M.; Banout, J. Factors associated with household food security in Zambia. Sustainability 2019, 11, 2715. [Google Scholar] [CrossRef]
- Christian, A.K.; Wilson, M.L.; Aryeetey, R.N.O.; Jones, A.D. Livestock ownership, household food security and childhood anaemia in rural Ghana. PLoS ONE 2019, 14, e0219310. [Google Scholar] [CrossRef] [PubMed]
- Adesogan, A.T.; Dahl, G.E. Milk Symposium Introduction: Dairy production in developing countries. J. Dairy Sci. 2020, 103, 9677–9680. [Google Scholar] [CrossRef]
- Azzarri, C.; Zezza, A.; Haile, B.; Cross, E. Does livestock ownership affect animal source foods consumption and child nutritional status? Evidence from rural Uganda. J. Dev. Stud. 2015, 51, 1034–1059. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. The Importance of Milk and other Animal-Source Foods for Children in Low-Income Countries. Food Nutr. Bull. 2011, 32, 227–243. [Google Scholar] [CrossRef]
- Headey, D. Animal sourced foods and child nutrition in South Asia: Policy priorities. LANSA Res. Brief Ser. 2018, 11. [Google Scholar]
- Headey, D.; Hirvonen, K.; Hoddinott, J. Animal Sourced Foods and Child Stunting; IFPRI: Washington, DC, USA, 2017. [Google Scholar]
- Iannotti, L. Milk and dairy programmes affecting nutrition. In Milk and Dairy Products in Human Nutrition; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; pp. 275–312. [Google Scholar]
- Long, J.K.; Murphy, S.P.; Weiss, R.E.; Nyerere, S.; Bwibo, N.O.; Neumann, C.G. Meat and milk intakes and toddler growth: A comparison feeding intervention of animal-source foods in rural Kenya. Public Health Nutr. 2011, 15, 1100–1107. [Google Scholar] [CrossRef]
- Miller, L.C.; Joshi, N.; Lohani, M.; Singh, R.; Bhatta, N.; Rogers, B.L.; Griffiths, J.K.; Ghosh, S.; Mahato, S.; Singh, P.; et al. Head growth of under-nourished children in rural Nepal: Association with demographics, health and diet. Paediatr. Child Health 2016, 36, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.C.; Neupane, S.; Joshi, N.; Lohani, M. MILK Symposium review: Milk consumption is associated with better height and weight in rural Nepali children over 60 months of age and better head circumference in children 24 to 60 months of age. J. Dairy Sci. 2020, 103, 9700–9714. [Google Scholar] [CrossRef] [PubMed]
- Mosites, E.M.; Rabinowitz, P.M.; Thumbi, S.M.; Montgomery, J.M.; Palmer, G.H.; May, S.; Rowhani-Rahbar, A.; Neuhouser, M.L.; Walson, J.L. The Relationship between Livestock Ownership and Child Stunting in Three Countries in Eastern Africa Using National Survey Data. PLoS ONE 2015, 10, e0136686. [Google Scholar] [CrossRef]
- Nicholson, C.F.; Mwangi, L.; Staal, S.J.; Thornton, P.K. Dairy Cow Ownership and Child Nutritional Status in Kenya; Cornell University: Ithaca, NY, USA, 2003. [Google Scholar]
- Wiley, A.S. Cow milk consumption, insulin-like growth factor-I, and human biology: A life history approach. Am. J. Hum. Biol. 2012, 24, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Kabunga, N.S.; Ghosh, S.; Webb, P. Does ownership of improved dairy cow breeds improve child nutrition? A pathway analysis for Uganda. PLoS ONE 2017, 12, e0187816. [Google Scholar] [CrossRef]
- Dumas, S.E.; Kassa, L.; Young, S.; Travis, A.J. Examining the association between livestock ownership typologies and child nutrition in the Luangwa Valley, Zambia. PLoS ONE 2018, 13, e0191339. [Google Scholar] [CrossRef]
- Iannotti, L.; Muehlhoff, E.; Mcmahon, D. Review of milk and dairy programmes affecting nutrition. J. Dev. Eff. 2013, 5, 82–115. [Google Scholar] [CrossRef]
- Rawlins, R.; Pimkina, S.; Barrett, C.; Pedersen, S.; Wydick, B. Got milk? The impact of Heifer International’s livestock donation programs in Rwanda on nutritional outcomes. Food Policy 2014, 44, 202–213. [Google Scholar] [CrossRef]
- Hoddinott, J.; Headey, D.; Dereje, M. Cows, missing milk markets, and nutrition in rural Ethiopia. J. Dev. Stud. 2015, 51, 958–975. [Google Scholar] [CrossRef]
- Allen, L.H.; Dror, D.K. Effects of Animal Source Foods, with Emphasis on Milk, in the Diet of Children in Low-Income Countries. Milk Milk Prod. Hum. Nutr. 2011, 67, 113–130. [Google Scholar]
- Grenov, B.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F. Role of milk and dairy products in growth of the child. Glob. Landsc. Nutr. Chall. Infants Child. 2020, 93, 77–90. [Google Scholar]
- Ouma, E.A.; Flax, V.; Twine, E.E.; Kamana, O.; Kariuki, J.B. Enhancing milk quality and consumption for improved income and nutrition in Rwanda. In Feed the Future Project Inception Workshop; ILRI: Kigali, Rwanda, 2017. [Google Scholar]
- Ruel, M.T. Milk intake is associated with better growth in Latin America: Evidence from the Demographic and Health Surveys. FASEB J. 2003, 17, A1199. [Google Scholar]
- Christian, P.; Smith, E.R. Adolescent undernutrition: Global burden, physiology, and nutritional risks. Ann. Nutr. Metab. 2018, 72, 316–328. [Google Scholar] [CrossRef]
- Darnton-Hill, I.; Mkparu, U.C. Micronutrients in pregnancy in low-and middle-income countries. Nutrients 2015, 7, 1744–1768. [Google Scholar] [CrossRef] [PubMed]
- Lander, R.L.; Hambidge, K.M.; Westcott, J.E.; Tejeda, G.; Diba, T.S.; Mastiholi, S.C.; Khan, U.S.; Garcés, A.; Figueroa, L.; Tshefu, A.; et al. Pregnant Women in Four Low-Middle Income Countries Have a High Prevalence of Inadequate Dietary Intakes That Are Improved by Dietary Diversity. Nutrients 2019, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Talegawkar, S.A.; Merialdi, M.; Caulfield, L.E. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr. 2012, 16, 1340–1353. [Google Scholar] [CrossRef]
- Ochola, S.; Masibo, P.K. Dietary Intake of Schoolchildren and Adolescents in Developing Countries. Ann. Nutr. Metab. 2014, 64 (Suppl. S2), 24–40. [Google Scholar] [CrossRef]
- Ruel, M.T. Operationalizing Dietary Diversity: A Review of Measurement Issues and Research Priorities. J. Nutr. 2003, 133, 3911S–3926S. [Google Scholar] [CrossRef]
- Adesogan, A.T.; Havelaar, A.H.; McKune, S.L.; Eilittä, M.; Dahl, G.E. Animal source foods: Sustainability problem or malnutrition and sustainability solution? Perspective matters. Glob. Food Secur. 2019, 25, 100325. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Hulett, J.L.; Weiss, R.E.; Bwibo, N.O.; Galal, O.M.; Drorbaugh, N.; Neumann, C.G. Animal source foods have a positive impact on the primary school test scores of Kenyan schoolchildren in a cluster-randomised, controlled feeding intervention trial. Br. J. Nutr. 2013, 111, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.G.; Murphy, S.P.; Gewa, C.; Grillenberger, M.; Bwibo, N.O. Meat Supplementation Improves Growth, Cognitive, and Behavioral Outcomes in Kenyan Children. J. Nutr. 2007, 137, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Habicht, J.P.; Victora, C.; Vaughan, J.P. Evaluation designs for adequacy, plausibility and probability of public health programme performance and impact. Int. J. Epidemiol. 1999, 28, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Nepal; New ERA; ICF. Nepal Demographic and Health Survey 2016: Key Indicators; Ministry of Health: Kathmandu, Nepal, 2017.
- Ritchie, H.; Mathieu, E.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus Pandemic (COVID-19). 2020. Available online: https://ourworldindata.org/coronavirus (accessed on 20 April 2022).
- Kennedy, G.; Ballard, T.; Dop, M.C. Guidelines for Measuring Household and Individual Dietary Diversity Rome: FAO. 2013. Available online: http://www.fao.org/3/a-i1983e.pdf (accessed on 20 April 2022).
- World Health Organization. Indicators for Assessing Infant and Young Child Feeding Practices: Conclusions of a Consensus Meeting Held 6–8 November 2007 in Washington DC, USA; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- World Health Organization. Indicators for Assessing Infant and Young Child Feeding Practices, Part 2: Measurement; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Steyn, N.P.; Nel, J.H.; Nantel, G.; Kennedy, G.; Labadarios, D. Food variety and dietary diversity scores in children: Are they good indicators of dietary adequacy? Public Health Nutr. 2007, 9, 644–650. [Google Scholar] [CrossRef]
- Allen, R.E.; Myers, A.L. Nutrition in toddlers. Am. Fam. Physician 2006, 74, 1527–1532. [Google Scholar]
- Lott, M.; Callahan, E.; Welker Duffy, E.; Story, M.; Daniels, S. Healthy Beverage Consumption in Early Childhood: Recommendations from Key National Health and Nutrition Organizations. Consensus Statement. Durham, NC: Healthy Eating Research. 2019. Available online: http://healthyeatingresearch.org (accessed on 17 April 2022).
- Food and Agricultural Organization. Compendium of Agricultural-Environmental Indicators, Statistics Division 2003. Available online: https://www.fao.org/3/j0945e/j0945e00.pdf (accessed on 20 April 2022).
- Pérez-Escamilla, R.; Cunningham, K.; Moran, V.H. COVID-19 and Maternal and Child Food and Nutrition Insecurity: A Complex Syndemic; Wiley Online Library: Hoboken, NJ, USA, 2020; p. e13036. [Google Scholar]
- Miller, L.; Joshi, N.; Lohani, M.; Rogers, B.; Mahato, S.; Neupane, S.; Neupane, S.; Ghosh, S.; Webb, P. Greater improvements in child growth and diet quality after a holistic community development intervention than after nutrition training alone. Ann. Nutr. Metab. 2017, 71, 412–413. [Google Scholar]
- Miller, L.C.; Neupane, S.; Joshi, N.; Shrestha, M.; Neupane, S.; Lohani, M.; Thorne-Lyman, A.L. Diet quality over time is associated with better development in rural Nepali children. Matern. Child Nutr. 2020, 16, e12964. [Google Scholar] [CrossRef]
- Kvestad, I.; Hysing, M.; Shrestha, M.; Ulak, M.; Thorne-Lyman, A.L.; Henjum, S.; Ueland, P.M.; Øyvind, M.; Fawzi, W.; Chandyo, R.K.; et al. Vitamin B-12 status in infancy is positively associated with development and cognitive functioning 5 y later in Nepalese children. Am. J. Clin. Nutr. 2017, 105, 1122–1131. [Google Scholar] [CrossRef]
- Neumann, C.G.; Bwibo, N.O.; Murphy, S.P.; Sigman, M.; Whaley, S.; Allen, L.H.; Guthrie, D.; Weiss, R.E.; Demment, M.W. Animal Source Foods Improve Dietary Quality, Micronutrient Status, Growth and Cognitive Function in Kenyan School Children: Background, Study Design and Baseline Findings. J. Nutr. 2003, 133, 3941S–3949S. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E. The Lancet Nutrition Interventions Review Group, the Maternal and Child Nutrition Study Group. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- Gittelsohn, J.; Thapa, M.; Landman, L.T. Cultural factors, caloric intake and micronutrient sufficiency in rural Nepali households. Soc. Sci. Med. 1997, 44, 1739–1749. [Google Scholar] [CrossRef]
- Nicholson, C.F.; Thornton, P.K.; Muinga, R.W. Household-level Impacts of Dairy Cow Ownership in Coastal Kenya. J. Agric. Econ. 2004, 55, 175–195. [Google Scholar] [CrossRef]
- Thorne-Lyman, A.; Spiegelman, D.; Fawzi, W.W. Is the strength of association between indicators of dietary quality and the nutritional status of children being underestimated? Matern. Child Nutr. 2014, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Data; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 6 May 2022).
- Choudhury, S.; Headey, D.D. Household dairy production and child growth: Evidence from Bangladesh. Econ. Hum. Biol. 2018, 30, 150–161. [Google Scholar] [CrossRef]
- Citrin, D.; Wangmo, T.; Shrestha, A.; Craig, S.R.; Rankin, K.; Murton, G. Why we cannot ignore Nepal’s COVID-19 crisis. Brit. Med. J. Opin. 2020. Available online: https://blogs.bmj.com/bmj/2021/09/17/why-we-cannot-ignore-nepals-covid-19-crisis/ (accessed on 6 May 2022).
- Osendarp, S.; Akuoku, J.; Black, R.; Headey, D.; Ruel, M.; Scott, N.; Shekar, M.; Walker, N.; Flory, A.; Haddad, L.; et al. The COVID-19 crisis will exacerbate maternal and child undernutrition and child mortality in low-and middle-income countries. Nat. Food 2021, 2, 476–484. [Google Scholar] [CrossRef]
- De Vries, A.; Kaylegian, K.; Dahl, G. MILK Symposium review: Improving the productivity, quality, and safety of milk in Rwanda and Nepal. J. Dairy Sci. 2020, 103, 9758–9773. [Google Scholar] [CrossRef]
- Sharma, B. Milk marketing and dairy value chain development in Nepal in relation with climate resilience effort in the present context. Nepal. Vet. J. 2017, 34, 144–151. [Google Scholar] [CrossRef][Green Version]
- Shingh, S.; Kalwar, C.S.; Poudel, S.; Tiwari, P.; Jha, S. A Study on Growth and Performance of Dairy Sector in Nepal. Int. J. Environ. Agric. Biotechnol. 2020, 5, 1154–1166. [Google Scholar]
- Bhattarai, N. Seasonal variation in milk yield, fat and SNF content of Murrah crossbred buffalo in mid-western terai region of Nepal. J. Agric. For. Univ. 2020, 4, 279. [Google Scholar]
- CASA Nepal Country Team. Dairy Sector Strategy–Nepal; UKAID: London, UK, 2020. [Google Scholar]
- Poudel, D.; Kandel, M.; Bhattarai, N.; Kaphle, K. Seasonal variation in milk yield and quality parameters in Murrah crossbred buffaloes of sub-tropical Nepal. In Proceedings of the International SAARC Youth Scientific Conference: Connecting Lives with Land, Water and Environment; Kathmandu: Ministry of Industry, Tourism, Forests, and Environment, Kathmandu, Nepal, 5–6 June 2019; Aryal, D., Ed.; Ministry of Industry, Tourism, Forests, and Environment: Kathmandu, Nepal, 2019; pp. 35–39. [Google Scholar]
| Child Characteristics | ||||
|---|---|---|---|---|
| Child gender (M:F) | 213:155 | |||
| Child gender % | 58%:42% | |||
| Parents’ education | ||||
| Mothers’ education (n = 309) | ||||
| None or basic | 32 (10%) | |||
| Some or completed primary school | 58 (19%) | |||
| Some or completed secondary school | 82 (27%) | |||
| School-leaving certificate or beyond | 137 (44%) | |||
| Fathers’ education (n = 309) | ||||
| None or basic | 6 (2%) | |||
| Some or completed primary school | 59 (19%) | |||
| Some or completed secondary school | 81 (26%) | |||
| School-leaving certificate or beyond | 163 (53%) | |||
| Household and child characteristics over time | ROUND 1 (n = 251) | ROUND 2 (n = 230) | ROUND 3 (n = 258) | Round 1 vs. Round 2 vs. Round 3 |
| Household | ||||
| Wealth score | −0.00 ± 1.00 | 0.09 ± 0.89 | −0.00 ± 1.00 | ns |
| Income per capita (NPR) | 65,925 ± 52,299 | 72,099 ± 55,741 | 73,240 ± 49,337 | ns |
| Animal score | 2.35 ± 2.36 | 2.51 ± 2.59 | 2.40 ± 2.49 | ns |
| Land ownership (m2) | 6272 ± 8198 | 5941 ± 5214 | 5884 ± 5171 | ns |
| Number of children <15 years | 2.04 ± 1.11 | 2.12 ± 1.05 | 2.18 ± 1.06 | ns |
| Child | (n = 299) | (n = 272) | (n = 340) | |
| Child age (months) | 34.3 ± 16.7 | 51.34 ± 21.1 | 47.7 ± 16.7 | <0.0001 |
| Round 1 (n = 301) | Round 2 (n = 272) | Round 3 (n = 341) | p 3 | |||
|---|---|---|---|---|---|---|
| Time | ||||||
| Child Key Diet Indicators | 24 h | DDS 1 | 4.5 ± 1.1 | 4.6 ± 1.1 | 4.3 ± 1.2 | *** |
| Consumed at least 1 ASF 2 | 98% | 93% | 89% | *** | ||
| # of ASF consumed 1 | 1.7 ± 0.8 | 1.8 ± 0.9 | 1.5 ± 0.9 | *** | ||
| Consumed eggs 2 | 38% | 32% | 22% | *** | ||
| Consumed meat 2 | 26% | 20% | 26% | ns | ||
| 7 days | # of times ASF consumed 1 | 16.4 ± 5.9 | 17.5 ± 8.1 | 15.3 ± 8.6 | ** | |
| # of times eggs consumed 1 | 2.2 ± 2.5 | 2.3 ± 2.6 | 1.5 ± 2.3 | *** | ||
| # of times meat consumed 1 | 0.7 ± 0.8 | 0.9 ± 0.9 | 1.2 ± 1.1 | *** | ||
| Child Milk-Specific indicators | 24 h | Consumed milk 2 | 97% | 89% | 79% | *** |
| Amount of milk consumed (mL) 1 | 298 ± 162 | 368 ± 204 | 297 ± 218 | *** | ||
| Milk volume sufficient for age? 2 | 21% | 30% | 22% | ** | ||
| Milk ml/kg 1 | 24 ± 14 | 29 ± 16 † | 22 ± 17 | *** | ||
| 7 days | # of times milk consumed 1 | 12.9 ± 5.1 | 12.5 ± 6.3 | 10.9 ± 6.9 | *** | |
| Consumed milk ≤7× 2 | 4% | 13% | 23% | *** | ||
| Family ^ Diet Indicators | 24 h | DDS 1 | 4.3 ± 1.1 | 4.5 ± 1.1 | 4.4 ± 1.1 | ns |
| # of ASF consumed 1 | 1.4 ± 0.8 | 1.6 ± 0.9 | 1.6 ± 1.0 | *** | ||
| Consumed at least 1 ASF 2 | 88% | 92% | 88% | ns | ||
| Consumed milk 2 | 81% | 85% | 77% | ns | ||
| Time | Indicator | Round 2 | Round 3 | |
|---|---|---|---|---|
| Child Key Diet Indicators | 24 h | DDS | 0.04 (−0.17, 0.25) | −0.227 * (-0.43, −0.02) |
| # ASF consumed | 0.09 (−0.09, 0.26) | −0.08 (−0.26, 0.09) | ||
| Consumed at least 1 ASF 1 | 0.07 ** (0.01, 0.34) | 0.04 *** (0.01, 0.18) | ||
| 7 days | # times ASF consumed | 2.03 ** (0.74, 3.32) | 0.48 (−0.82, 1.77) | |
| Child Milk-specific indicators | 24 h | Consumed milk 1 | 0.15 *** (0.05, 0.43) | 0.07 *** (0.02, 0.19) |
| Amount of milk consumed (mL) | 50.37 ** (16.25, 84.48) | 3.02 (−31.13, 37.17) | ||
| Milk volume sufficient for age? 1 | 2.78*** (1.57, 4.93) | 1.63 (0.92, 2.88) | ||
| Milk mL/kg | 3.56 * (0.46, 6.65) | −0.32 (−2.98, 2.33) | ||
| 7 days | # times milk consumed | 0.19 (−0.86, 1.24) | −0.94 (−1.99, 0.1) | |
| Consumed milk ≤7× 1 | 0.26 *** (0.14, 0.47) | 0.16 *** (0.09, 0.28) | ||
| Family Diet Indicators † | 7 days | DDS | 0.16 (−0.05, 0.36) | 0.04 (−0.17, 0.24) |
| # ASF consumed | 0.26 ** (0.08, 0.43) | 0.27 ** (0.1, 0.45) | ||
| Consumed at least 1 ASF 1 | 1.32 (0.65, 2.7) | 0.89 (0.47, 1.7) | ||
| Consumed milk 1 | 1 (0.54, 1.83) | 0.64 (0.36, 1.14) |
| Time | Indicator | Has at Least 1 Adult Cow or Buffalo | Milk Production >3.5 L/Day | |
|---|---|---|---|---|
| Child Key Diet Indicators | 24 h | DDS | −0.02 (−0.26, 0.23) | 0.16 (−0.01, 0.33) |
| # ASF consumed | 0.11 (−0.1, 0.31) | 0.14 (0.00, 0.28) | ||
| Consumed at least 1 ASF 1 | 0.89 (0.51, 1.55) | 1.12 (0.76, 1.65) | ||
| 7 days | # times ASF consumed | 1.60 (−0.05, 3.24) | 2.21 *** (1.09, 3.34) | |
| Child Milk-specific indicators | 24 h | Consumed milk 1 | 2.88 * (1.29, 6.43) | 7.45 *** (2.97, 18.69) |
| Amount of milk consumed (mL) | 58.72 ** (15.44, 102.01) | 67.98 *** (38.41, 97.55) | ||
| Milk volume sufficient for age? 1 | 2.31 * (1.08, 4.94) | 2.12 ** (1.31, 3.43) | ||
| Milk mL/kg | 4.66 * (0.91, 8.40) | 4.33 ** (1.85, 6.81) | ||
| 7 days | # times milk consumed | 1.60 * (0.28, 2.93) | 1.99 *** (1.09, 2.9) | |
| Consumed milk ≤7× 1 | 1.2 (0.64, 2.27) | 1.24 (0.79, 1.96) | ||
| Family Diet Indicators † | 7 days | DDS | 0.2 (−0.03, 0.44) | 0.31 *** (0.15, 0.48) |
| Consumed at least 1 ASF 1 | 4.77 *** (2.56, 8.89) | 6.116 *** (3.06, 12.17) | ||
| # ASF consumed | 0.35 ** (0.14, 0.55) | 0.24 *** (0.11, 0.37) | ||
| Consumed milk1 | 5.81 *** (3.15, 10.7) | 11.88 *** (6.17, 22.88) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, L.C.; Neupane, S.; Joshi, N.; Lohani, M.; Sah, K.; Shrestha, B. Dairy Animal Ownership and Household Milk Production Associated with Better Child and Family Diet in Rural Nepal during the COVID-19 Pandemic. Nutrients 2022, 14, 2074. https://doi.org/10.3390/nu14102074
Miller LC, Neupane S, Joshi N, Lohani M, Sah K, Shrestha B. Dairy Animal Ownership and Household Milk Production Associated with Better Child and Family Diet in Rural Nepal during the COVID-19 Pandemic. Nutrients. 2022; 14(10):2074. https://doi.org/10.3390/nu14102074
Chicago/Turabian StyleMiller, Laurie C., Sumanta Neupane, Neena Joshi, Mahendra Lohani, Keshav Sah, and Bhola Shrestha. 2022. "Dairy Animal Ownership and Household Milk Production Associated with Better Child and Family Diet in Rural Nepal during the COVID-19 Pandemic" Nutrients 14, no. 10: 2074. https://doi.org/10.3390/nu14102074
APA StyleMiller, L. C., Neupane, S., Joshi, N., Lohani, M., Sah, K., & Shrestha, B. (2022). Dairy Animal Ownership and Household Milk Production Associated with Better Child and Family Diet in Rural Nepal during the COVID-19 Pandemic. Nutrients, 14(10), 2074. https://doi.org/10.3390/nu14102074






