Exclusion Diets in Functional Dyspepsia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
3. Results
3.1. FODMAP in Patients with Functional Dyspepsia
3.2. Dyspepsia and Gluten
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology 2016, 150, 1262–1279.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talley, N.J. Functional dyspepsia: New insights into pathogenesis and therapy. Korean J. Intern. Med. 2016, 31, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Mahadeva, S.; Carbone, M.F.; Lacy, B.E.; Talley, N.J. Functional dyspepsia. Lancet 2020, 396, 1689–1702. [Google Scholar] [CrossRef]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef] [PubMed]
- Wauters, L.; Dickman, R.; Drug, V.; Mulak, A.; Serra, J.; Enck, P.; Tack, J.; Accarino, A.; Barbara, G.; Bor, S.; et al. United European Gastroenterology (UEG) and European Society for Neurogastroenterology and Motility (ESNM) consensus on functional dyspepsia. Neurogastroenterol. Motil. 2021, 33, e14238. [Google Scholar] [CrossRef]
- Gathaiya, N.; Locke, G.R., 3rd; Camilleri, M.; Schleck, C.D.; Zinsmeister, A.R.; Talley, N.J. Novel associations with dyspepsia: A community-based study of familial aggregation, sleep dysfunction and somatization. Neurogastroenterol. Motil. 2009, 21, 922-e69. [Google Scholar] [CrossRef] [Green Version]
- Pesce, M.; Cargiolli, M.; Cassarano, S.; Polese, B.; De Conno, B.; Aurino, L.; Mancino, N.; Sarnelli, G. Diet and functional dyspepsia: Clinical correlates and therapeutic perspectives. World J. Gastroenterol. 2020, 26, 456–465. [Google Scholar] [CrossRef]
- Dehghanizade, Z.; Zargar, Y.; Honarmand, M.M.; Kadkhodaie, A.; Baygi, M.E. The effectiveness of cognitive behavior stress management on functional dyspepsia symptoms. J. Adv. Med Educ. Prof. 2015, 3, 45–49. [Google Scholar]
- Faramarzi, M.; Azadfallah, P.; Book, H.E.; Tabatabaei, K.R.; Taheri, H.; Shokri-Shirvani, J. A randomized controlled trial of brief psychoanalytic psychotherapy in patients with functional dyspepsia. Asian J. Psychiatry 2013, 6, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Popa, S.-L.; Chiarioni, G.; David, L.; Dumitrascu, D.L. The Efficacy of Hypnotherapy in the Treatment of Functional Dyspepsia. Am. J. Ther. 2019, 26, e704–e713. [Google Scholar] [CrossRef]
- Chen, J.D.Z.; Ni, M.; Yin, J. Electroacupuncture treatments for gut motility disorders. Neurogastroenterol. Motil. 2018, 30, e13393. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.V.B.; Lorena, S.L.S.; Almeida, J.R.D.S.; Mesquita, M.A. Food Intolerance, Diet Composition, and Eating Patterns in Functional Dyspepsia Patients. Am. J. Dig. Dis. 2009, 55, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Stanghellini, V.; De Ponti, F.; de Giorgio, R.; Barbara, G.; Tosetti, C.; Corinaldesi, R. New Developments in the Treatment of Functional Dyspepsia. Drugs 2003, 63, 869–892. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tan, V.P. The low-FODMAP diet in the management of functional dyspepsia in East and Southeast Asia. J. Gastroenterol. Hepatol. 2017, 32, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, P.; Zhang, L.; Hou, X. A Low-FODMAP Diet Improves the Global Symptoms and Bowel Habits of Adult IBS Patients: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 683191. [Google Scholar] [CrossRef]
- Popa, S.L.; Pop, C.; Dumitrascu, D.L. Diet Advice for Crohn’s Disease: FODMAP and Beyond. Nutrients 2020, 12, 3751. [Google Scholar] [CrossRef]
- Duncanson, K.; Burns, G.; Pryor, J.; Keely, S.; Talley, N.J. Mechanisms of Food-Induced Symptom Induction and Dietary Management in Functional Dyspepsia. Nutrients 2021, 13, 1109. [Google Scholar] [CrossRef]
- Tsega, E.; Endeshaw, Y.; Mengesha, B.; Tedla, B. The role of milk and lactose intolerance in Ethiopian patients with non-ulcer dyspepsia: A case control study. Ethiop. Med. J. 1989, 27, 135–145. [Google Scholar]
- Wortmann, A.C.; Simon, D.; Mazzoleni, L.E.; Sander, G.B.; Francesconi, C.F.M.; Nabinger, D.D.; Grott, C.S.; Rech, T.F.; Mazzoleni, F.; Lunge, V.R.; et al. The association between adult-type hypolactasia and symptoms of functional dyspepsia. Genet. Mol. Biol. 2018, 41, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Mishkin, D.; Sablauskas, L.; Yalovsky, M.; Mishkin, S. Fructose and sorbitol malabsorption in ambulatory patients with functional dyspepsia: Comparison with lactose maldigestion/malabsorption. Dig. Dis. Sci. 1997, 42, 2591–2598. [Google Scholar] [PubMed]
- Pryor, J.; Burns, G.L.; Duncanson, K.; Horvat, J.C.; Walker, M.M.; Talley, N.J.; Keely, S. Functional Dyspepsia and Food: Immune Overlap with Food Sensitivity Disorders. Curr. Gastroenterol. Rep. 2020, 22, 51. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.D.E.; Duncanson, K.; Jones, M.P.; Walker, M.M.; Keely, S.; Talley, N.J. Wheat Sensitivity and Functional Dyspepsia: A Pilot, Double-Blind, Randomized, Placebo-Controlled Dietary Crossover Trial with Novel Challenge Protocol. Nutrients 2020, 12, 1947. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Nevin, A.N.; Duff, C.; Kendall, B.J.; Holtmann, G.J. Epigastric symptom response to low FODMAP dietary advice compared with standard dietetic advice in individuals with functional dyspepsia. Neurogastroenterol. Motil. 2021, 33, e14148. [Google Scholar] [CrossRef] [PubMed]
- Adibi, P.; Esmaillzadeh, A.; Daghaghzadeh, H.; Keshteli, A.H.; Feizi, A.; Haghighatdoost, F.; Jafari, M. Low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet is associated with increased risk of uninvestigated chronic dyspepsia and its symptoms in adults. Minerva Gastroenterol. 2021. [Google Scholar] [CrossRef]
- Goyal, O.; Nohria, S.; Batta, S.; Dhaliwal, A.; Goyal, P.; Sood, A. Low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet versus traditional dietary advice for functional dyspepsia: A randomized controlled trial. J. Gastroenterol. Hepatol. 2021, 37, 301–309. [Google Scholar] [CrossRef]
- Tejedor, M.; Alcalde, D.; Cruces, C.; Hernando, E.; López-Martín, M.C.; Briz, R.; Calvache, A.; Barranco, R.; Castillo, L.A.; Chico, I.; et al. Functional gastrointestinal disorders: Real-life results of a multidisciplinary non-pharmacological approach based on group-consultations. Rev. Esp. Enferm. Dig. 2021, 113, 627–634. [Google Scholar] [CrossRef]
- King, J.A.; Jeong, J.; Underwood, F.E.; Quan, J.; Panaccione, N.; Windsor, J.W.; Coward, S.; Debruyn, J.; Ronksley, P.E.; Shaheen, A.-A.; et al. Incidence of Celiac Disease Is Increasing Over Time: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2020, 115, 507–525. [Google Scholar] [CrossRef]
- Ford, A.C.; Ching, E.; Moayyedi, P. Meta-analysis: Yield of diagnostic tests for coeliac disease in dyspepsia. Aliment. Pharmacol. Ther. 2009, 30, 28–36. [Google Scholar] [CrossRef]
- Chiarioni, G.; Bassotti, G.; Germani, U.; Battaglia, E.; Brentegani, M.T.; Morelli, A.; Vantini, I. Gluten-free diet normalizes mouth-to-cecum transit of a caloric meal in adult patients with celica disease. Dig. Dis. Sci. 1997, 42, 2100–2105. [Google Scholar]
- Santolaria, S.; Alcedo, J.; Cuartero, B.; Diez, I.; Abascal, M.; García-Prats, M.D. Spectrum of gluten-sensitive enteropathy in patients with dysmotility-like dyspepsia. Gastroenterol. Hepatol. 2013, 36, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A. Wheat allergy: Diagnosis and management. J. Asthma Allergy 2016, 9, 13–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinninella, E.; Cintoni, M.; Mele, M.C.; Gasbarrini, A. Irritable Bowel Syndrome (IBS) and Non-Celiac Gluten Sensitivity (NCGS): Where Is the Culprit Hiding? Nutritional Tips for Gastroenterologists. Nutrients 2019, 11, 2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, M.D.E.; Walker, M.M.; Jones, M.P.; Koloski, N.A.; Keely, S.; Talley, N.J. Wheat Intolerance and Chronic Gastrointestinal Symptoms in an Australian Population-based Study: Association Between Wheat Sensitivity, Celiac Disease and Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2018, 113, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Shahbazkhani, B.; Fanaeian, M.M.; Farahvash, M.J.; Aletaha, N.; Alborzi, F.; Elli, L.; Shahbazkhani, A.; Zebardast, J.; Rostami-Nejad, M. Prevalence of Non-Celiac Gluten Sensitivity in Patients with Refractory Functional Dyspepsia: A Randomized Double-blind Placebo Controlled Trial. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elli, L.; Tomba, C.; Branchi, F.; Roncoroni, L.; Lombardo, V.; Bardella, M.T.; Ferretti, F.; Conte, D.; Valiante, F.; Fini, L.; et al. Evidence for the Presence of Non-Celiac Gluten Sensitivity in Patients with Functional Gastrointestinal Symptoms: Results from a Multicenter Randomized Double-Blind Placebo-Controlled Gluten Challenge. Nutrients 2016, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Shen, J.; Kim, J.J.; He, H.; Chen, B.; Dai, N. Impact of gluten consumption in patients with functional dyspepsia: A case-control study. J. Gastroenterol. Hepatol. 2018, 33, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Rej, A.; Aziz, I.; Tornblom, H.; Sanders, D.S.; Simrén, M. The role of diet in irritable bowel syndrome: Implications for dietary advice. J. Intern. Med. 2019, 286, 490–502. [Google Scholar] [CrossRef] [Green Version]
- Mounsey, A.; Barzin, A.; Rietz, A. Functional dyspepsia: Evaluation and management. Am. Fam. Phys. 2020, 101, 84–88. [Google Scholar]
- Saito, Y.A.; Locke, G.R., 3rd; Weaver, A.L.; Zinsmeister, A.R.; Talley, N.J. Diet and functional gastrointestinal disorders: A population-based case-control study. Am. J. Gastroenterol. 2005, 100, 2743–2748. [Google Scholar] [CrossRef]
- Friedlander, P.H. Food and indigestion. An investigation of possible relationships. BMJ 1959, 2, 1454–1458. [Google Scholar] [CrossRef] [PubMed]
- Kaess, H.; Kellermann, M.; Castro, A. Food intolerance in duodenal ulcer patients, non ulcer dyspeptic patients and healthy subjects. A prospective study. Klin. Wochenschr. 1988, 66, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Filipović, B.F.; Randjelovic, T.; Kovacevic, N.; Milinić, N.; Markovic, O.; Gajić, M.; Filipović, B.R. Laboratory parameters and nutritional status in patients with functional dyspepsia. Eur. J. Intern. Med. 2011, 22, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Feinle-Bisset, C.; Azpiroz, F. Dietary and lifestyle factors in functional dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 150–157. [Google Scholar] [CrossRef]
- Piessevaux, H.; De Winter, B.; Louis, E.; Muls, V.; De Looze, D.; Pelckmans, P.; Deltenre, M.; Urbain, D.; Tack, J. Dyspeptic symptoms in the general population: A factor and cluster analysis of symptom groupings. Neurogastroenterol. Motil. 2009, 21, 378–388. [Google Scholar] [CrossRef]
- Castillo, E.J.; Camilleri, M.; Locke, G.R.; Burton, D.D.; Stephens, D.A.; Geno, D.M.; Zinsmeister, A.R. A community-based, controlled study of the epidemiology and pathophysiology of dyspepsia. Clin. Gastroenterol. Hepatol. 2004, 2, 985–996. [Google Scholar] [CrossRef]
- Bisschops, R.; Karamanolis, G.; Arts, J.; Caenepeel, P.; Verbeke, K.; Janssens, J.; Tack, J. Relationship between symptoms and ingestion of a meal in functional dyspepsia. Gut 2008, 57, 1495–1503. [Google Scholar] [CrossRef]
- Mullan, A.; Kavanagh, P.; O’Mahony, P.; Joy, T.; Gleeson, F.; Gibney, M.J. Food and nutrient intakes and eating patterns in functional and organic dyspepsia. Eur. J. Clin. Nutr. 1994, 48, 97–105. [Google Scholar]
- Pilichiewicz, A.N.; Horowitz, M.; Holtmann, G.J.; Talley, N.J.; Feinle-Bisset, C. Relationship between symptoms and dietary patterns in patients with functional dyspepsia. Clin. Gastroenterol. Hepatol. 2009, 7, 317–322. [Google Scholar] [CrossRef]
- Duncanson, K.R.; Talley, N.J.; Walker, M.M.; Burrows, T.L. Food and functional dyspepsia: A systematic review. J. Hum. Nutr. Diet. 2018, 31, 390–407. [Google Scholar] [CrossRef]
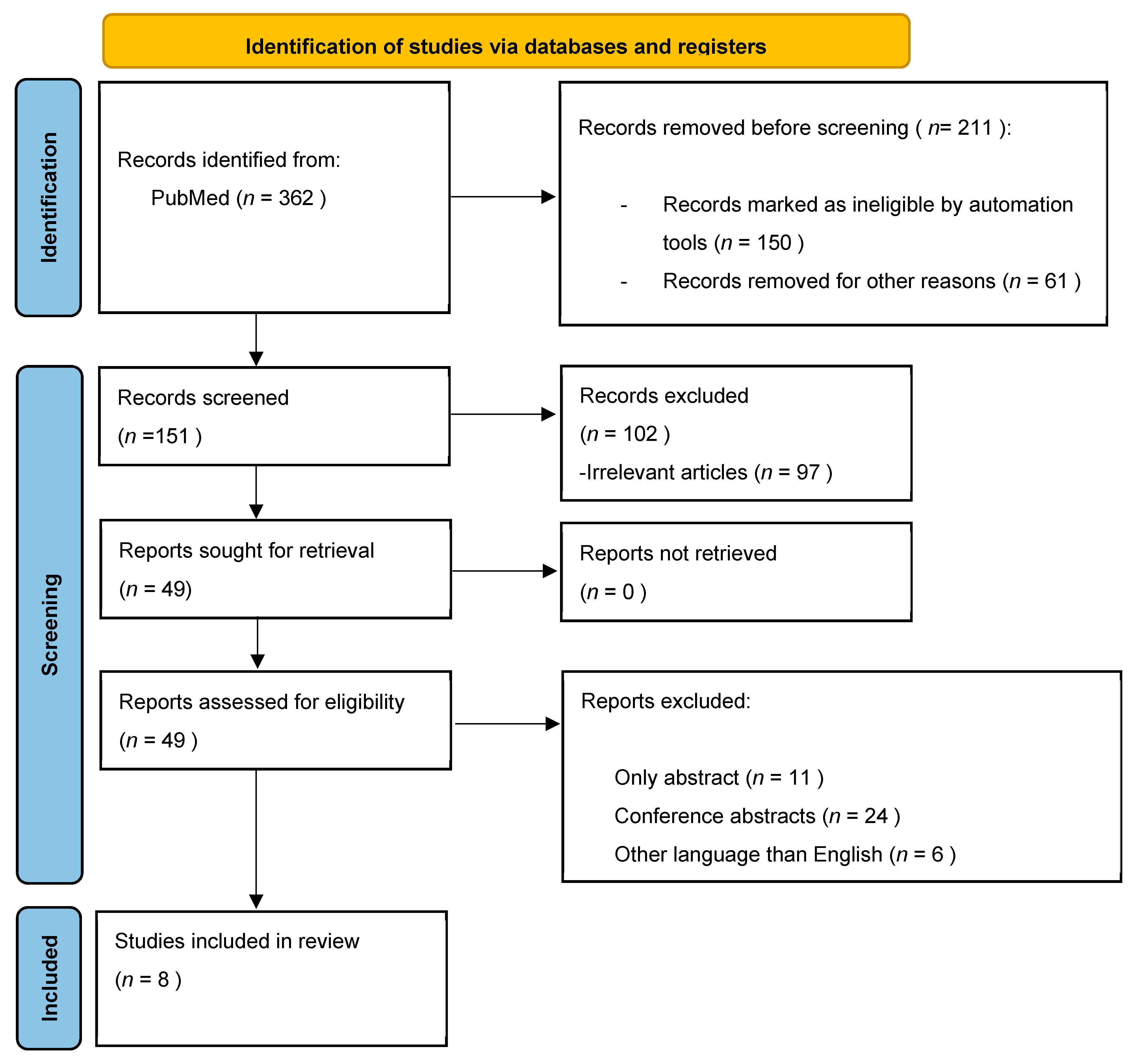
| Symptoms | Type of Food |
|---|---|
| Early satiety | Red meat, bananas, bread, cakes, pasta, sausages, fried foods, beans, onions, mayonnaise, milk, chocolate, eggs, sweets, oranges |
| Bloating | Soft drinks, onions, beans, bananas |
| Epigastric pain Epigastric burning | Cheese, Coffee, onion, pepper, milk, chocolate, pineapple |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, S.L.; Dumitrascu, D.I.; Pop, C.; Surdea-Blaga, T.; Ismaiel, A.; Chiarioni, G.; Dumitrascu, D.L.; Brata, V.D.; Grad, S. Exclusion Diets in Functional Dyspepsia. Nutrients 2022, 14, 2057. https://doi.org/10.3390/nu14102057
Popa SL, Dumitrascu DI, Pop C, Surdea-Blaga T, Ismaiel A, Chiarioni G, Dumitrascu DL, Brata VD, Grad S. Exclusion Diets in Functional Dyspepsia. Nutrients. 2022; 14(10):2057. https://doi.org/10.3390/nu14102057
Chicago/Turabian StylePopa, Stefan Lucian, Dinu Iuliu Dumitrascu, Cristina Pop, Teodora Surdea-Blaga, Abdulrahman Ismaiel, Giuseppe Chiarioni, Dan Lucian Dumitrascu, Vlad Dumitru Brata, and Simona Grad. 2022. "Exclusion Diets in Functional Dyspepsia" Nutrients 14, no. 10: 2057. https://doi.org/10.3390/nu14102057
APA StylePopa, S. L., Dumitrascu, D. I., Pop, C., Surdea-Blaga, T., Ismaiel, A., Chiarioni, G., Dumitrascu, D. L., Brata, V. D., & Grad, S. (2022). Exclusion Diets in Functional Dyspepsia. Nutrients, 14(10), 2057. https://doi.org/10.3390/nu14102057









