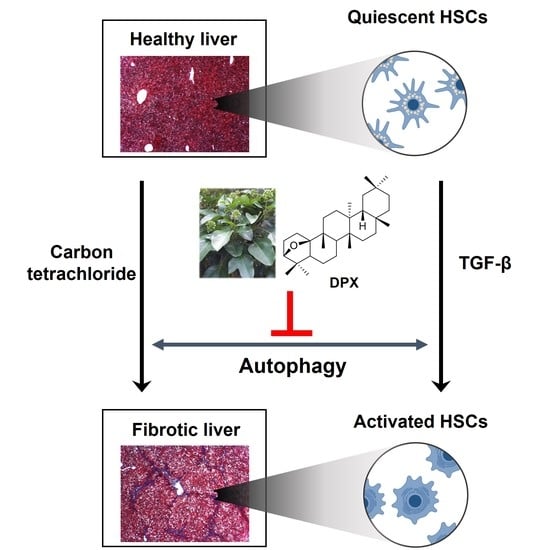
Dendropanoxide, a Triterpenoid from Dendropanax morbifera, Ameliorates Hepatic Fibrosis by Inhibiting Activation of Hepatic Stellate Cells through Autophagy Inhibition
Abstract
:1. Introduction
2. Materials and Methods
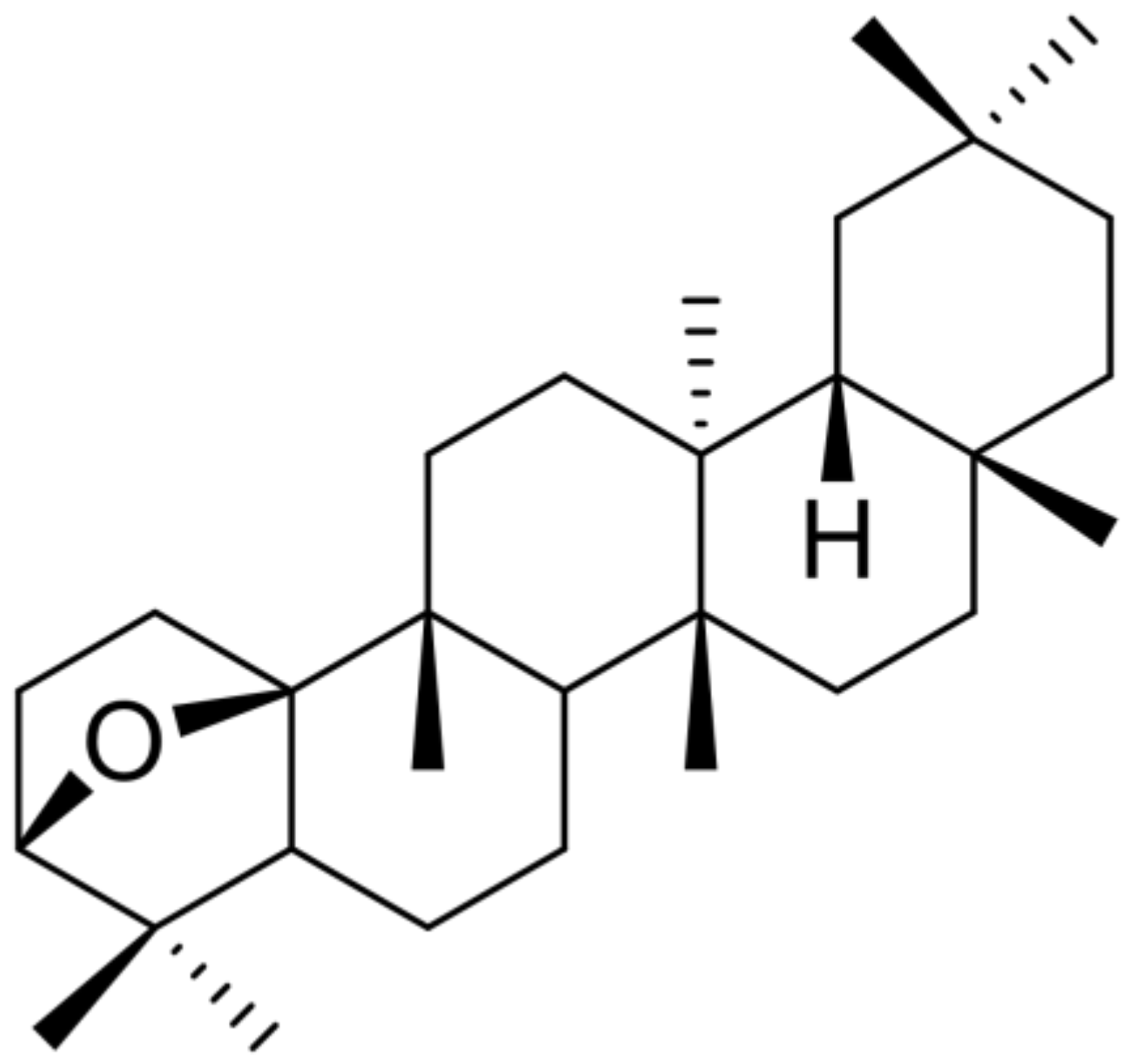
2.1. Extraction and Isolation of DPX
2.2. Chemicals
2.3. Cell Lines and Culture
2.4. Animal Experiments
2.5. Liver Histology and Blood Analysis
2.6. Cell Viability Assay
2.7. Comparative Quantitative Real-Time PCR (qRT-PCR)
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
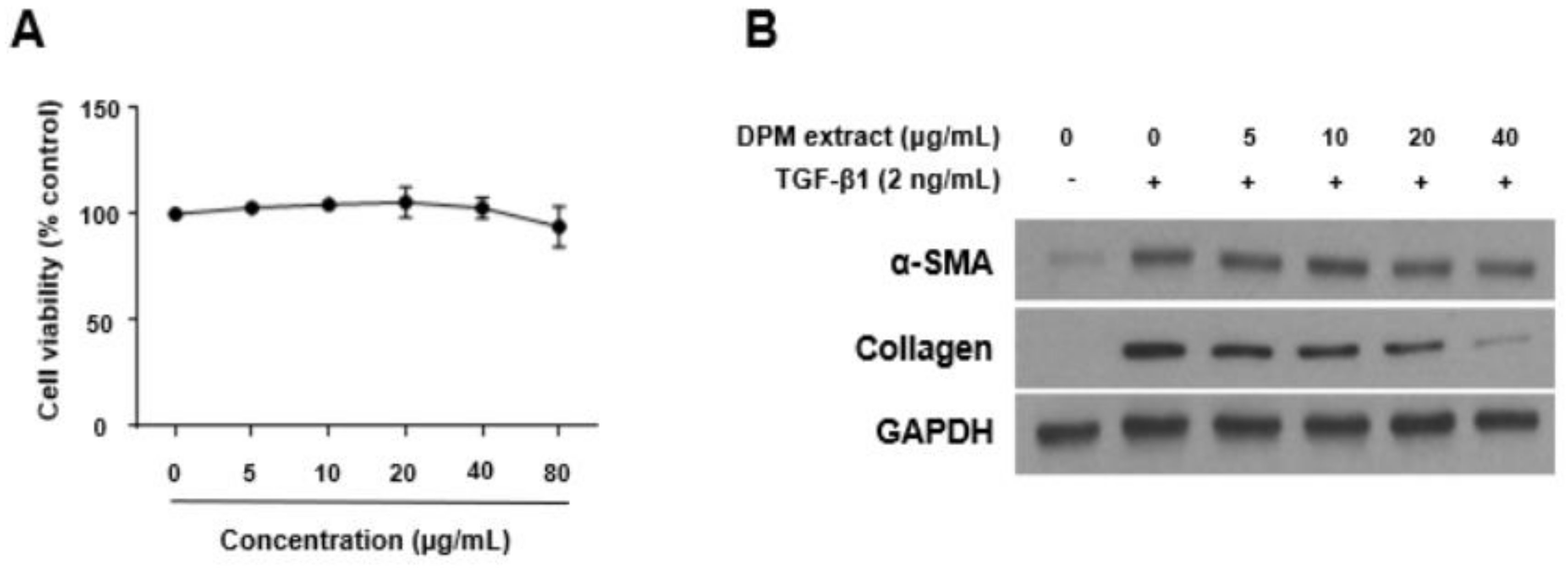
3.1. Effects of DPM Extract on Activated Hepatic Stellate Cells
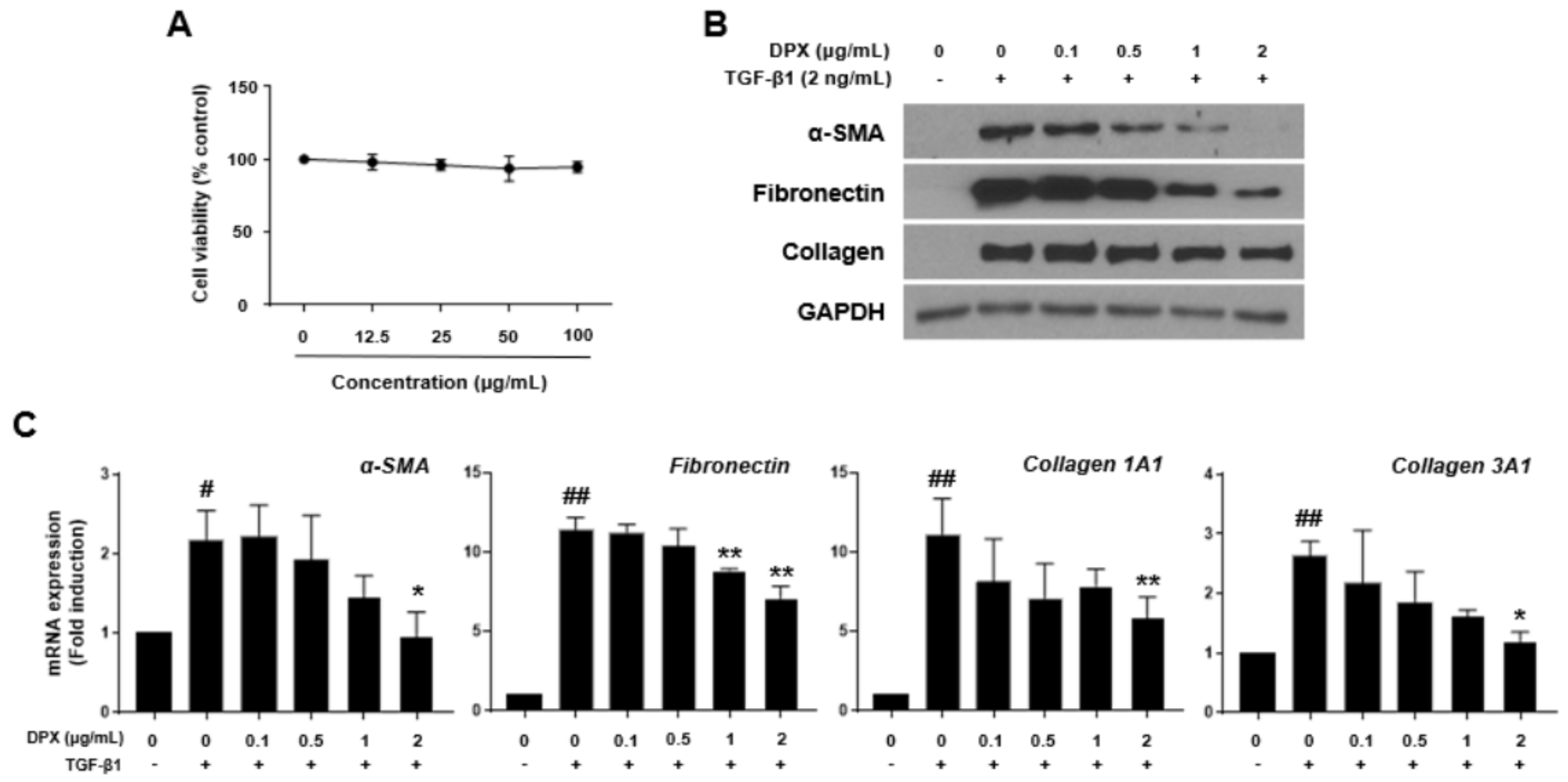
3.2. Isolation of DPX and Its Effects on Activated Hepatic Stellate Cells
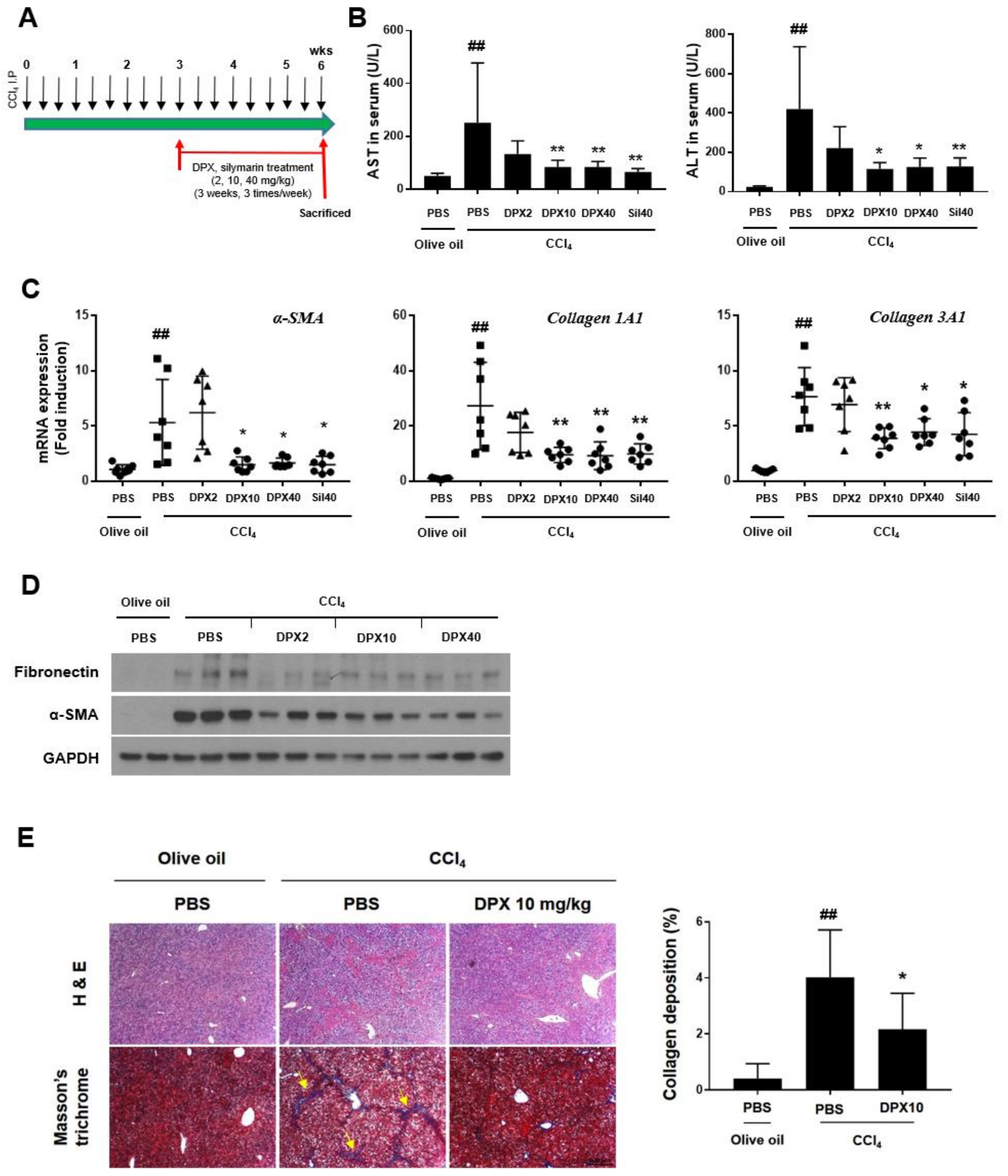
3.3. Anti-Fibrotic Effects of DPX in Hepatic Fibrosis Mice
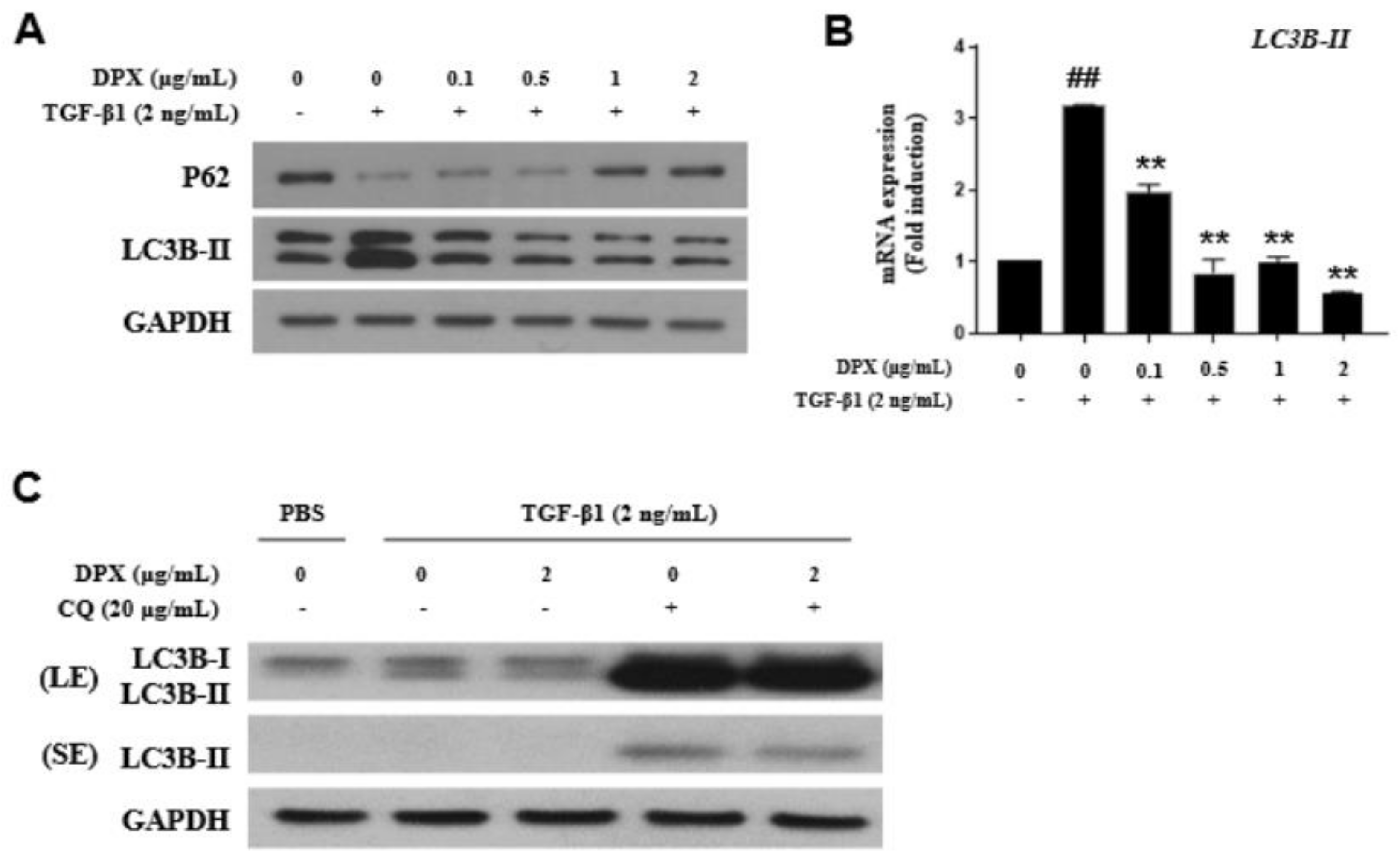
3.4. Anti-Fibrotic Effects of DPX by Inhibiting Autophagy Inhibition
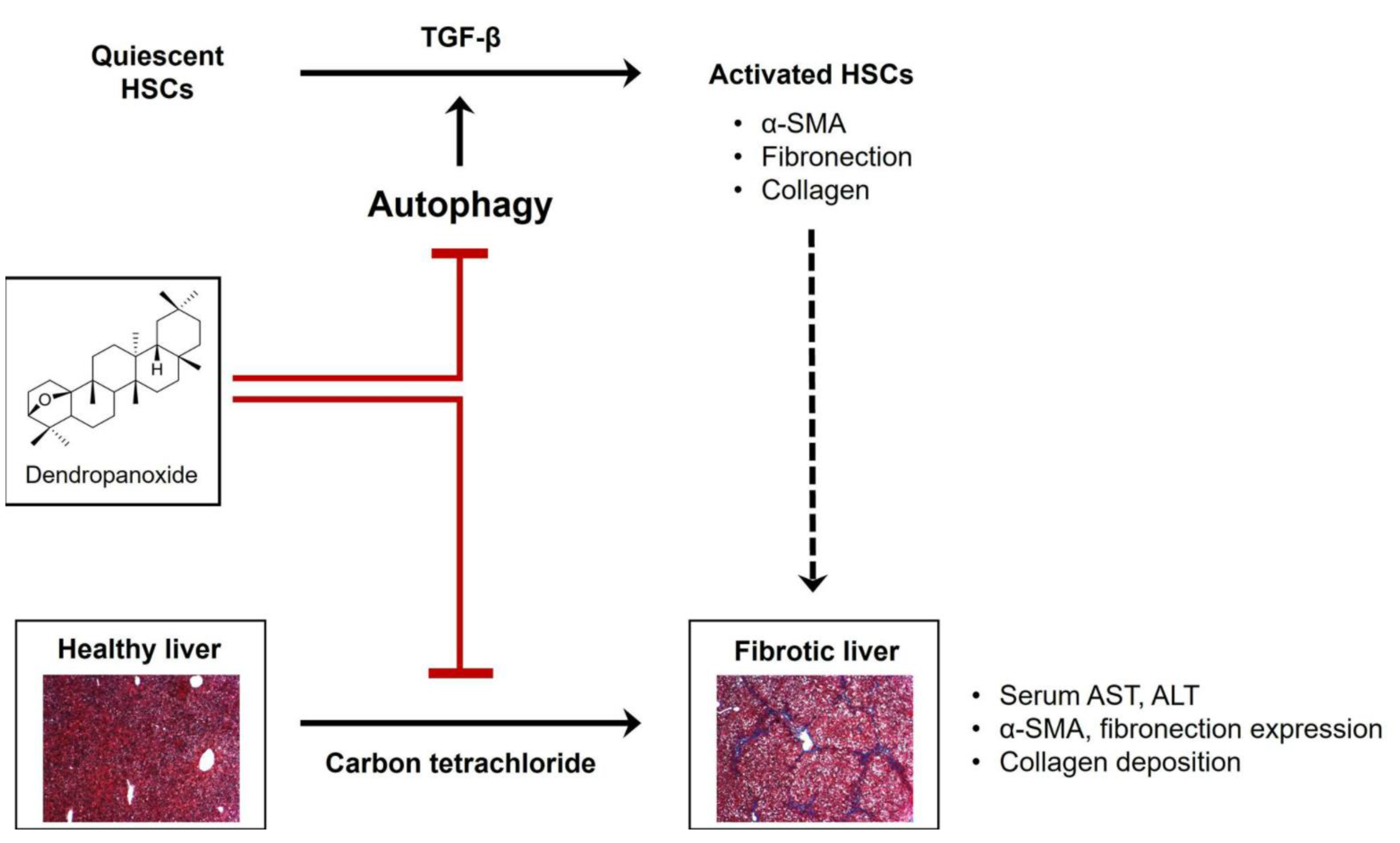
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harris, R.; Harman, D.J.; Card, T.R.; Aithal, G.P.; Guha, I.N. Prevalence of clinically significant liver disease within the general population, as defined by non-invasive markers of liver fibrosis: A systematic review. Lancet Gastroenterol. Hepatol. 2017, 2, 288–297. [Google Scholar] [CrossRef]
- Povero, D.; Busletta, C.; Novo, E.; di Bonzo, L.V.; Cannito, S.; Paternostro, C.; Parola, M. Liver fibrosis: A dynamic and potentially reversible process. Histol. Histopathol. 2010, 25, 1075–1091. [Google Scholar]
- Moreira, R.K. Hepatic stellate cells and liver fibrosis. Arch. Pathol. Lab. Med. 2007, 131, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Yuan, W.G.; He, P.; Lei, J.H.; Wang, C.X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512–10522. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Schmitt-Graff, A.; Kruger, S.; Bochard, F.; Gabbiani, G.; Denk, H. Modulation of alpha smooth muscle actin and desmin expression in perisinusoidal cells of normal and diseased human livers. Am. J. Pathol. 1991, 138, 1233–1242. [Google Scholar] [PubMed]
- Nouchi, T.; Tanaka, Y.; Tsukada, T.; Sato, C.; Marumo, F. Appearance of alpha-smooth-muscle-actin-positive cells in hepatic fibrosis. Liver 1991, 11, 100–105. [Google Scholar] [CrossRef]
- Yamaoka, K.; Nouchi, T.; Marumo, F.; Sato, C. Alpha-smooth-muscle actin expression in normal and fibrotic human livers. Dig. Dis. Sci. 1993, 38, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Guido, M.; Rugge, M.; Chemello, L.; Leandro, G.; Fattovich, G.; Giustina, G.; Cassaro, M.; Alberti, A. Liver stellate cells in chronic viral hepatitis: The effect of interferon therapy. J. Hepatol. 1996, 24, 301–307. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Cursio, R.; Colosetti, P.; Codogno, P.; Cuervo, A.M.; Shen, H.M. The role of autophagy in liver diseases: Mechanisms and potential therapeutic targets. BioMed Res. Int. 2015, 2015, 480508. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.Q.; Fan, X.M. Autophagy: A new therapeutic target for liver fibrosis. World J. Hepatol. 2015, 7, 1982–1986. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Park, D.H.; Song, S.H.; Yoon, I.S.; Cho, S.S. Development and Validation of a HPLC-UV Method for Extraction Optimization and Biological Evaluation of Hot-Water and Ethanolic Extracts of Dendropanax morbifera Leaves. Molecules 2018, 23, 650. [Google Scholar] [CrossRef]
- Kim, W.; Kim, D.W.; Yoo, D.Y.; Jung, H.Y.; Nam, S.M.; Kim, J.W.; Hong, S.M.; Kim, D.W.; Choi, J.H.; Moon, S.M.; et al. Dendropanax morbifera Leveille extract facilitates cadmium excretion and prevents oxidative damage in the hippocampus by increasing antioxidant levels in cadmium-exposed rats. BMC Complement. Altern. Med. 2014, 14, 428. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Lee, J.S.; Akram, M.; Kim, K.A.; Shin, Y.J.; Yu, J.H.; Bae, O.N. Protective activity of Dendropanax morbifera against cisplatin-induced acute kidney injury. Kidney Blood Press Res. 2015, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Kim, K.A.; Kim, E.S.; Syed, A.S.; Kim, C.Y.; Lee, J.S.; Bae, O.N. Potent Anti-inflammatory and Analgesic Actions of the Chloroform Extract of Dendropanax morbifera Mediated by the Nrf2/HO-1 Pathway. Biol. Pharm. Bull. 2016, 39, 728–736. [Google Scholar] [CrossRef]
- Kim, W.; Yim, H.S.; Yoo, D.Y.; Jung, H.Y.; Kim, J.W.; Choi, J.H.; Yoon, Y.S.; Kim, D.W.; Hwang, I.K. Dendropanax morbifera Leveille extract ameliorates cadmium-induced impairment in memory and hippocampal neurogenesis in rats. BMC Complement. Altern. Med. 2016, 16, 452. [Google Scholar] [CrossRef] [PubMed]
- Heo, M.-G.; Byun, J.-H.; Kim, J.; Choung, S.-Y. Treatment of Dendropanax morbifera leaves extract improves diabetic phenotype and inhibits diabetes induced retinal degeneration in db/db mice. J. Funct. Foods 2018, 46, 136–146. [Google Scholar] [CrossRef]
- Moon, H.I. Antidiabetic effects of dendropanoxide from leaves of Dendropanax morbifera Leveille in normal and streptozotocin-induced diabetic rats. Hum. Exp. Toxicol. 2011, 30, 870–875. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, M.Y.; Park, W.H.; Moon, H.I. Antiatherogenic activity of Dendropanax morbifera essential oil in rats. Pharmazie 2009, 64, 547–549. [Google Scholar]
- Yang, H.Y.; Kim, K.S.; Lee, Y.H.; Park, J.H.; Kim, J.H.; Lee, S.Y.; Kim, Y.M.; Kim, I.S.; Kacew, S.; Lee, B.M.; et al. Dendropanax morbifera Ameliorates Thioacetamide-Induced Hepatic Fibrosis via TGF-beta1/Smads Pathways. Int. J. Biol. Sci. 2019, 15, 800–811. [Google Scholar] [CrossRef]
- Tori, M.; Matsuda, R.; Sono, M.; Asakawa, Y. 13C NMR assignment of dammarane triterpenes and dendropanoxide: Application of 2D long-range 13C—1H correlation spectra. Magn. Reson. Chem. 1988, 26, 581–590. [Google Scholar] [CrossRef]
- Kim, M.; Park, Y.J.; Lim, H.S.; Lee, H.H.; Kim, T.H.; Lee, B. The Clinical Effects of Dendropanax Morbifera on Postmenopausal Symptoms: Review Article. J. Menopausal. Med. 2017, 23, 146–155. [Google Scholar] [CrossRef]
- Baiocchini, A.; Montaldo, C.; Conigliaro, A.; Grimaldi, A.; Correani, V.; Mura, F.; Ciccosanti, F.; Rotiroti, N.; Brenna, A.; Montalbano, M.; et al. Extracellular Matrix Molecular Remodeling in Human Liver Fibrosis Evolution. PLoS ONE 2016, 11, e0151736. [Google Scholar] [CrossRef]
- Puche, J.E.; Saiman, Y.; Friedman, S.L. Hepatic stellate cells and liver fibrosis. Compr. Physiol. 2013, 3, 1473–1492. [Google Scholar] [PubMed]
- Dewidar, B.; Soukupova, J.; Fabregat, I.; Dooley, S. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis: Updated. Curr. Pathobiol. Rep. 2015, 3, 291–305. [Google Scholar] [CrossRef]
- Scholten, D.; Trebicka, J.; Liedtke, C.; Weiskirchen, R. The carbon tetrachloride model in mice. Lab. Anim 2015, 49, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Slater, T.F.; Cheeseman, K.H.; Ingold, K.U. Carbon tetrachloride toxicity as a model for studying free-radical mediated liver injury. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985, 311, 633–645. [Google Scholar] [PubMed]
- Kim, K.Y.; Choi, I.; Kim, S.S. Progression of hepatic stellate cell activation is associated with the level of oxidative stress rather than cytokines during CCl4-induced fibrogenesis. Mol. Cells 2000, 10, 289–300. [Google Scholar]
- Nevzorova, Y.A.; Bangen, J.M.; Hu, W.; Haas, U.; Weiskirchen, R.; Gassler, N.; Huss, S.; Tacke, F.; Sicinski, P.; Trautwein, C.; et al. Cyclin E1 controls proliferation of hepatic stellate cells and is essential for liver fibrogenesis in mice. Hepatology 2012, 56, 1140–1149. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, K.S.; An, H.K.; Kim, C.H.; Moon, H.I.; Lee, Y.C. Dendropanoxide induces autophagy through ERK1/2 activation in MG-63 human osteosarcoma cells and autophagy inhibition enhances dendropanoxide-induced apoptosis. PLoS ONE 2013, 8, e83611. [Google Scholar]
- Settembre, C.; Fraldi, A.; Medina, D.L.; Ballabio, A. Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 2013, 14, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gea, V.; Ghiassi-Nejad, Z.; Rozenfeld, R.; Gordon, R.; Fiel, M.I.; Yue, Z.; Czaja, M.J.; Friedman, S.L. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 2012, 142, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Thoen, L.F.; Guimaraes, E.L.; Dolle, L.; Mannaerts, I.; Najimi, M.; Sokal, E.; van Grunsven, L.A. A role for autophagy during hepatic stellate cell activation. J. Hepatol. 2011, 55, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Bjorkoy, G.; Lamark, T.; Brech, A.; Outzen, H.; Perander, M.; Overvatn, A.; Stenmark, H.; Johansen, T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 2005, 171, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
| Gene | Species | Forward | Reverse |
|---|---|---|---|
| α-SMA | Human | CTGGCATCGTGCTGGACTCT | GATCTCGGCCAGCCAGATC |
| Col 1A1 | Human | GGCAACAGCCGCTTCACCTAC | GCGGGAGGACTTGGTGGTTTT |
| Col 3A1 | Human | CACGGAAACACTGGTGGACAGATT | ATGCCAGCTGCACATCAAGGAC |
| Fibronectin | Human | CAGTGGGAGACCTCGAGAAG | TCCCTCGGAACATCAGAAAC |
| LC3B-II | Human | CGCACCTTCGAACAAGAG | CTCACCCTTGTATCGTTCTATTA |
| α-SMA | Mouse | GTTCAGTGGTGCCTCTGTCA | ACTGGGACGACATGGAAAAG |
| Col 1A1 | Mouse | TTCGGACTAGACATTGG | GGGTTGTTCGTCTGTTTC |
| Col 3A1 | Mouse | ACGTAGATGAATTGGGATGCAG | GGGTTGGGGCAGTCTAGTG |
| Olive Oil + Olive Oil | Olive oil + Carbon Tetrachloride | DPX 2 + Carbon Tetrachloride | DPX 10 + Carbon Tetrachloride | DPX 40 + Carbon Tetrachloride | Silymarin 40 + Carbon Tetrachloride | |
|---|---|---|---|---|---|---|
| Initial body weight (g) | 22.78 ± 0.50 | 23.33 ± 1.20 | 23.29 ± 0.69 | 22.8 ± 1.06 | 22.0 ± 0.58 | 22.93 ± 1.52 |
| Final body weight (g) | 26.34 ± 0.80 | 25.91 ± 1.27 | 25.31 ± 0.69 | 24.36 ± 1.11 | 23.59 ± 0.92 | 24.81 ± 0.98 |
| Liver weight (g) | 1.35 ± 0.10 | 1.6 ± 0.17 | 1.51 ± 0.09 | 1.44 ± 0.15 | 1.17 ± 0.08 | 1.30 ± 0.12 |
| Liver weight /body weight (×100) | 5.11 ± 0.34 | 6.18 ± 0.49 ## | 5.98 ± 0.18 | 5.92 ± 0.46 | 4.95 ± 0.32 ** | 5.24 ± 0.34 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-J.; Kim, D.-M.; Choi, H.-B.; Jeong, M.-H.; Kwon, S.-H.; Kim, H.-R.; Kwak, J.-H.; Chung, K.-H. Dendropanoxide, a Triterpenoid from Dendropanax morbifera, Ameliorates Hepatic Fibrosis by Inhibiting Activation of Hepatic Stellate Cells through Autophagy Inhibition. Nutrients 2022, 14, 98. https://doi.org/10.3390/nu14010098
Park Y-J, Kim D-M, Choi H-B, Jeong M-H, Kwon S-H, Kim H-R, Kwak J-H, Chung K-H. Dendropanoxide, a Triterpenoid from Dendropanax morbifera, Ameliorates Hepatic Fibrosis by Inhibiting Activation of Hepatic Stellate Cells through Autophagy Inhibition. Nutrients. 2022; 14(1):98. https://doi.org/10.3390/nu14010098
Chicago/Turabian StylePark, Yong-Joo, Dong-Min Kim, Hye-Been Choi, Mi-Ho Jeong, Seung-Hwan Kwon, Ha-Ryong Kim, Jong-Hwan Kwak, and Kyu-Hyuck Chung. 2022. "Dendropanoxide, a Triterpenoid from Dendropanax morbifera, Ameliorates Hepatic Fibrosis by Inhibiting Activation of Hepatic Stellate Cells through Autophagy Inhibition" Nutrients 14, no. 1: 98. https://doi.org/10.3390/nu14010098
APA StylePark, Y.-J., Kim, D.-M., Choi, H.-B., Jeong, M.-H., Kwon, S.-H., Kim, H.-R., Kwak, J.-H., & Chung, K.-H. (2022). Dendropanoxide, a Triterpenoid from Dendropanax morbifera, Ameliorates Hepatic Fibrosis by Inhibiting Activation of Hepatic Stellate Cells through Autophagy Inhibition. Nutrients, 14(1), 98. https://doi.org/10.3390/nu14010098