Soybean-Derived Peptides Attenuate Hyperlipidemia by Regulating Trans-Intestinal Cholesterol Excretion and Bile Acid Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Antibodies, and Reagents
2.2. Cell Culture and Treatment
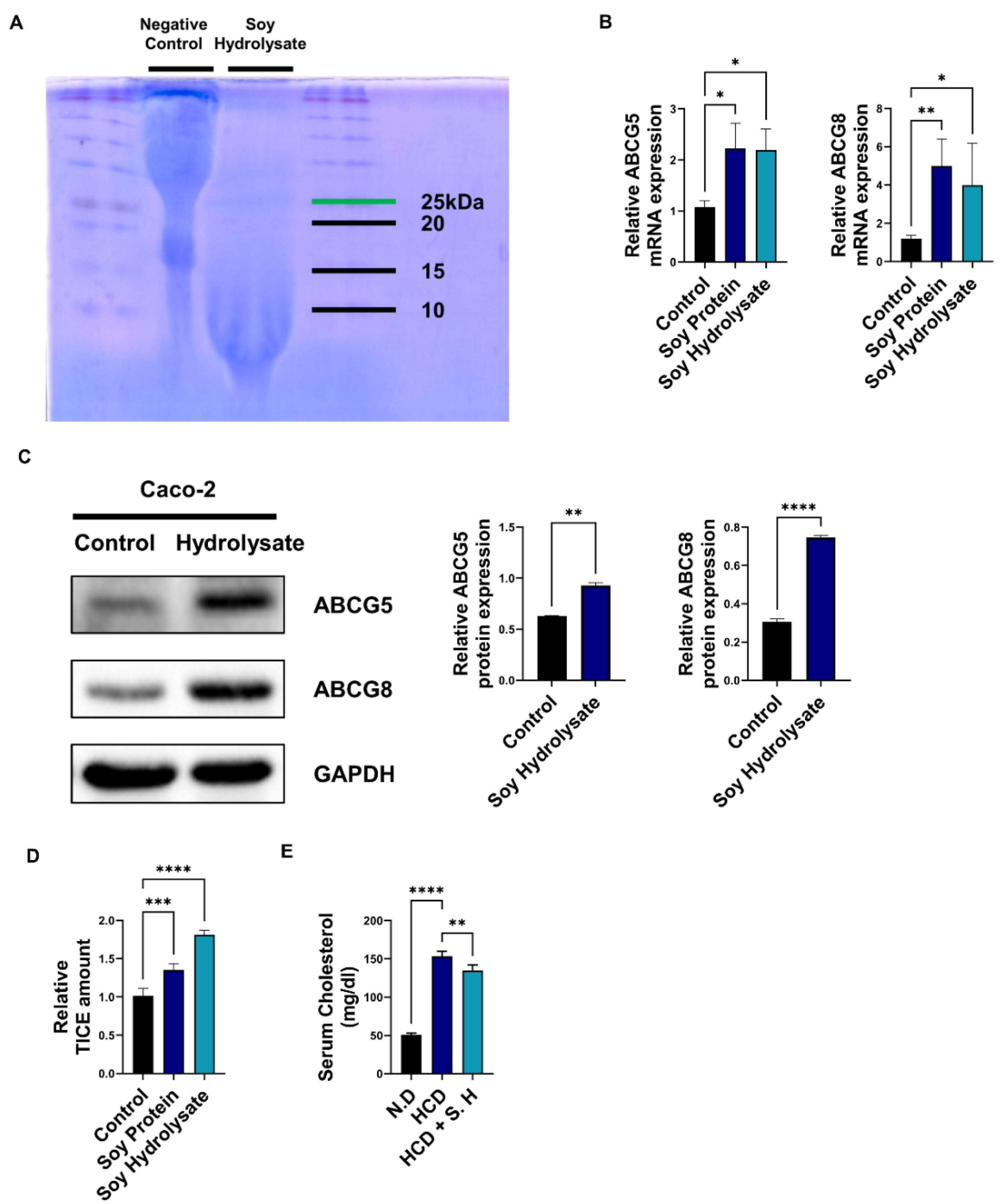
2.3. Soy Hydrolysis
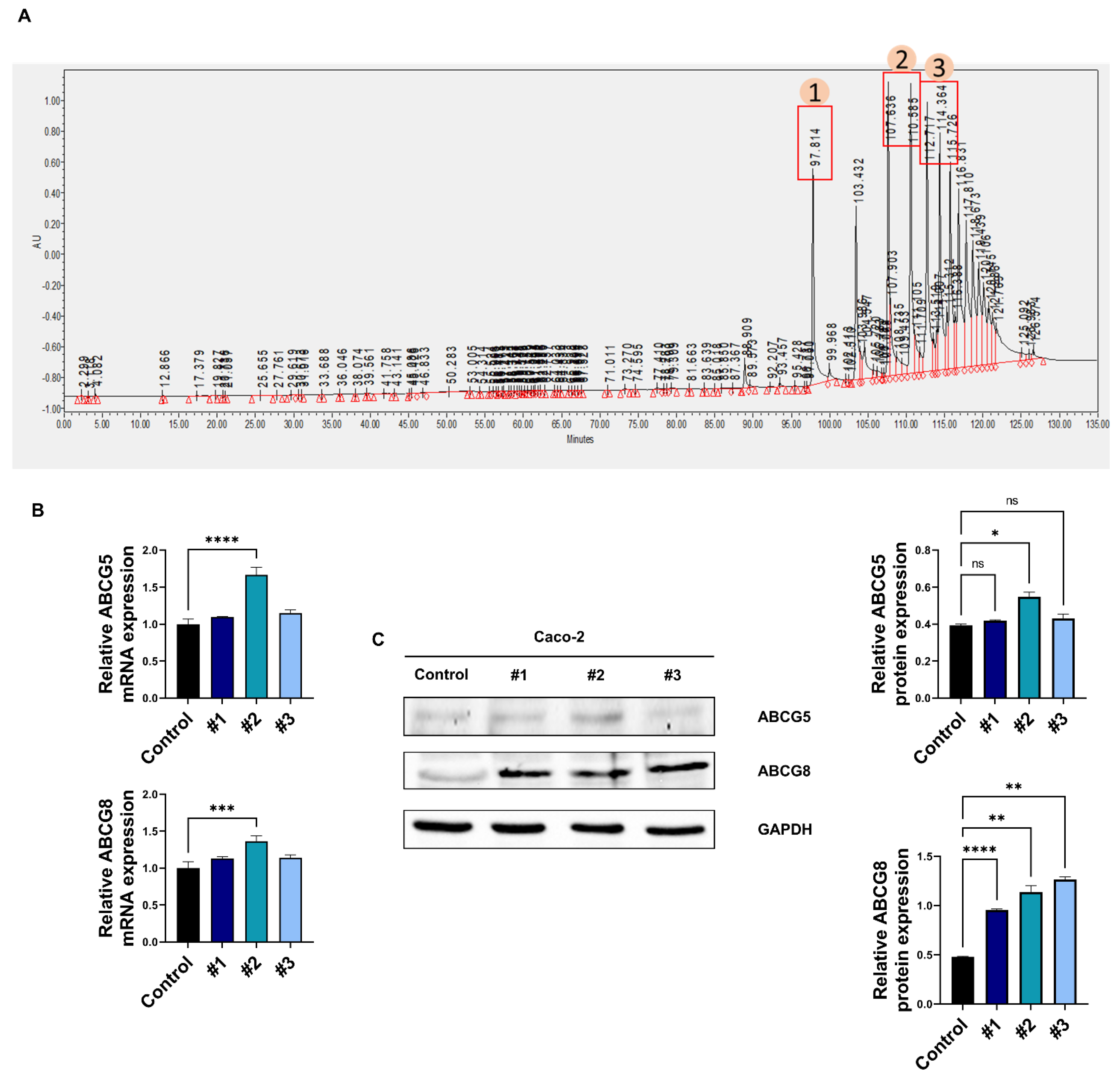
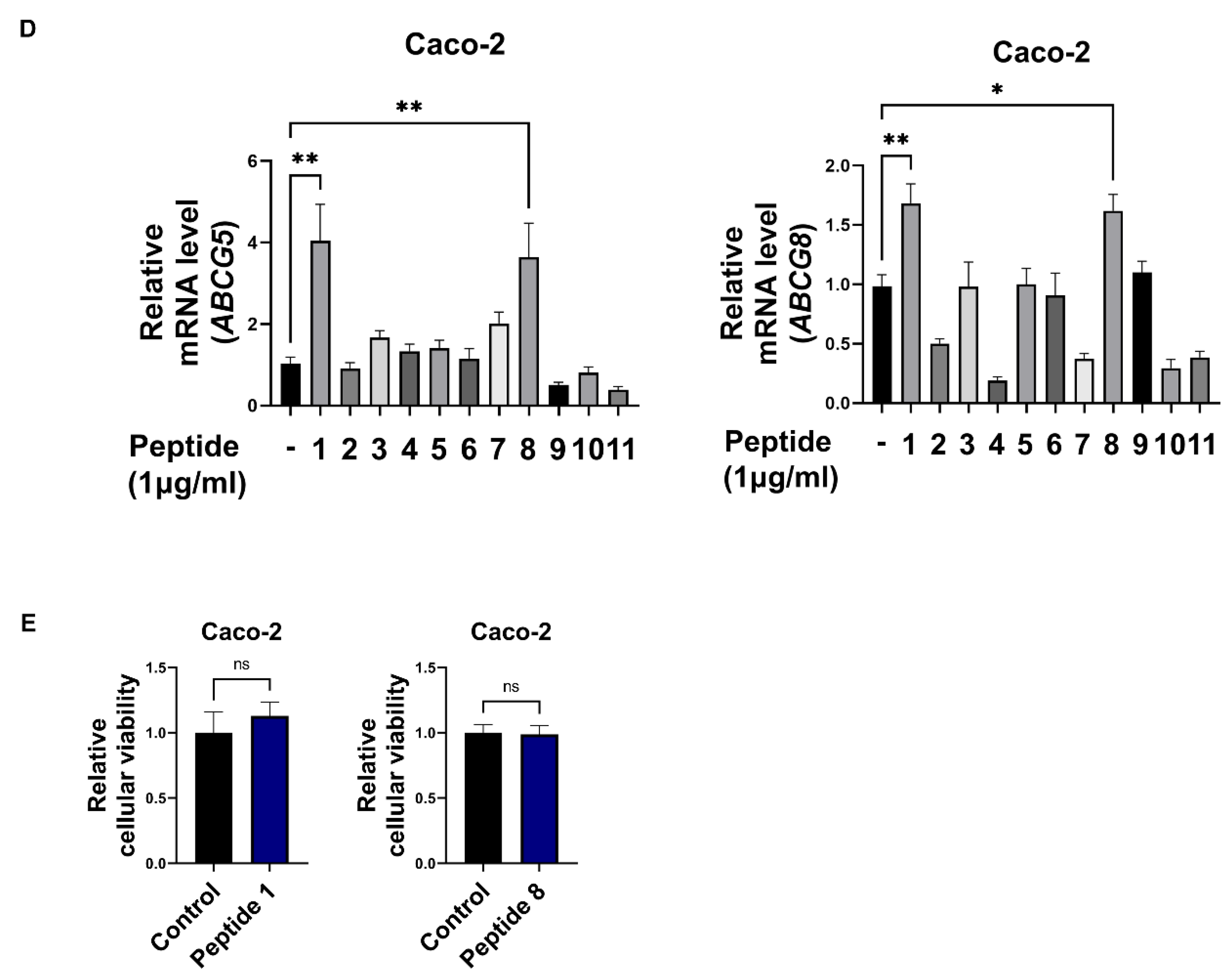
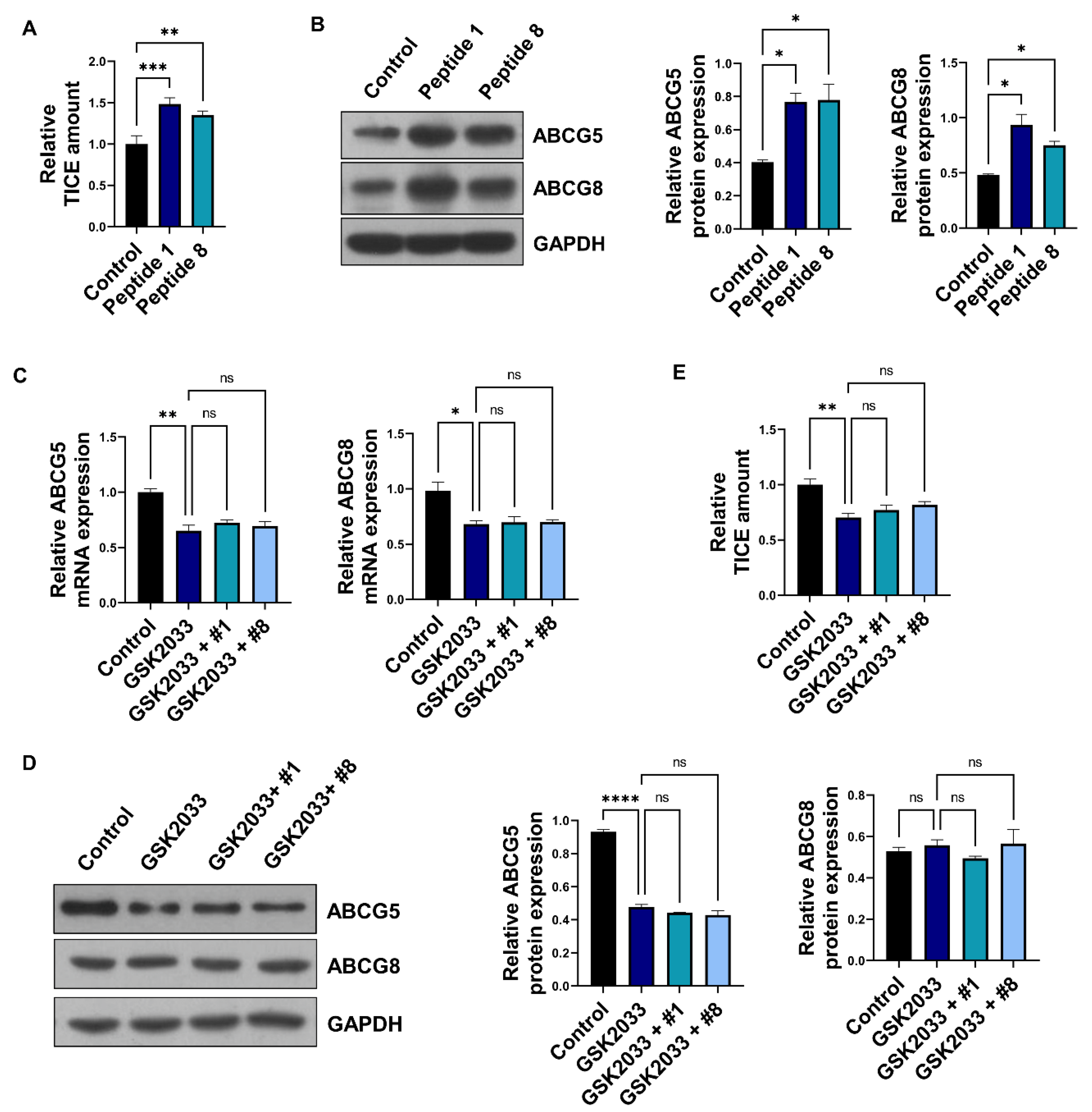
2.4. Total RNA Isolation and qRT-PCR
2.5. Western Blotting
2.6. Cholesterol Assay
2.7. In Vitro TICE Assay
2.8. High-Performance Liquid Chromatography (HPLC) Analysis of Soy Hydrolysates
2.9. Peptide Sequencing and Synthesis
2.10. Cellular Viability Assay
2.11. Animal Care Protocol
2.12. Enzyme-Linked Immunosorbent Assay (ELISA)
2.13. Statistical Analysis
3. Results
3.1. Soy Hydrolysates Upregulate TICE and Downregulate Cholesterol Levels
3.2. Soy Hydrolysate-Derived Bioactive Peptides Induce TICE
3.3. Soybean-Derived Peptides Upregulate TICE via LXRα Signaling
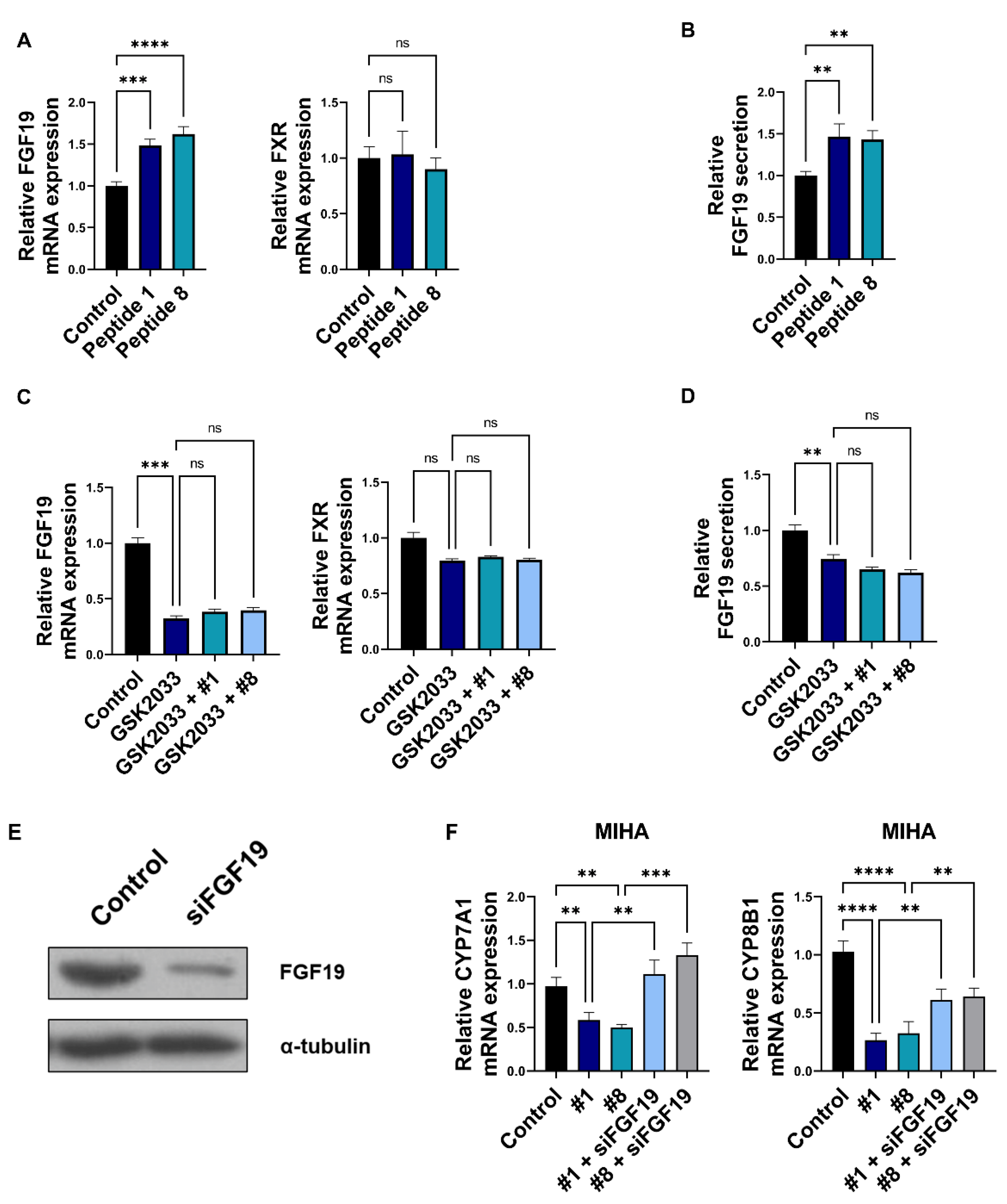
3.4. Bioactive Peptides Regulate Bile Acid Synthesis via Regulation of Enterocyte-Derived FGF19
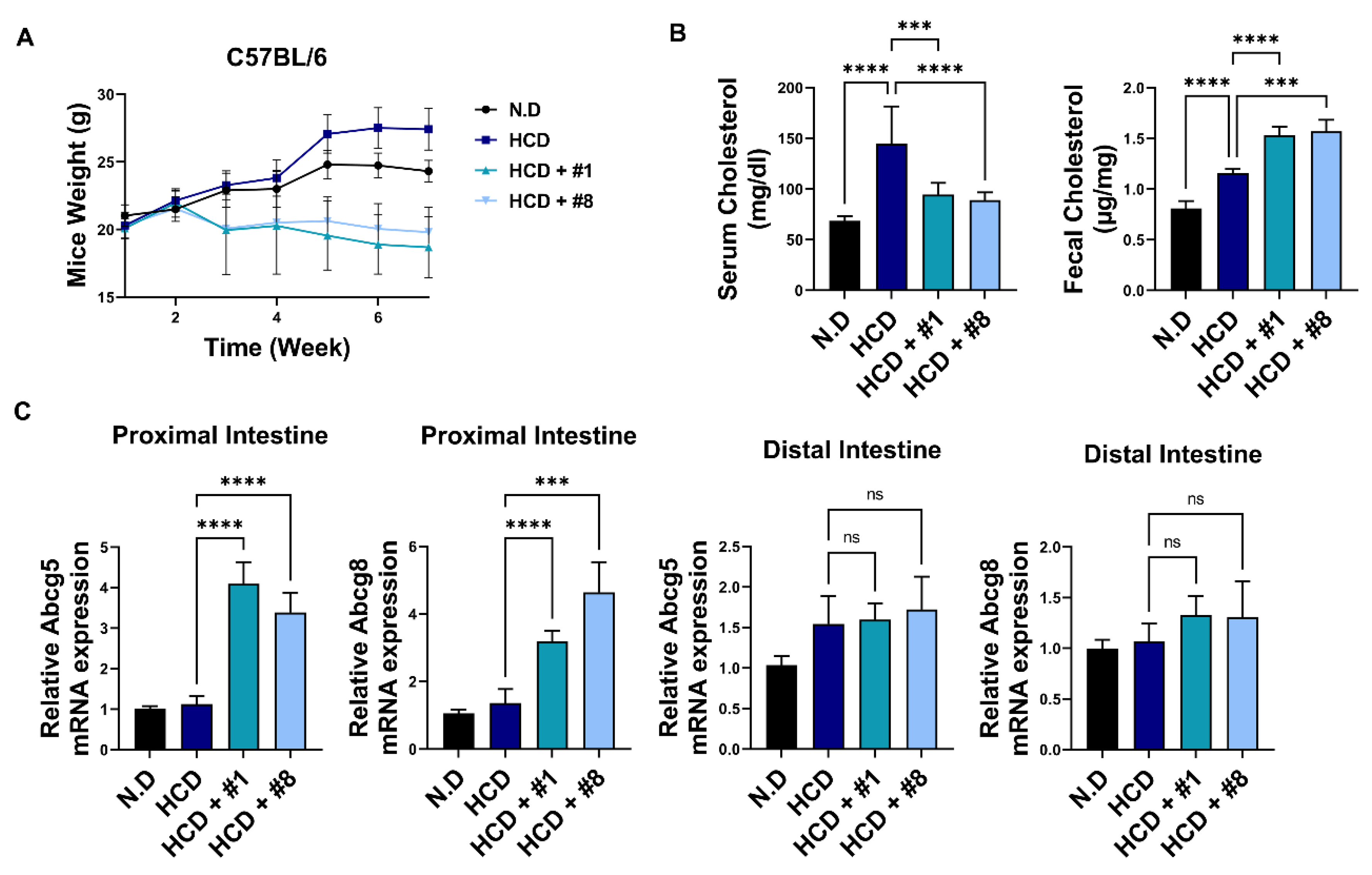
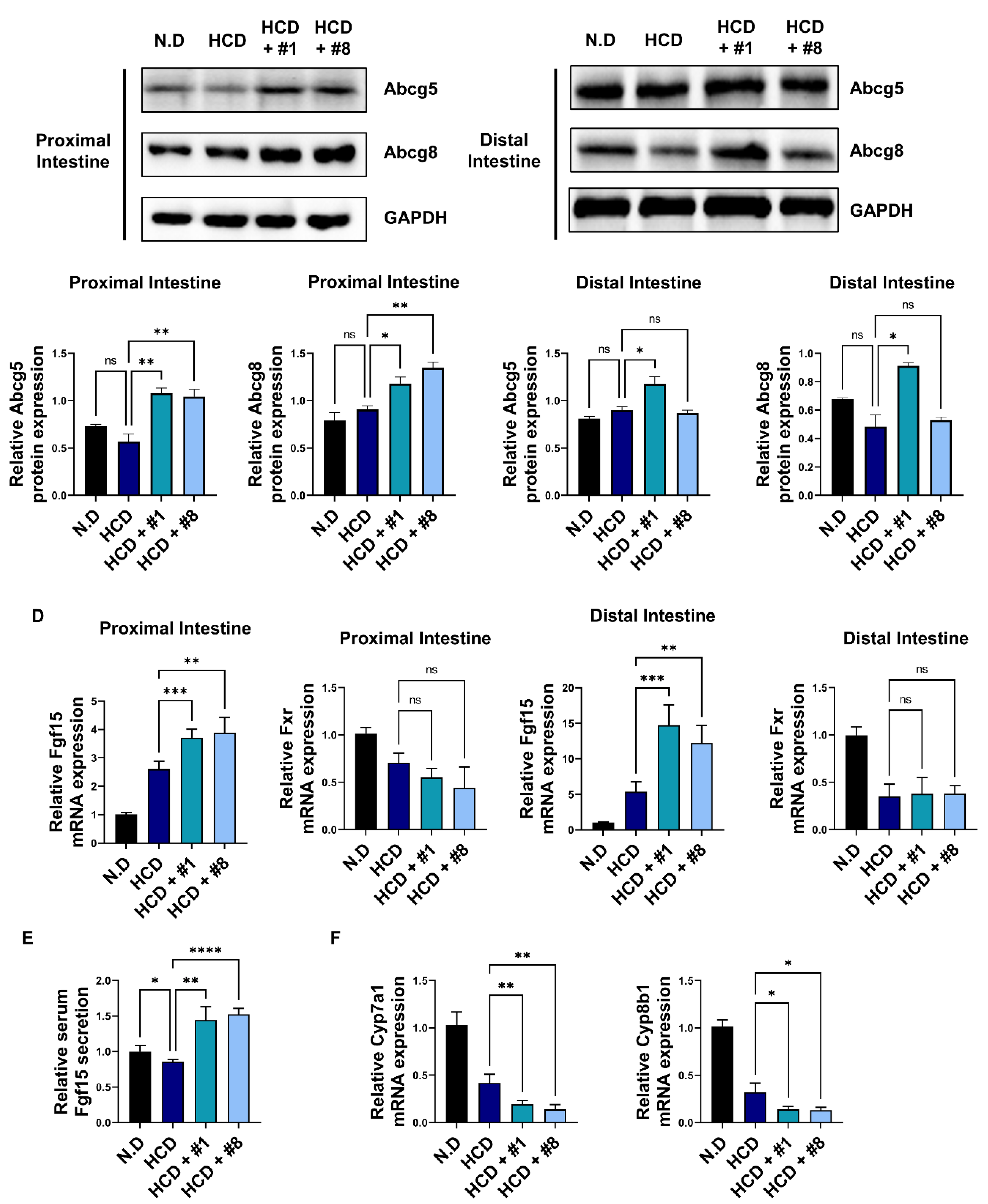
3.5. Bioactive Peptides Attenuate Cholesterol-Derived Obesity and Hyperlipidemia
4. Discussion
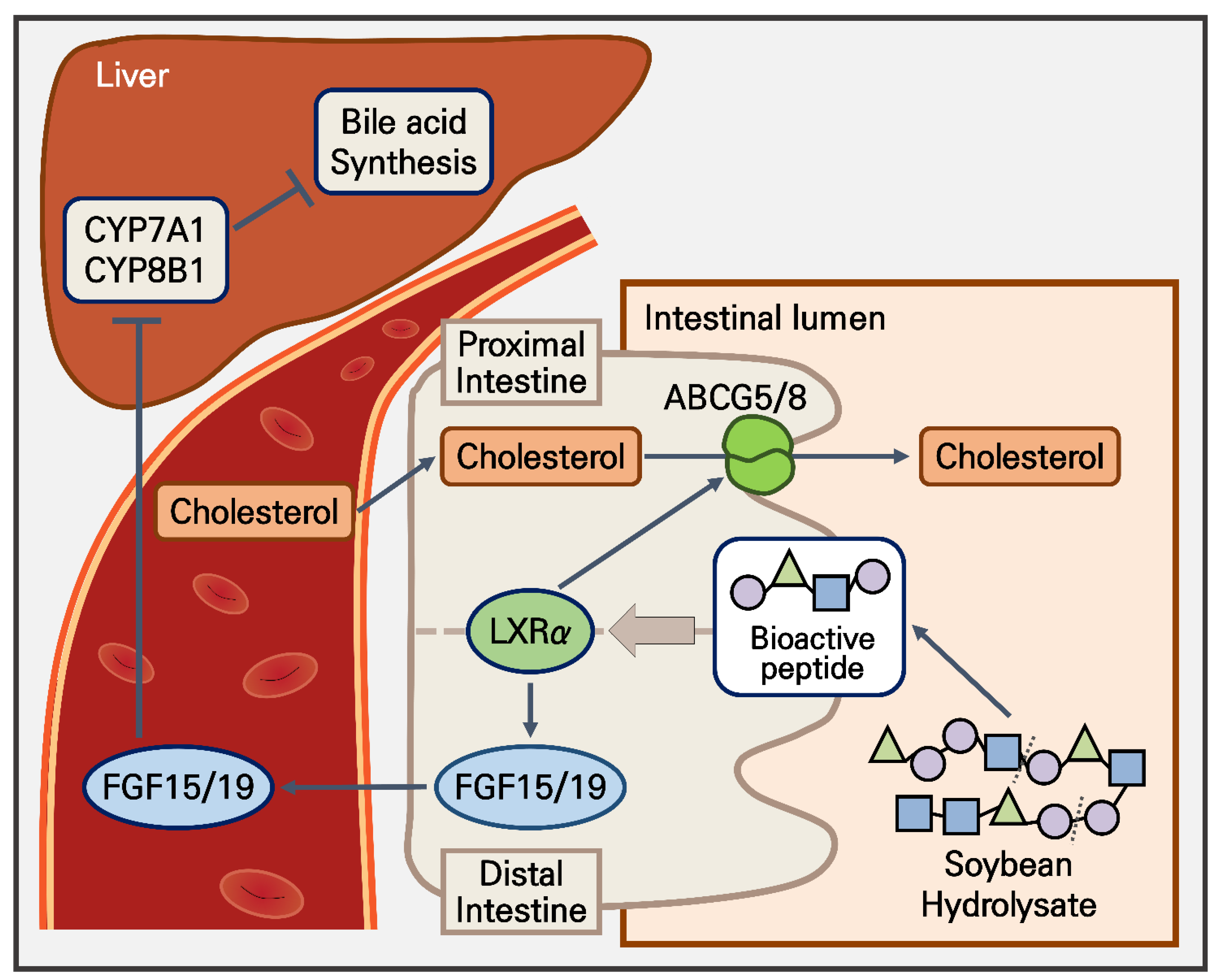
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chou, Y.; Ma, J.; Su, X.; Zhong, Y. Emerging insights into the relationship between hyperlipidemia and the risk of diabetic retinopathy. Lipids Health Dis. 2020, 19, 241. [Google Scholar] [CrossRef]
- Rabar, S.; Harker, M.; O’Flynn, N.; Wierzbicki, A.S. Lipid modification and cardiovascular risk assessment for the primary and secondary prevention of cardiovascular disease: Summary of updated NICE guidance. BMJ 2014, 349, g4356. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R. Plasma high density lipoproteins: Therapeutic targeting and links to atherogenic inflammation. Atherosclerosis 2018, 276, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Last, A.R.; Ference, J.D.; Menzel, E.R. Hyperlipidemia: Drugs for Cardiovascular Risk Reduction in Adults. Am. Fam. Physician 2017, 95, 78–87. [Google Scholar] [PubMed]
- Friesen, J.A.; Rodwell, V.W. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol. 2004, 5, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, P.D.; Panza, G.; Zaleski, A.; Taylor, B. Statin-Associated Side Effects. J. Am. Coll. Cardiol. 2016, 67, 2395–2410. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.H.; Kim, H.; Lee, S.H.; Cho, J.H.; Lee, H.; Yim, H.W.; Yoon, K.H.; Kim, H.S.; Kim, J.H. Low-density lipoprotein cholesterol reduction and target achievement after switching from statin monotherapy to statin/ezetimibe combination therapy: Real-world evidence. J. Clin. Pharm. Ther. 2021, 46, 134–142. [Google Scholar] [CrossRef]
- Beshir, S.A.; Hussain, N.; Elnor, A.A.; Said, A.S.A. Umbrella Review on Non-Statin Lipid-Lowering Therapy. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 437–452. [Google Scholar] [CrossRef]
- Toyoda, Y.; Takada, T.; Yamanashi, Y.; Suzuki, H. Pathophysiological importance of bile cholesterol reabsorption: Hepatic NPC1L1-exacerbated steatosis and decreasing VLDL-TG secretion in mice fed a high-fat diet. Lipids Health Dis. 2019, 18, 234. [Google Scholar] [CrossRef] [Green Version]
- Van der Velde, A.E.; Vrins, C.L.; van den Oever, K.; Kunne, C.; Oude Elferink, R.P.; Kuipers, F.; Groen, A.K. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology 2007, 133, 967–975. [Google Scholar] [CrossRef]
- Blanchard, C.; Moreau, F.; Cariou, B.; Le May, C. Trans-intestinal cholesterol excretion (TICE): A new route for cholesterol excretion. Med. Sci. 2014, 30, 896–901. [Google Scholar] [CrossRef] [Green Version]
- de Boer, J.F.; Schonewille, M.; Boesjes, M.; Wolters, H.; Bloks, V.W.; Bos, T.; van Dijk, T.H.; Jurdzinski, A.; Boverhof, R.; Wolters, J.C.; et al. Intestinal Farnesoid X Receptor Controls Transintestinal Cholesterol Excretion in Mice. Gastroenterology 2017, 152, 1126–1138.e1126. [Google Scholar] [CrossRef] [Green Version]
- Grefhorst, A.; Verkade, H.J.; Groen, A.K. The TICE Pathway: Mechanisms and Lipid-Lowering Therapies. Methodist Debakey Cardiovasc. J. 2019, 15, 70–76. [Google Scholar] [CrossRef]
- Le May, C.; Berger, J.M.; Lespine, A.; Pillot, B.; Prieur, X.; Letessier, E.; Hussain, M.M.; Collet, X.; Cariou, B.; Costet, P. Transintestinal cholesterol excretion is an active metabolic process modulated by PCSK9 and statin involving ABCB1. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1484–1493. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, N.M.; van Wouw, S.A.E.; Ottenhoff, R.; Nelson, J.K.; Kingma, J.; Scheij, S.; Moeton, M.; Zelcer, N. Regulation of intestinal LDLR by the LXR-IDOL axis. Atherosclerosis 2020, 315, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Ruiz, F.; Mancera-Andrade, E.I.; Iqbal, H.M. Marine-Derived Bioactive Peptides for Biomedical Sectors: A Review. Protein Pept. Lett. 2017, 24, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Huma, N.; Butt, M.S.; Aleem, M.; Abbas, M. Therapeutic potential of dairy bioactive peptides: A contemporary perspective. Crit. Rev. Food Sci. Nutr. 2018, 58, 105–115. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive Peptides Derived from Seaweed Protein and Their Health Benefits: Antihypertensive, Antioxidant, and Antidiabetic Properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Youn, B. Hypolipidemic Roles of Casein-Derived Peptides by Regulation of Trans-Intestinal Cholesterol Excretion and Bile Acid Synthesis. Nutrients 2020, 12, 3058. [Google Scholar] [CrossRef]
- Ng, S.P.; Nomura, W.; Mohri, S.; Takahashi, H.; Jheng, H.F.; Ara, T.; Nagai, H.; Ito, T.; Kawada, T.; Goto, T. Soy hydrolysate enhances the isoproterenol-stimulated lipolytic pathway through an increase in β-adrenergic receptor expression in adipocytes. Biosci. Biotechnol. Biochem. 2019, 83, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J. Soy bioactive peptides and the gut microbiota modulation. Appl. Microbiol. Biotechnol. 2020, 104, 9009–9017. [Google Scholar] [CrossRef] [PubMed]
- Daliri, E.B.; Ofosu, F.K.; Chelliah, R.; Park, M.H.; Kim, J.H.; Oh, D.H. Development of a Soy Protein Hydrolysate with an Antihypertensive Effect. Int. J. Mol. Sci. 2019, 20, 1496. [Google Scholar] [CrossRef] [Green Version]
- Son, B.; Jeon, J.; Lee, S.; Kim, H.; Kang, H.; Youn, H.; Jo, S.; Youn, B. Radiotherapy in combination with hyperthermia suppresses lung cancer progression via increased NR4A3 and KLF11 expression. Int. J. Radiat. Biol. 2019, 95, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, W.; Kwon, T.; Youn, H.; Kim, J.S.; Youn, B. Plasminogen activator inhibitor-1 enhances radioresistance and aggressiveness of non-small cell lung cancer cells. Oncotarget 2016, 7, 23961–23974. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Lee, S.; Kim, K.; Jeon, J.; Kang, S.G.; Youn, H.; Kim, H.Y.; Youn, B. Downregulated CLIP3 induces radioresistance by enhancing stemness and glycolytic flux in glioblastoma. J. Exp. Clin. Cancer Res. 2021, 40, 282. [Google Scholar] [CrossRef]
- Son, B.; Lee, S.; Kim, H.; Kang, H.; Kim, J.; Youn, H.; Nam, S.Y.; Youn, B. Low dose radiation attenuates inflammation and promotes wound healing in a mouse burn model. J. Dermatol. Sci. 2019, 96, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Dugardin, C.; Briand, O.; Touche, V.; Schonewille, M.; Moreau, F.; Le May, C.; Groen, A.K.; Staels, B.; Lestavel, S. Retrograde cholesterol transport in the human Caco-2/TC7 cell line: A model to study trans-intestinal cholesterol excretion in atherogenic and diabetic dyslipidemia. Acta Diabetol. 2017, 54, 191–199. [Google Scholar] [CrossRef]
- Park, G.; Son, B.; Kang, J.; Lee, S.; Jeon, J.; Kim, J.H.; Yi, G.R.; Youn, H.; Moon, C.; Nam, S.Y.; et al. LDR-Induced miR-30a and miR-30b Target the PAI-1 Pathway to Control Adverse Effects of NSCLC Radiotherapy. Mol. Ther. 2019, 27, 342–354. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, C.; Gleddie, S.; Xiao, C.W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [Green Version]
- Karnjanapratum, S.; O’Callaghan, Y.C.; Benjakul, S.; O’Brien, N. Antioxidant, immunomodulatory and antiproliferative effects of gelatin hydrolysate from unicorn leatherjacket skin. J. Sci. Food Agric. 2016, 96, 3220–3226. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Sugihara, F.; Wang, X. Ingestion of bioactive collagen hydrolysates enhance facial skin moisture and elasticity and reduce facial ageing signs in a randomised double-blind placebo-controlled clinical study. J. Sci. Food Agric. 2016, 96, 4077–4081. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Kim, I.H.; Nam, T.J. Bioactive peptide from Pyropia yezoensis and its anti-inflammatory activities. Int. J. Mol. Med. 2015, 36, 1701–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Repa, J.J.; Berge, K.E.; Pomajzl, C.; Richardson, J.A.; Hobbs, H.; Mangelsdorf, D.J. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J. Biol. Chem. 2002, 277, 18793–18800. [Google Scholar] [CrossRef] [Green Version]
- Jakulj, L.; van Dijk, T.H.; de Boer, J.F.; Kootte, R.S.; Schonewille, M.; Paalvast, Y.; Boer, T.; Bloks, V.W.; Boverhof, R.; Nieuwdorp, M.; et al. Transintestinal Cholesterol Transport Is Active in Mice and Humans and Controls Ezetimibe-Induced Fecal Neutral Sterol Excretion. Cell Metab. 2016, 24, 783–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinet, E.M.; Basso, M.D.; Halpern, A.R.; Yates, D.W.; Steffan, R.J.; Clerin, V.; Resmini, C.; Keith, J.C.; Berrodin, T.J.; Feingold, I.; et al. LXR ligand lowers LDL cholesterol in primates, is lipid neutral in hamster, and reduces atherosclerosis in mouse. J. Lipid Res. 2009, 50, 2358–2370. [Google Scholar] [CrossRef] [Green Version]
- Dumeus, S.; Shibu, M.A.; Lin, W.T.; Wang, M.F.; Lai, C.H.; Shen, C.Y.; Lin, Y.M.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Bioactive Peptide Improves Diet-Induced Hepatic Fat Deposition and Hepatocyte Proinflammatory Response in SAMP8 Ageing Mice. Cell. Physiol. Biochem. 2018, 48, 1942–1952. [Google Scholar] [CrossRef]
- Yu, X.H.; Qian, K.; Jiang, N.; Zheng, X.L.; Cayabyab, F.S.; Tang, C.K. ABCG5/ABCG8 in cholesterol excretion and atherosclerosis. Clin. Chim. Acta 2014, 428, 82–88. [Google Scholar] [CrossRef]
- Yaklich, R.W. beta-Conglycinin and glycinin in high-protein soybean seeds. J. Agric. Food Chem. 2001, 49, 729–735. [Google Scholar] [CrossRef]
- Wang, T.; Qin, G.X.; Sun, Z.W.; Zhao, Y. Advances of research on glycinin and β-conglycinin: A review of two major soybean allergenic proteins. Crit. Rev. Food Sci. Nutr. 2014, 54, 850–862. [Google Scholar] [CrossRef]
- Zheng, S.; Qin, G.; Chen, J.; Zhang, F. Acidic polypeptides A(1a), A(3) and A(4) of Gly m 6 (glycinin) are allergenic for piglets. Vet. Immunol. Immunopathol. 2018, 202, 147–152. [Google Scholar] [CrossRef]
- Fassini, P.G.; Noda, R.W.; Ferreira, E.S.; Silva, M.A.; Neves, V.A.; Demonte, A. Soybean glycinin improves HDL-C and suppresses the effects of rosuvastatin on hypercholesterolemic rats. Lipids Health Dis. 2011, 10, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriyama, T.; Kishimoto, K.; Nagai, K.; Urade, R.; Ogawa, T.; Utsumi, S.; Maruyama, N.; Maebuchi, M. Soybean beta-conglycinin diet suppresses serum triglyceride levels in normal and genetically obese mice by induction of beta-oxidation, downregulation of fatty acid synthase, and inhibition of triglyceride absorption. Biosci. Biotechnol. Biochem. 2004, 68, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Joseph, J.F.; Durairaj, P.; Parr, M.K.; Bureik, M. Conversion of chenodeoxycholic acid to cholic acid by human CYP8B1. Biol. Chem. 2019, 400, 625–628. [Google Scholar] [CrossRef]
- Al-Dury, S.; Marschall, H.U. Ileal Bile Acid Transporter Inhibition for the Treatment of Chronic Constipation, Cholestatic Pruritus, and NASH. Front. Pharmacol. 2018, 9, 931. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.M.; Harper Calderon, J.E.H.; Jimenez, Y.; Barringer, K.; Carbonaro, M.; Molina-Portela, M.D.P.; Thurston, G.; Li, Z.; Daly, C. Monomeric/dimeric forms of Fgf15/FGF19 show differential activity in hepatocyte proliferation and metabolic function. FASEB J. 2021, 35, e21286. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Seok, S.; Zhang, Y.; Ma, J.; Kong, B.; Guo, G.; Kemper, B.; Kemper, J.K. Intestinal FGF15/19 physiologically repress hepatic lipogenesis in the late fed-state by activating SHP and DNMT3A. Nat. Commun. 2020, 11, 5969. [Google Scholar] [CrossRef]
- Wang, Y.; Gunewardena, S.; Li, F.; Matye, D.J.; Chen, C.; Chao, X.; Jung, T.; Zhang, Y.; Czerwiński, M.; Ni, H.M.; et al. An FGF15/19-TFEB regulatory loop controls hepatic cholesterol and bile acid homeostasis. Nat. Commun. 2020, 11, 3612. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Jia, W. Fibroblast growth factor 21: A novel metabolic regulator from pharmacology to physiology. Front. Med. 2013, 7, 25–30. [Google Scholar] [CrossRef]
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| Human ABCG5 | 5′-AGCAAGGAACGGGAAATAGA-3′ | 5′-CAGGAGAACACCCAGTTTAGAG-3′ |
| Human ABCG8 | 5′-GATACAGCCGCCCTCTTTT-3′ | 5′-GCCCGTCTTCCAGTTCATAG-3′ |
| Human FGF19 | 5′-AGATCAAGGCAGTCGCTCTG-3′ | 5′-AAAGCACAGTCTTCCTCCGA-3′ |
| Human FXR | 5′-AAAGTTGTGTAAGATTCACCAGCCT-3′ | 5′-GGTCGTTTACTCTCCATGACATCA-3′ |
| Human CYP7A1 | 5′-GACCACATCTTTGATTTGG-3′ | 5′-CCGTTTGCCTTCTCCTAA-3′ |
| Human CYP8B1 | 5′-GCCTGTCCTTTGTAATGCTGA-3′ | 5′-GAAGCGAAAGAGGCTGTCC-3′ |
| Human GAPDH | 5′-ATGACATCAAGAAGGTGGTG-3′ | 5′-CATACCAGGAAATGAGCTTG-3′ |
| Mouse Abcg5 | 5′-CTTCGACAAAATTGCCATCC-3′ | 5′-GAAAGGAACCGTGGGTAAGG-3′ |
| Mouse Abcg8 | 5′-TGGTCAGTCCAACACTCTGG-3′ | 5′-ACTGGGTTGCCCATTTATCC-3′ |
| Mouse Fgf15 | 5′-GAGGACCAAAACGAACGAAATT-3′ | 5′-ACGTCCTTGATGGCAATCG-3′ |
| Mouse Fxr | 5′-AAATGAGGGCTGCAAAGGTTTCT-3′ | 5′-TGCCCCCGTTCTTACACTTG-3′ |
| Mouse Cyp7a1 | 5′-TACAGAGTGCTGGCCAAGAG-3′ | 5′-GCTGTCCGGATATTCAAGGA-3′ |
| Mouse Cyp8b1 | 5′-CCTCTGGACAAGGGTTTTGTG-3′ | 5′-GCACCGTGAAGACATCCCC-3′ |
| Mouse Gapdh | 5′-CGACTTCAACAGCAACTCCCACTCTTCC-3′ | 5′-TGGGTGGTCCAGGGTTTCTTACTCCTT-3′ |
| No. | Sequence | Original Protein |
|---|---|---|
| 1 | ALEPDHRVESEGGL | Glycinin |
| 2 | NALEPDHRVESEGGL | Glycinin |
| 3 | FVDAQPQQKEEGN | Beta-conglycinin alpha’-subunit |
| 4 | VDAQPQQKEEGN | Beta-conglycinin alpha’-subunit |
| 5 | VVNPDNDENLRM | Beta-conglycinin alpha’-subunit |
| 6 | YVVNPDNDENLRM | Beta-conglycinin alpha’-subunit |
| 7 | SLVNNDDRDSY | Beta-conglycinin alpha-subunit |
| 8 | SLVNNDDRDSYRLQSGDAL | Beta-conglycinin alpha-subunit |
| 9 | VGLKEQQQEQQQEEQPLEVR | Beta-conglycinin alpha-subunit |
| 10 | TISSEDEPFNLRS | Beta-conglycinin beta-subunit |
| 11 | FPFELPSEERG | Sucrose binding protein homolog S-64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Shin, E.; Kang, H.; Youn, H.; Youn, B. Soybean-Derived Peptides Attenuate Hyperlipidemia by Regulating Trans-Intestinal Cholesterol Excretion and Bile Acid Synthesis. Nutrients 2022, 14, 95. https://doi.org/10.3390/nu14010095
Lee H, Shin E, Kang H, Youn H, Youn B. Soybean-Derived Peptides Attenuate Hyperlipidemia by Regulating Trans-Intestinal Cholesterol Excretion and Bile Acid Synthesis. Nutrients. 2022; 14(1):95. https://doi.org/10.3390/nu14010095
Chicago/Turabian StyleLee, Haksoo, Eunguk Shin, Hyunkoo Kang, HyeSook Youn, and BuHyun Youn. 2022. "Soybean-Derived Peptides Attenuate Hyperlipidemia by Regulating Trans-Intestinal Cholesterol Excretion and Bile Acid Synthesis" Nutrients 14, no. 1: 95. https://doi.org/10.3390/nu14010095






