American Ginseng Attenuates Eccentric Exercise-Induced Muscle Damage via the Modulation of Lipid Peroxidation and Inflammatory Adaptation in Males
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Procedures
2.3. Administration of AG Extract or Placebo
2.4. peak Testing and 60% peak DH Running
2.5. GPRS
2.6. Blood Sample Collection
2.7. Biochemical Measurements
2.8. Statistical Analyses
3. Results
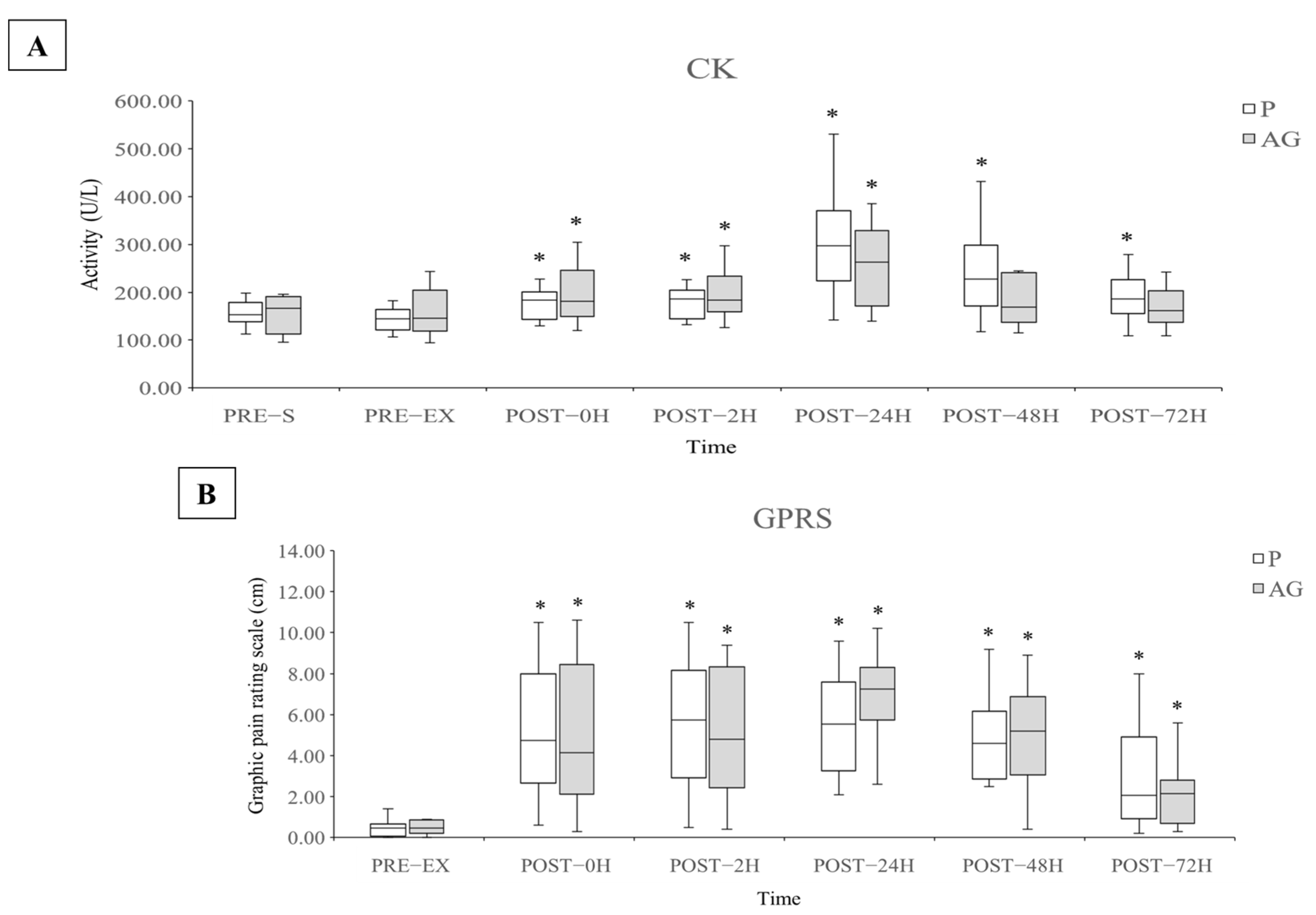
3.1. Muscle Damage and Soreness
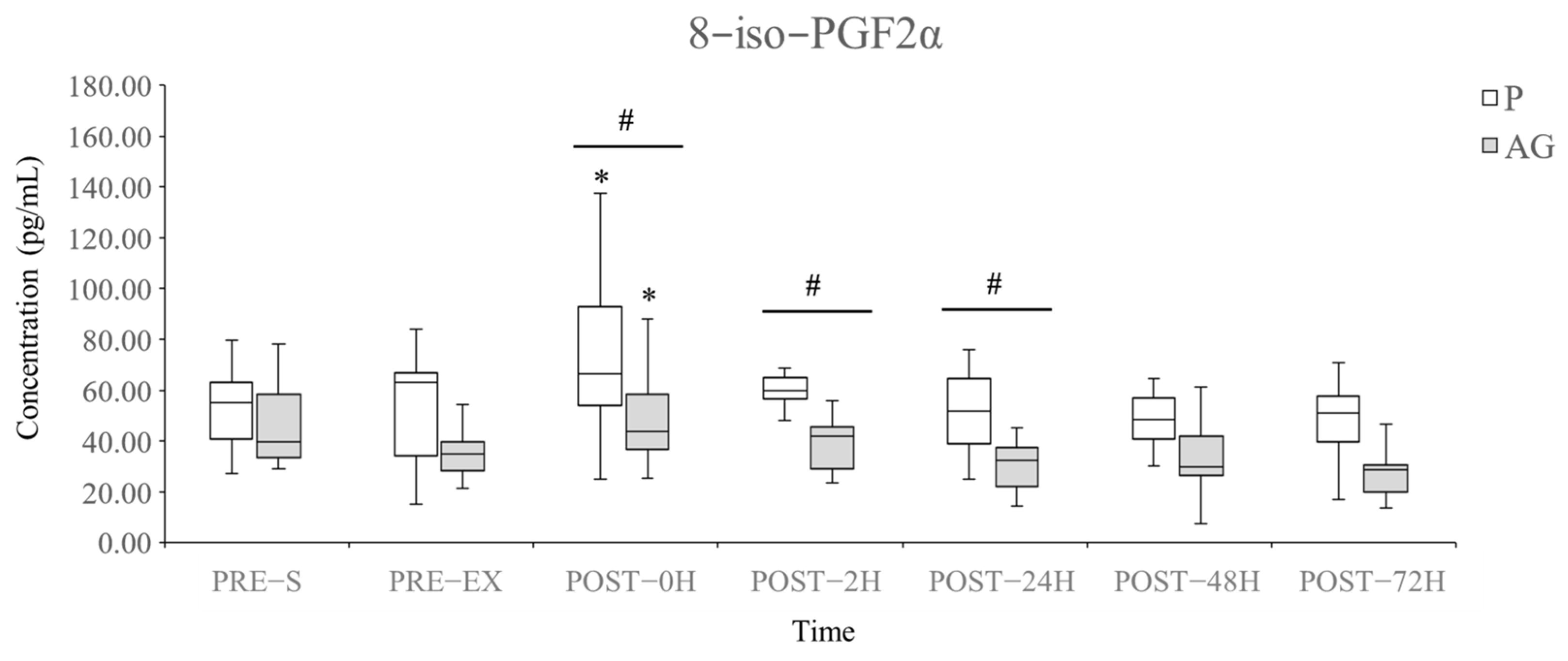
3.2. Lipid Peroxidation
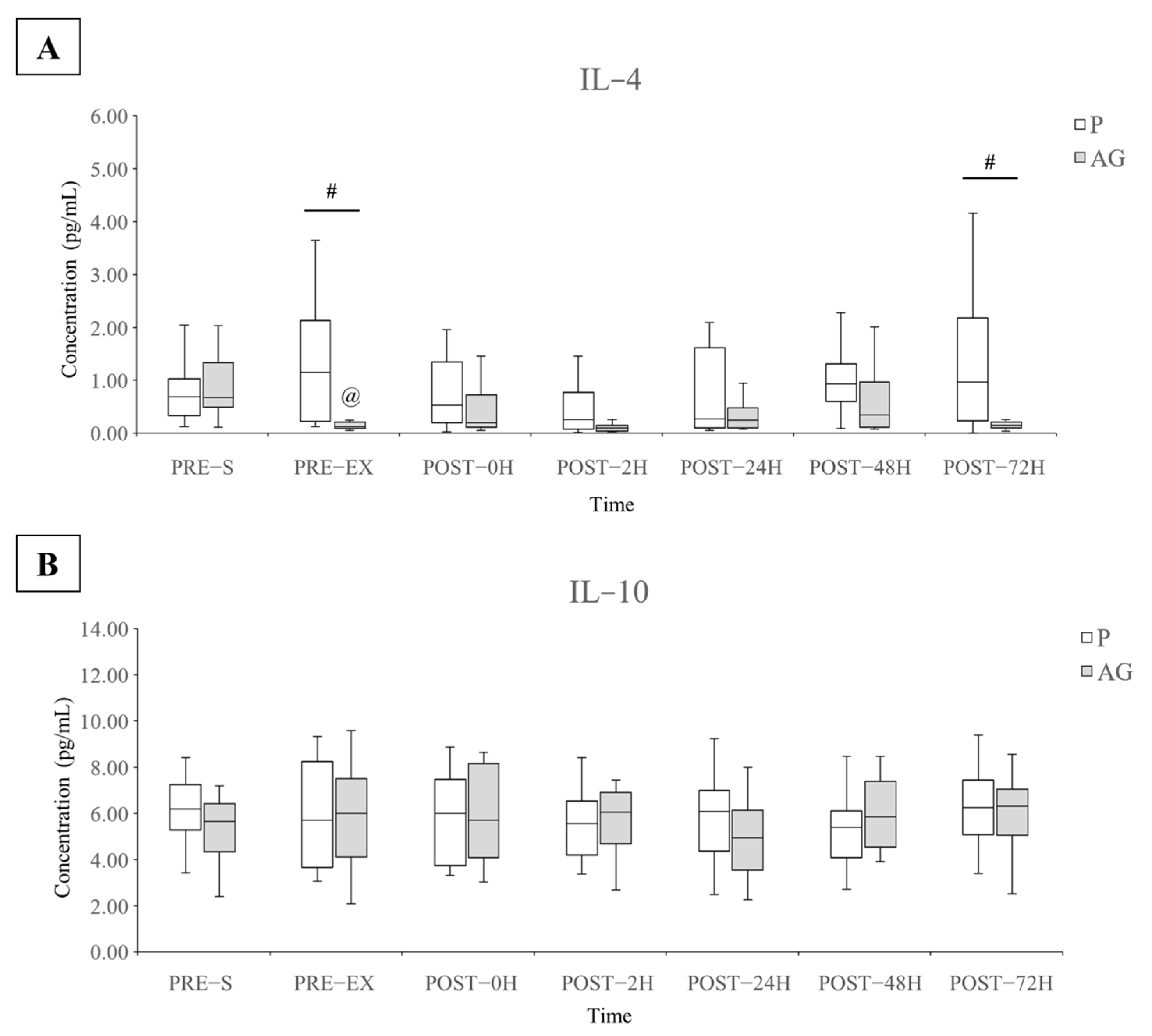
3.3. Inflammatory Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fredsted, A.; Clausen, T.; Overgaard, K. Effects of step exercise on muscle damage and muscle Ca2+ content in men and women. J. Strength Cond. Res. 2008, 22, 1136–1146. [Google Scholar] [CrossRef]
- Hyldahl, R.D.; Chen, T.C.; Nosaka, K. Mechanisms and mediators of the skeletal muscle repeated bout effect. Exerc. Sport Sci. Rev. 2017, 45, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Neubauer, O.; DellaGatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Bontemps, B.; Vercruyssen, F.; Gruet, M.; Louis, J. Downhill running: What are the effects and how can we adapt? A narrative review. Sports Med. 2020, 50, 2083–2110. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Mikkelsen, U.R.; Raastad, T.; Peake, J.M. Leucocytes, cytokines and satellite cells: What role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012, 18, 42–97. [Google Scholar]
- Morawin, B.; Turowski, D.; Naczk, M.; Siatkowski, I.; Zembron-Lacny, A. The combination of α-lipoic acid intake with eccentric exercise modulates erythropoietin release. Biol. Sport 2014, 31, 179–185. [Google Scholar] [CrossRef]
- Sacheck, J.M.; Milbury, P.E.; Cannon, J.G.; Roubenoff, R.; Blumberg, J.B. Effect of vitamin E and eccentric exercise on selected biomarkers of oxidative stress in young and elderly men. Free Radic. Biol. Med. 2003, 34, 1575–1588. [Google Scholar] [CrossRef]
- Righi, N.C.; Schuch, F.B.; De Nardi, A.T.; Pippi, C.M.; de Almeida Righi, G.; Puntel, G.O.; da Silva, A.M.V.; Signori, L.U. Effects of vitamin C on oxidative stress, inflammation, muscle soreness, and strength following acute exercise: Meta-analyses of randomized clinical trials. Eur. J. Nutr. 2020, 59, 2827–2839. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.-C.; Martínez, A.; Santangelo, G.; Pallardó, F.V.; Sastre, J.; Viña, J. Oxidative stress in marathon runners: Interest of antioxidant supplementation. Br. J. Nutr. 2006, 96 (Suppl. 1), S31–S33. [Google Scholar] [CrossRef]
- Yang, W.; Hu, P. Skeletal muscle regeneration is modulated by inflammation. J. Orthop. Transl. 2018, 13, 25–32. [Google Scholar] [CrossRef]
- Suzuki, K. Cytokine response to exercise and its modulation. Antioxidants 2018, 7, 17. [Google Scholar] [CrossRef]
- Chazaud, B.; Brigitte, M.; Yacoub-Youssef, H.; Arnold, L.; Gherardi, R.; Sonnet, C.; Lafuste, P.; Chretien, F. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc. Sport Sci. Rev. 2009, 37, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Toft, A.D. Effects of exercise on lymphocytes and cytokines. Br. J. Sports Med. 2000, 34, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Szczuka, D.; Nowak, A.; Zakłos-Szyda, M.; Kochan, E.; Szymańska, G.; Motyl, I.; Blasiak, J. American ginseng (Panax quinquefolium L.) as a source of bioactive phytochemicals with pro-health properties. Nutrients 2019, 11, 1041. [Google Scholar] [CrossRef]
- Qi, L.-W.; Wang, C.-Z.; Yuan, C.-S. Ginsenosides from American ginseng: Chemical and pharmacological diversity. Phytochemistry 2011, 72, 689–699. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Ho, M.-C.; Lin, L.-C.; Su, B.; Hsu, M.-C. American ginseng supplementation attenuates creatine kinase level induced by submaximal exercise in human beings. World J. Gastroenterol. 2005, 11, 5327–5331. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.-L.; Mao, X.-Y.; Liu, S.; Chen, W.-Z.; Huang, D.-D.; Zhang, C.-J.; Chen, B.-C.; Shen, X.; Yu, Z. Ginsenoside Rb1 improves postoperative fatigue syndrome by reducing skeletal muscle oxidative stress through activation of the PI3K/Akt/Nrf2 pathway in aged rats. Eur. J. Pharmacol. 2014, 740, 480–487. [Google Scholar] [CrossRef]
- Xie, J.-T.; Shao, Z.-H.; VandenHoek, T.L.; Chang, W.-T.; Li, J.; Mehendale, S.; Wang, C.-Z.; Hsu, C.-W.; Becker, L.B.; Yin, J.-J.; et al. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur. J. Pharmacol. 2006, 532, 201–207. [Google Scholar] [CrossRef]
- Wang, Q.-W.; Yu, X.-F.; Xu, H.-L.; Jiang, Y.-C.; Zhao, X.-Z.; Sui, D.-Y. Ginsenoside Re attenuates isoproterenol-induced myocardial injury in rats. Evid. Based Complement. Alternat. Med. 2018, 2018, 8637134. [Google Scholar] [CrossRef]
- Xia, R.; Zhao, B.; Wu, Y.; Hou, J.-B.; Zhang, L.; Xu, J.-J.; Xia, Z.-Y. Ginsenoside Rb1 preconditioning enhances eNOS expression and attenuates myocardial ischemia/reperfusion injury in diabetic rats. J. Biomed. Biotechnol. 2011, 2011, 767930. [Google Scholar] [CrossRef]
- Julian, V.; Thivel, D.; Costes, F.; Touron, J.; Boirie, Y.; Pereira, B.; Perrault, H.; Duclos, M.; Richard, R. Eccentric training improves body composition by inducing mechanical and metabolic adaptations: A promising approach for overweight and Obese Individuals. Front. Physiol. 2018, 9, 1013. [Google Scholar] [CrossRef] [PubMed]
- Lavender, A.P.; Nosaka, K. Changes in fluctuation of isometric force following eccentric and concentric exercise of the elbow flexors. Eur. J. Appl. Physiol. 2006, 96, 235–240. [Google Scholar] [CrossRef]
- Harty, P.S.; Cottet, M.L.; Malloy, J.K.; Kerksick, C.M. Nutritional and supplementation strategies to prevent and attenuate exercise-induced muscle damage: A brief review. Sports Med.-Open 2019, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Denegar, C.R.; Perrin, D.H. Effect of transcutaneous electrical nerve stimulation, cold, and a combination treatment on pain, decreased range of motion, and strength loss associated with delayed onset muscle soreness. J. Athl. Train. 1992, 27, 200–206. [Google Scholar]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Academic Press: New York, NY, USA, 1988. [Google Scholar]
- Wang, L.; Zhao, H.; Zhai, Z.-Z.; Qu, L.-X. Protective effect and mechanism of ginsenoside Rg1 in cerebral ischaemia-reperfusion injury in mice. Biomed. Pharmacother. 2018, 99, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Yu, W.; Lin, Z.; Chen, Q.; Shi, J.; Dong, Y.; Duan, K.; Bai, X.; Xu, L.; Li, J.; et al. Anti-inflammatory effect of ginsenoside Rb1 contributes to the recovery of gastrointestinal motility in the rat model of postoperative ileus. Biol. Pharm. Bull. 2014, 37, 1788–1794. [Google Scholar] [CrossRef][Green Version]
- Hu, J.-N.; Xu, X.-Y.; Li, W.; Wang, Y.-M.; Liu, Y.; Wang, Z.; Wang, Y.-P. Ginsenoside Rk1 ameliorates paracetamol-induced hepatotoxicity in mice through inhibition of inflammation, oxidative stress, nitrative stress and apoptosis. J. Ginseng Res. 2019, 43, 10–19. [Google Scholar] [CrossRef]
- Cheng, W.; Jing, J.; Wang, Z.; Wu, D.; Huang, Y. Chondroprotective effects of ginsenoside Rg1 in human osteoarthritis chondrocytes and a rat model of anterior cruciate ligament transection. Nutrients 2017, 9, 263. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; U.S. Department of Health & Human Services: Washington, DC, USA, 2005; pp. 1–27.
- Li, X.; Wang, G.; Sun, J.; Hao, H.; Xiong, Y.; Yan, B.; Zheng, Y.; Sheng, L. Pharmacokinetic and absolute bioavailability study of total panax notoginsenoside, a typical multiple constituent traditional chinese medicine (TCM) in rats. Biol. Pharm. Bull. 2007, 30, 847–851. [Google Scholar] [CrossRef]
- Xu, Q.F.; Fang, X.L.; Chen, D.F. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J. Ethnopharmacol. 2003, 84, 187–192. [Google Scholar] [CrossRef]
- Joo, K.-M.; Lee, J.-H.; Jeon, H.-Y.; Park, C.-W.; Hong, D.-K.; Jeong, H.-J.; Lee, S.J.; Lee, S.-Y.; Lim, K.-M. Pharmacokinetic study of ginsenoside Re with pure ginsenoside Re and ginseng berry extracts in mouse using ultra performance liquid chromatography/mass spectrometric method. J. Pharm. Biomed. Anal. 2010, 51, 278–283. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Lee, J.; Park, J.-H.; Lee, C.-H.; Choi, M.-K.; Song, I.-S. Effect of lactic acid bacteria on the pharmacokinetics and metabolism of ginsenosides in mice. Pharmaceutics 2021, 13, 1496. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Smith, D.L.; Yang, Z.; Gao, S.; Yin, T.; Jiang, Z.-H.; You, M.; Gibbs, R.A.; Petrosino, J.F.; Hu, M. Bioactivity and bioavailability of ginsenosides are dependent on the glycosidase activities of the A/J mouse intestinal microbiome defined by pyrosequencing. Pharm. Res. 2013, 30, 836–846. [Google Scholar] [CrossRef]
- Zhou, Q.-L.; Zhu, D.-N.; Yang, Y.-F.; Xu, W.; Yang, X.-W. Simultaneous quantification of twenty-one ginsenosides and their three aglycones in rat plasma by a developed UFLC-MS/MS assay: Application to a pharmacokinetic study of red ginseng. J. Pharm. Biomed. Anal. 2017, 137, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Gu, L.-Q.; Xin, Y.-F.; Bai, Y.-S.; Zhang, S.; Gao, H.-Y.; Xu, P.-S.; Ma, Z.-F.; You, Z.-Q.; Wang, Z.; et al. Potential accumulation of protopanaxadiol-type ginsenosides in six-months toxicokinetic study of SHENMAI injection in dogs. Regul. Toxicol. Pharmacol. 2017, 83, 5–12. [Google Scholar] [CrossRef]
- Qi, B.; Zhang, L.; Zhang, Z.; Ouyang, J.; Huang, H. Effects of ginsenosides-Rb1 on exercise-induced oxidative stress in forced swimming mice. Pharmacogn. Mag. 2014, 10, 458–463. [Google Scholar] [CrossRef]
- Estaki, M.; Noble, E.G. North American ginseng protects against muscle damage and reduces neutrophil infiltration after an acute bout of downhill running in rats. Appl. Physiol. Nutr. Metab. 2015, 40, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Chtourou, H.; Hammouda, O.; Turki, M.; Ayedi, F.; Kallel, C.; AbdelKarim, O.; Hoekelmann, A.; Souissi, N. Relationship between biomarkers of muscle damage and redox status in response to a weightlifting training session: Effect of time-of-day. Physiol. Int. 2016, 103, 243–261. [Google Scholar] [CrossRef]
- Park, K.-S.; Sedlock, D.A.; Navalta, J.W.; Lee, M.-G.; Kim, S.-H. Leukocyte apoptosis and pro-/anti-apoptotic proteins following downhill running. Eur. J. Appl. Physiol. 2011, 111, 2349–2357. [Google Scholar] [CrossRef]
- Smith, L.L.; McKune, A.J.; Semple, S.J.; Sibanda, E.; Steel, H.; Anderson, R. Changes in serum cytokines after repeated bouts of downhill running. Appl. Physiol. Nutr. Metab. 2007, 32, 233–240. [Google Scholar] [CrossRef]
- Nosaka, K.; Newton, M.; Sacco, P. Delayed-onset muscle soreness does not reflect the magnitude of eccentric exercise-induced muscle damage. Scand. J. Med. Sci. Sports 2002, 12, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, M.I.; Kilduff, L.P.; McEneny, J.; Dietzig, R.E.; Benton, D. Phosphatidylserine supplementation and recovery following downhill running. Med. Sci. Sports Exerc. 2006, 38, 1617–1625. [Google Scholar] [CrossRef]
- Retamoso, L.T.; Silveira, M.E.P.; Lima, F.D.; Busanello, G.L.; Bresciani, G.; Ribeiro, L.R.; Chagas, P.M.; Nogueira, C.W.; Braga, A.C.M.; Furian, A.F.; et al. Increased xanthine oxidase-related ROS production and TRPV1 synthesis preceding DOMS post-eccentric exercise in rats. Life Sci. 2016, 152, 52–59. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.C.C.; Perez, A.C.; Prieto, J.G.; Duarte, I.D.G.; Alvarez, A.I. Protection of Panax ginseng in injured muscles after eccentric exercise. J. Ethnopharmacol. 2005, 97, 211–214. [Google Scholar] [CrossRef]
- Hsu, M.-F.; Yu, S.-H.; Korivi, M.; Jean, W.-H.; Lee, S.-D.; Huang, C.-Y.; Liao, Y.-H.; Lu, J.; Kuo, C.-H. Hormetic property of ginseng steroids on anti-oxidant status against exercise challenge in rat skeletal muscle. Antioxidants 2017, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Voces, J.; Cabral de Oliveira, A.C.; Prieto, J.G.; Vila, L.; Perez, A.C.; Duarte, I.D.G.; Alvarez, A.I. Ginseng administration protects skeletal muscle from oxidative stress induced by acute exercise in rats. Braz. J. Med. Biol. Res. 2004, 37, 1863–1871. [Google Scholar] [CrossRef]
- de Oliveira, A.C.C.; Perez, A.C.; Merino, G.; Prieto, J.G.; Alvarez, A.I. Protective effects of Panax ginseng on muscle injury and inflammation after eccentric exercise. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 369–377. [Google Scholar] [CrossRef]
- Lim, K.H.; Ko, D.; Kim, J.-H. Cardioprotective potential of Korean Red Ginseng extract on isoproterenol-induced cardiac injury in rats. J. Ginseng Res. 2013, 37, 273–282. [Google Scholar] [CrossRef]
- Steward, C.J.; Zhou, Y.; Keane, G.; Cook, M.D.; Liu, Y.; Cullen, T. One week of magnesium supplementation lowers IL-6, muscle soreness and increases post-exercise blood glucose in response to downhill running. Eur. J. Appl. Physiol. 2019, 119, 2617–2627. [Google Scholar] [CrossRef]
- Drobnic, F.; Riera, J.; Appendino, G.; Togni, S.; Franceschi, F.; Valle, X.; Pons, A.; Tur, J. Reduction of delayed onset muscle soreness by a novel curcumin delivery system (Meriva®): A randomised, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2014, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- McFarlin, B.K.; Venable, A.S.; Henning, A.L.; Sampson, J.N.B.; Pennel, K.; Vingren, J.L.; Hill, D.W. Reduced inflammatory and muscle damage biomarkers following oral supplementation with bioavailable curcumin. BBA Clin. 2016, 5, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Suzuki, K.; Wilson, G.; Hordern, M.; Nosaka, K.; Mackinnon, L.; Coombes, J.S. Exercise-induced muscle damage, plasma cytokines, and markers of neutrophil activation. Med. Sci. Sports Exerc. 2005, 37, 737–745. [Google Scholar] [CrossRef]
- Philippe, M.; Junker, G.; Gatterer, H.; Melmer, A.; Burtscher, M. Acute effects of concentric and eccentric exercise matched for energy expenditure on glucose metabolism in healthy females: A randomized crossover trial. Springerplus 2016, 5, 1455. [Google Scholar] [CrossRef]
- Howard, E.E.; Pasiakos, S.M.; Blesso, C.N.; Fussell, M.A.; Rodriguez, N.R. Divergent roles of inflammation in skeletal muscle recovery from injury. Front. Physiol. 2020, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Malm, C.; Sjödin, T.L.B.; Sjöberg, B.; Lenkei, R.; Renström, P.; Lundberg, I.E.; Ekblom, B. Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J. Physiol. 2004, 556, 983–1000. [Google Scholar] [CrossRef]
- Cornish, S.M.; Johnson, S.T. Systemic cytokine response to three bouts of eccentric exercise. Results Immunol. 2014, 4, 23–29. [Google Scholar] [CrossRef][Green Version]
- Kanda, K.; Sugama, K.; Hayashida, H.; Sakuma, J.; Kawakami, Y.; Miura, S.; Yoshioka, H.; Mori, Y.; Suzuki, K. Eccentric exercise-induced delayed-onset muscle soreness and changes in markers of muscle damage and inflammation. Exerc. Immunol. Rev. 2013, 19, 72–85. [Google Scholar]
- Peake, J.M.; Della Gatta, P.; Suzuki, K.; Nieman, D.C. Cytokine expression and secretion by skeletal muscle cells: Regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015, 21, 8–25. [Google Scholar] [CrossRef]
- Steensberg, A.; Keller, C.; Starkie, R.L.; Osada, T.; Febbraio, M.A.; Pedersen, B.K. IL-6 and TNF-α expression in, and release from, contracting human skeletal muscle. Am. J. Physiol. Metab. 2002, 283, E1272–E1278. [Google Scholar] [CrossRef] [PubMed]
- Borge, B.A.S.; Kalland, K.-H.; Olsen, S.; Bletsa, A.; Berggreen, E.; Wiig, H. Cytokines are produced locally by myocytes in rat skeletal muscle during endotoxemia. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H735–H744. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Characterization of exercise-induced cytokine release, the impacts on the body, the mechanisms and modulations. Int. J. Sport. Exerc. Med. 2019, 5, 122. [Google Scholar] [CrossRef]
- Jung, J.H.; Kang, T.K.; Oh, J.H.; Jeong, J.U.; Ko, K.P.; Kim, S.T. The effect of Korean red ginseng on symptoms and inflammation in patients with allergic rhinitis. Ear Nose Throat J. 2020, 100, S712–S719. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Deng, J.S.; Chang, Y.S.; Huang, G.J. Ginsenoside Rh2 ameliorates lipopolysaccharide-induced acute lung injury by regulating the TLR4/PI3K/Akt/mTOR, Raf-1/MEK/ERK, and Keap1/Nrf2/HO-1 signaling pathways in mice. Nutrients 2018, 10, 1208. [Google Scholar] [CrossRef]
- Daseke, M.J.; Tenkorang-Impraim, M.A.A.; Ma, Y.; Chalise, U.; Konfrst, S.R.; Garrett, M.R.; DeLeon-Pennell, K.Y.; Lindsey, M.L. Exogenous IL-4 shuts off pro-inflammation in neutrophils while stimulating anti-inflammation in macrophages to induce neutrophil phagocytosis following myocardial infarction. J. Mol. Cell. Cardiol. 2020, 145, 112–121. [Google Scholar] [CrossRef]
- DellaGatta, P.A.; Garnham, A.P.; Peake, J.M.; Cameron-Smith, D. Effect of exercise training on skeletal muscle cytokine expression in the elderly. Brain Behav. Immun. 2014, 39, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qin, X.; Zhang, X.; Liu, B.; Chen, M. Upregulation of IL-4 signaling contributes to aerobic exercise-induced insulin sensitivity. Biochem. Biophys. Res. Commun. 2020, 525, 662–667. [Google Scholar] [CrossRef]
- Horsley, V.; Jansen, K.M.; Mills, S.T.; Pavlath, G.K. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 2003, 113, 483–494. [Google Scholar] [CrossRef]
- Silva, L.A.; Bom, K.F.; Tromm, C.B.; Rosa, G.L.; Mariano, I.; Pozzi, B.G.; Tuon, T.; Stresck, E.L.; Souza, C.T.; Pinho, R.A. Effect of eccentric training on mitochondrial function and oxidative stress in the skeletal muscle of rats. Braz. J. Med. Biol. Res. 2013, 46, 14–20. [Google Scholar] [CrossRef][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Lin, Y.-A.; Chen, S.-L.; Hsu, M.-C.; Hsu, C.-C. American Ginseng Attenuates Eccentric Exercise-Induced Muscle Damage via the Modulation of Lipid Peroxidation and Inflammatory Adaptation in Males. Nutrients 2022, 14, 78. https://doi.org/10.3390/nu14010078
Lin C-H, Lin Y-A, Chen S-L, Hsu M-C, Hsu C-C. American Ginseng Attenuates Eccentric Exercise-Induced Muscle Damage via the Modulation of Lipid Peroxidation and Inflammatory Adaptation in Males. Nutrients. 2022; 14(1):78. https://doi.org/10.3390/nu14010078
Chicago/Turabian StyleLin, Ching-Hung, Yi-An Lin, Shu-Li Chen, Mei-Chich Hsu, and Cheng-Chen Hsu. 2022. "American Ginseng Attenuates Eccentric Exercise-Induced Muscle Damage via the Modulation of Lipid Peroxidation and Inflammatory Adaptation in Males" Nutrients 14, no. 1: 78. https://doi.org/10.3390/nu14010078
APA StyleLin, C.-H., Lin, Y.-A., Chen, S.-L., Hsu, M.-C., & Hsu, C.-C. (2022). American Ginseng Attenuates Eccentric Exercise-Induced Muscle Damage via the Modulation of Lipid Peroxidation and Inflammatory Adaptation in Males. Nutrients, 14(1), 78. https://doi.org/10.3390/nu14010078





