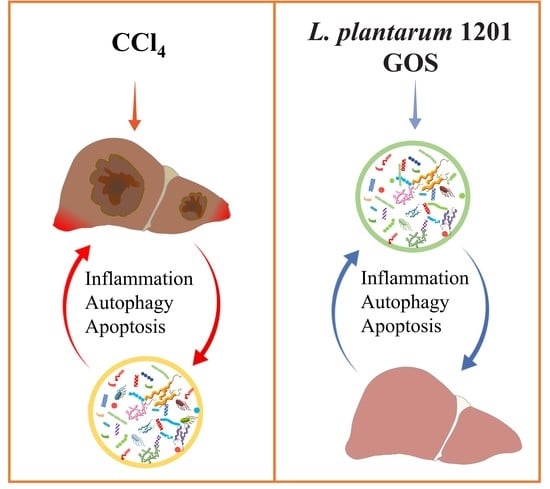
Protective Effect of Lactiplantibacillus plantarum 1201 Combined with Galactooligosaccharide on Carbon Tetrachloride-Induced Acute Liver Injury in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Lactobacillus Strain and Culture Conditions
2.2. Antioxidant Ability of the Lactobacillus Strain
2.2.1. Preparation Culture Supernatant and the Intracellular Extract
2.2.2. DPPH Radical Scavenging Assay
2.2.3. Reducing Power Assay
2.2.4. Hydroxyl Free Radical Scavenging Activity Assay
2.3. Growth Experiments
2.4. Animals
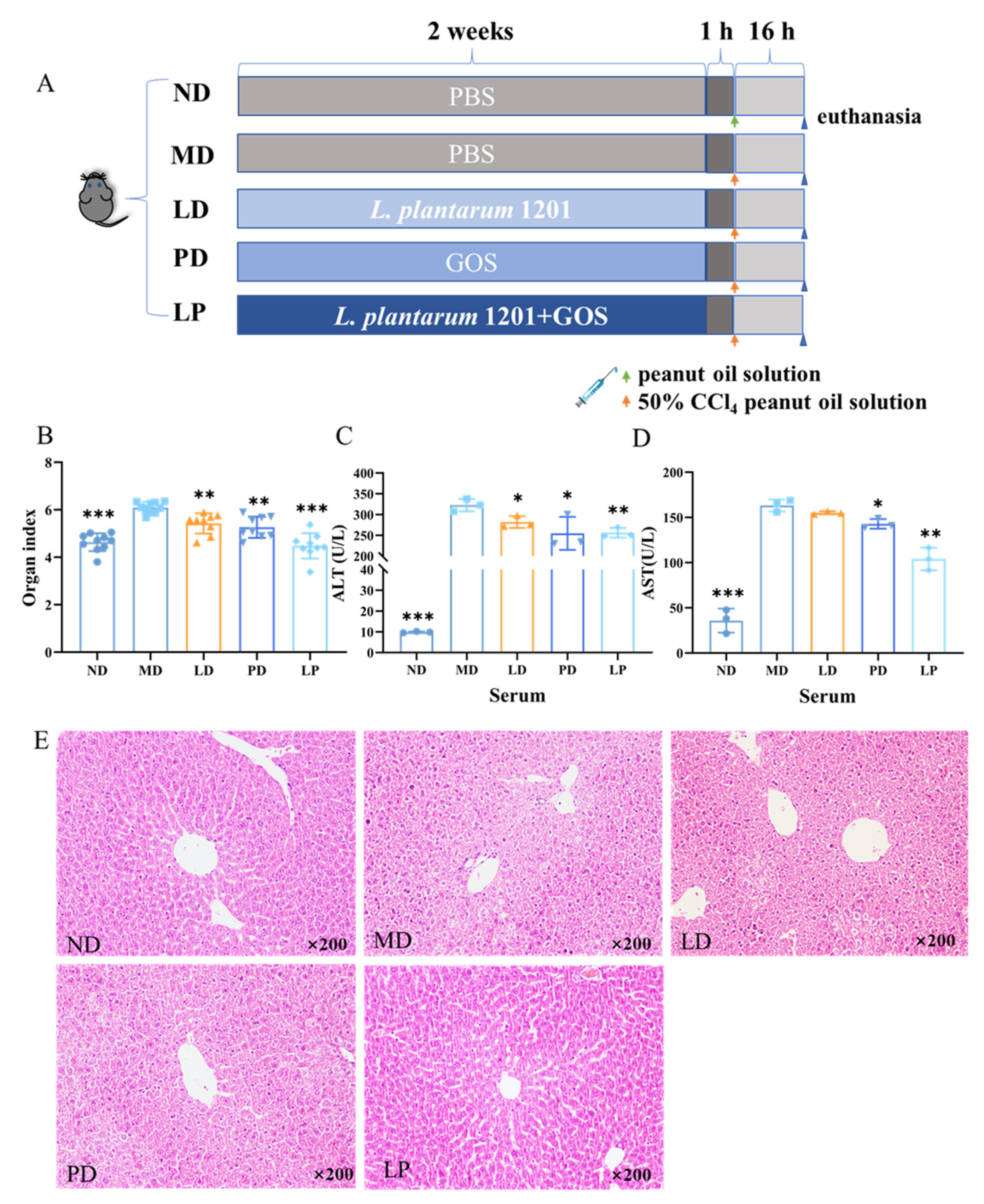
2.5. Experimental Groups
2.6. Organ Index Determination
2.7. Aminotransferase Measurement
2.8. Examination of Oxidation Markers
2.9. Hematoxylin and Eosin Staining
2.10. DNA Extraction from the Mouse Cecal Contents and 16S rRNA Gene Sequencing
2.11. Gene Expression
2.12. Enzyme-Linked Immunosorbent Assay
2.13. Statistical Analysis
3. Results
3.1. GOS Promoted the Growth of L. plantarum 1201 with High-Quality Antioxidant Power
3.2. Synbiotics Alleviated the Dysfunction and Pathological Damage of the Liver in CCl4-Induced ALI Mice
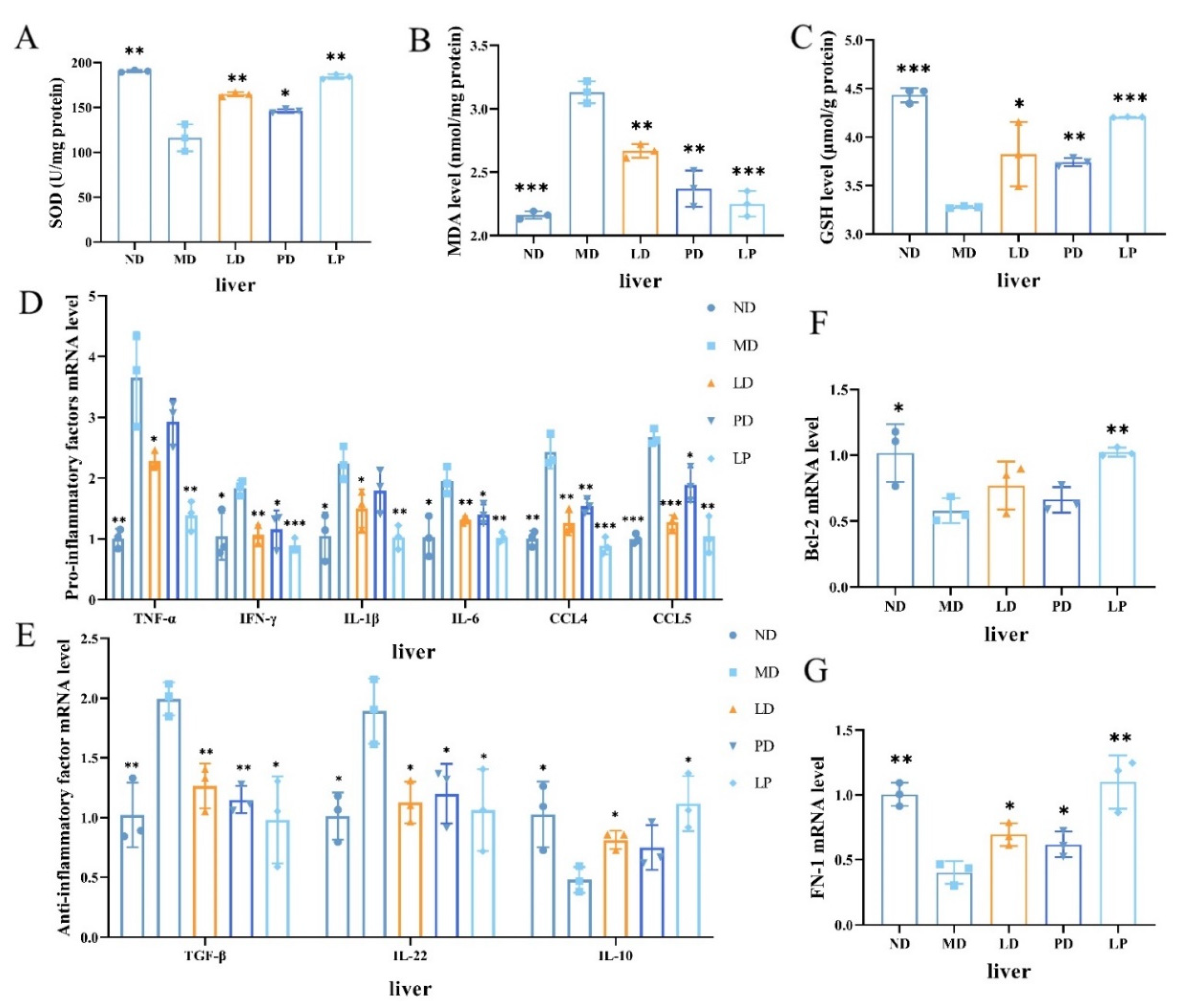
3.3. Synbiotics Reduced Oxidative Stress and Relieved Liver Inflammation in CCl4-Induced ALI Mice
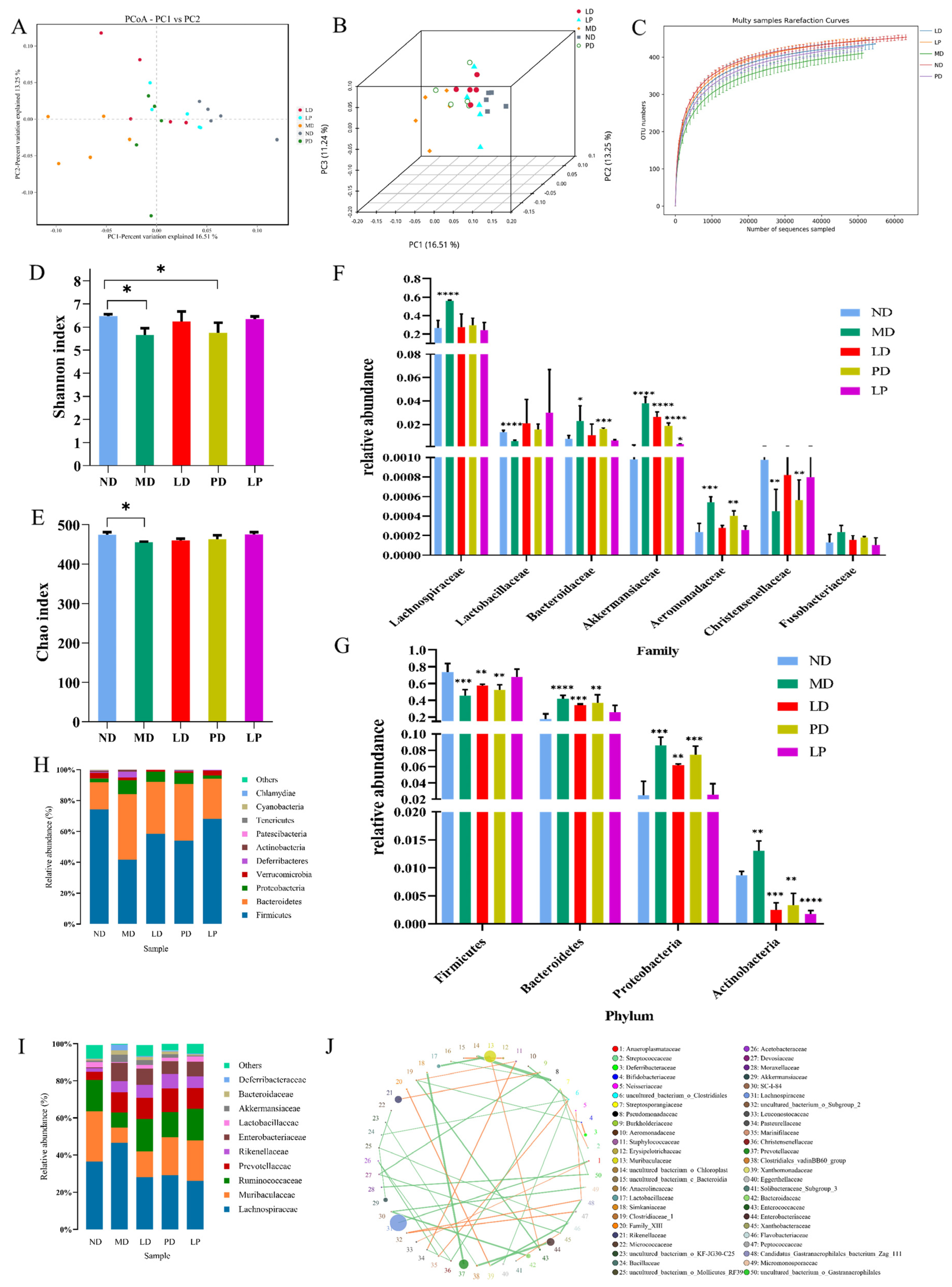
3.4. Synbiotics Alleviated Intestinal Flora Disturbance in CCl4-Induced ALI Mice
3.5. Synbiotics Relieved Intestinal Inflammation in CCl4-Induced ALI Mice
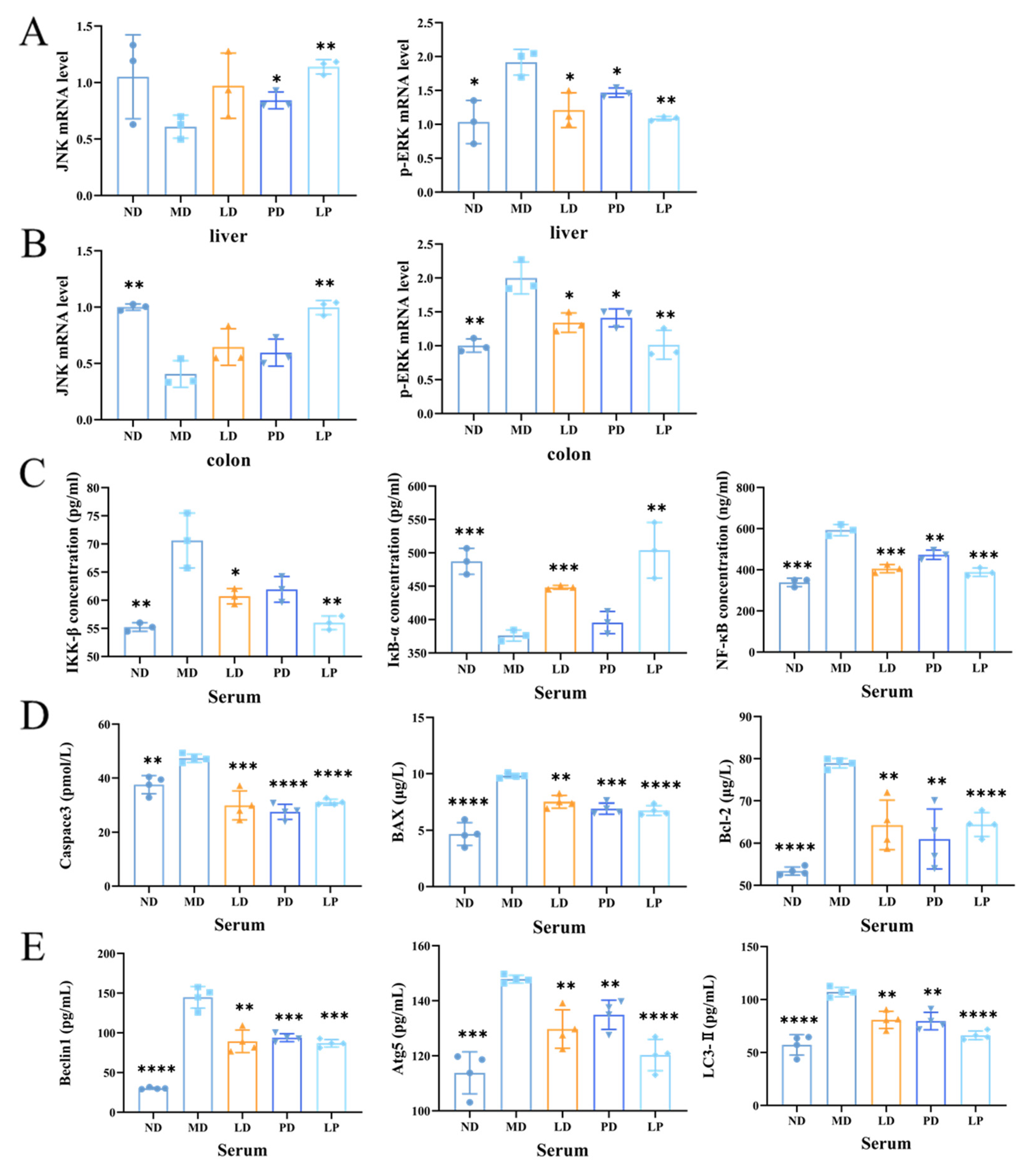
3.6. Synbiotics Inhibited the Apoptotic Signaling Pathway and the Autophagic Signaling Pathway through Inhibiting the MAPK/NF-κB Signaling Pathway in CCl4-Induced ALI Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taub, R. Liver regeneration: From myth to mechanism. Nat. Rev. Mol. Cell Biol. 2004, 5, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Cetin, A.; Kaynar, L.; Kocyigit, I.; Hacioglu, S.; Saraymen, R.; Ozturk, A.; Sari, I.; Sagdic, O. Role of grape seed extract on methotrexate induced oxidative stress in rat liver. Am. J. Chin. Med. 2008, 36, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Xu, Y.; Xu, U.; Cong, X.; Yin, L.; Hua, L.; Peng, J. Mechanism investigation of dioscin against CCl4-induced acute liver damage in mice. Env. Toxicol. Pharm. 2012, 34, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Han, W.; Shi, H.; Ren, F.; Chen, D.; Chen, Y.; Duan, Z. Augmenter of liver regeneration protects against carbon tetrachloride-induced liver injury by promoting autophagy in mice. Oncotarget 2017, 8, 12637–12648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeschke, H.; McGill, M.; Ramachandran, A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 2012, 44, 88–106. [Google Scholar] [CrossRef] [Green Version]
- Urano, Y.; Oda, S.; Tsuneyama, K.; Yokoi, T. Comparative hepatic transcriptome analyses revealed possible pathogenic mechanisms of fasiglifam (TAK-875)-induced acute liver injury in mice. Chem. Biol. Interact. 2018, 296, 185–197. [Google Scholar] [CrossRef]
- Arjinajarn, P.; Chueakula, N.; Pongchaidecha, A.; Jaikumkao, K.; Chatsudthipong, V.; Mahatheeranont, S.; Norkaew, O.; Chattipakorn, N.; Lungkaphin, A. Anthocyanin-rich Riceberry bran extract attenuates gentamicin-induced hepatotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Biomed. Pharmacother. 2017, 92, 412–420. [Google Scholar] [CrossRef]
- Tabassum, H.; Parvez, S.; Pasha, S.T.; Banerjee, B.D.; Raisuddin, S. Protective effect of lipoic acid against methotrexate-induced oxidative stress in liver mitochondria. Food Chem. Toxicol. 2010, 48, 1973–1979. [Google Scholar] [CrossRef]
- Dong, X.; Feng, X.; Liu, J.; Xu, Y.; Pan, Q.; Ling, Z.; Yu, J.; Yang, J.; Li, L.; Cao, H. Characteristics of Intestinal Microecology during Mesenchymal Stem Cell-Based Therapy for Mouse Acute Liver Injury. Stem Cells Int. 2019, 2019, 2403793. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, Z.; Huo, D.; Shao, Y. Consumption of Goats’ Milk Protects Mice from Carbon Tetrachloride-Induced Acute Hepatic Injury and Improves the Associated Gut Microbiota Imbalance. Front. Immunol. 2018, 9, 1034. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, J.; Li, Y.; Dong, S.; Liu, H.; Chen, J.; Wang, Y.; Zhao, S.; Zhang, Y.; Zhang, H. Probiotic Lactobacillus casei Zhang reduces pro-inflammatory cytokine production and hepatic inflammation in a rat model of acute liver failure. Eur. J. Nutr. 2016, 55, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sung, C.; Lee, N.; Ni, Y.; Pihlajamäki, J.; Panagiotou, G.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315. [Google Scholar] [CrossRef] [Green Version]
- Chassaing, B.; Etienne-Mesmin, L.; Gewirtz, A. Microbiota-liver axis in hepatic disease. Hepatology 2014, 59, 328–339. [Google Scholar] [CrossRef] [Green Version]
- Zhong, G.; Wan, F.; Lan, J.; Jiang, X.; Wu, S.; Pan, J.; Tang, Z.; Hu, L. Arsenic exposure induces intestinal barrier damage and consequent activation of gut-liver axis leading to inflammation and pyroptosis of liver in ducks. Sci. Total. Environ. 2021, 788, 147780. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, H.; Kim, S.; Lee, J.; Ha, J.; Choi, Y.; Oh, H.; Kim, Y.; Lee, Y.; Choi, K.; et al. Clostridioides difficileIntestinal Can Cause Liver Injury through the Occurrence of Inflammation and Damage to Hepatocytes. BioMed Res. Int. 2020, 2020, 7929610. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vrese, M.; Schrezenmeir, J. Probiotics, Prebiotics, and Synbiotics. In Food Biotechnology; Stahl, U., Donalies, U.E.B., Nevoigt, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–66. [Google Scholar]
- Williams, N. Probiotics. Am. J. Health Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, S.; Johnson, D.; Campbell, K.; Williams, S.; Fenning, A.; Saluja, S.; Irwin, C. Effect of probiotics and synbiotics consumption on serum concentrations of liver function test enzymes: A systematic review and meta-analysis. Eur. J. Nutr. 2018, 57, 2037–2053. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Li, P.; Liu, Y.; Zhang, Y. Efficacy of Probiotics and Synbiotics in Patients with Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Dig. Dis. Sci. 2019, 64, 3402–3412. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Yi, R.; Mu, J.; Zhao, X.; Yang, Z. Lactobacillus Hepatoprotective Effects of on Carbon Tetrachloride-Induced Acute Liver Injury in Mice. Int. J. Mol. Sci. 2018, 19, 2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Fu, Y.; Zhang, H.; Wang, J.; Zhu, J.; Wang, Y.; Guo, Y.; Wang, G.; Xu, T.; Chu, M.; et al. The hepatoprotective effect of the probiotic Clostridium butyricum against carbon tetrachloride-induced acute liver damage in mice. Food Funct. 2017, 8, 4042–4052. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Steed, H.; Macfarlane, S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 2008, 104, 305–344. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, M.; Zhang, P.; Fan, S.; Huang, J.; Yu, S.; Zhang, C.; Li, H. Fucoidan and galactooligosaccharides ameliorate high-fat diet-induced dyslipidemia in rats by modulating the gut microbiota and bile acid metabolism. Nutrition 2019, 65, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.; Kuo, Y.; Tseng, Y.; Lee, M.; Chen, H. Beneficial effects of fructo-oligosaccharides supplementation on fecal bifidobacteria and index of peroxidation status in constipated nursing-home residents—a placebo-controlled, diet-controlled trial. Nutrition 2011, 27, 323–328. [Google Scholar] [CrossRef]
- Chu, H.; Tao, X.; Sun, Z.; Hao, W.; Wei, X. Galactooligosaccharides protects against DSS-induced murine colitis through regulating intestinal flora and inhibiting NF-kappaB pathway. Life Sci. 2020, 242, 117220. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Maldonado-Gómez, M.; Martínez, I. To engraft or not to engraft: An ecological framework for gut microbiome modulation with live microbes. Curr. Opin. Biotechnol. 2018, 49, 129–139. [Google Scholar] [CrossRef]
- Ma, C.; Wasti, S.; Huang, S.; Zhang, Z.; Mishra, R.; Jiang, S.; You, Z.; Wu, Y.; Chang, H.; Wang, Y.; et al. The gut microbiome stability is altered by probiotic ingestion and improved by the continuous supplementation of galactooligosaccharide. Gut Microbes 2020, 12, 1785252. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Wang, M.; Zhao, Y.; Liu, S.; Chen, L.; Xu, H. Protective Effect of Lactobacillus rhamnosus GG on TiO2 Nanoparticles-Induced Oxidative Stress Damage in the Liver of Young Rats. Nanomaterials 2021, 11, 803. [Google Scholar] [CrossRef]
- Shi, Y.; Cui, X.; Gu, S.; Yan, X.; Li, R.; Xia, S.; Chen, H.; Ge, J. Antioxidative and Probiotic Activities of Lactic Acid Bacteria Isolated from Traditional Artisanal Milk Cheese from Northeast China. Probiotics Antimicrob. Proteins 2019, 11, 1086–1099. [Google Scholar] [CrossRef] [PubMed]
- Pieniz, S.; Andreazza, R.; Anghinoni, T.; Camargo, F.; Brandelli, A. Probiotic potential, antimicrobial and antioxidant activities of Enterococcus durans strain LAB18s. Food Control 2014, 37, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Adebayo-Tayo, B.; Ishola, R.; Oyewunmi, T. Characterization, antioxidant and immunomodulatory potential on exopolysaccharide produced by wild type and mutant Weissella confusa strains. Biotechnol. Rep. 2018, 19, e00271. [Google Scholar] [CrossRef] [PubMed]
- Salli, K.; Söderling, E.; Hirvonen, J.; Gürsoy, U.; Ouwehand, A. Streptococcus mutansInfluence of 2′-fucosyllactose and galacto-oligosaccharides on the growth and adhesion of. Br. J. Nutr. 2020, 124, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M. Prebiotics: The Concept Revisited. J. Nutr. 2007, 137, 830S–837S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capurso, L. Thirty Years of Lactobacillus rhamnosus GG: A Review. J. Clin. Gastroenterol. 2019, 53, S1–S41. [Google Scholar] [CrossRef]
- Shen, X.; Tang, Y.; Yang, R.; Yu, L.; Fang, T.; Duan, J. The protective effect of Zizyphus jujube fruit on carbon tetrachloride-induced hepatic injury in mice by anti-oxidative activities. J. Ethnopharmacol. 2009, 122, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Yu, Y.; Bai, F.; Wang, L.; Yang, D.; Zhang, C.; Qin, C.; Yang, M.; Zhang, D.; Zhu, Y.; et al. Effect of fecal microbiota transplantation on neurological restoration in a spinal cord injury mouse model: Involvement of brain-gut axis. Microbiome 2021, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, C.; Powell, D.; Kalman, D. Layered defense: How mucus and tight junctions seal the intestinal barrier. J. Mol. Med. 2017, 95, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Diao, J.X.; Ou, J.Y.; Dai, H.; Li, H.Y.; Yang, Y.G. Antioxidant and Antiapoptotic Polyphenols from Green Tea Extract Ameliorate CCl4-Induced Acute Liver Injury in Mice. Chin. J. Integr. Med. 2020, 26, 736–744. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Y.; Zhang, Q.; Liu, B.; Wang, F.; Li, J.; Zhu, R. Hepatoprotective effects of baicalein against CCl4-induced acute liver injury in mice. World J. Gastroenterol. 2012, 18, 6605–6613. [Google Scholar] [CrossRef]
- Huang, R.; Tao, X.; Wan, C.; Li, S.; Xu, H.; Xu, F.; Shah, N.; Wei, H. In vitro probiotic characteristics of Lactobacillus plantarum ZDY 2013 and its modulatory effect on gut microbiota of mice. J. Dairy Sci. 2015, 98, 5850–5861. [Google Scholar] [CrossRef]
- Trocha, M.; Merwid-Lad, A.; Szuba, A.; Sozański, T.; Magdalan, J.; Szelag, A.; Kopacz, M.; Kuźniar, A.; Nowak, D. Effect of quercetin-5′-sulfonic acid sodium salt on SOD activity and ADMA/DDAH pathway in extracorporeal liver perfusion in rats. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2012, 21, 423–431. [Google Scholar]
- Ibrahim, M.; Mikail, M.; Ahmed, I.; Hazali, N.; Abdul, R.; Abdul, G.; Hashim, R.; Arief, S.; Md-Isa, M.; Draman, S. Comparison of the effects of three different Baccaurea angulata whole fruit juice doses on plasma, aorta and liver MDA levels, antioxidant enzymes and total antioxidant capacity. Eur. J. Nutr. 2018, 57, 1817–1828. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Kanikarla-Marie, P.; Warden, C.; Micinski, D. L-cysteine supplementation upregulates glutathione (GSH) and vitamin D binding protein (VDBP) in hepatocytes cultured in high glucose and in vivo in liver, and increases blood levels of GSH, VDBP, and 25-hydroxy-vitamin D in Zucker diabetic fatty rats. Mol. Nutr. Food Res. 2016, 60, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Li, C.; Zhang, Y.; Zheng, H.; Cheng, Y.; Zhu, J.; Chen, X.; Zhu, Z.; Piao, J.; Li, F. Targeted Manganese doped silica nano GSH-cleaner for treatment of Liver Cancer by destroying the intracellular redox homeostasis. Theranostics 2020, 10, 9865–9887. [Google Scholar] [CrossRef] [PubMed]
- López-Navarro, M.; Jarquín-Martínez, M.; Sánchez-Labastida, L.; Ramírez-Rosales, D.; Godínez-Victoria, M.; Quintas-Granados, L.; Trujillo-Ferrara, J. Decoding Aging: Understanding the Complex Relationship among Aging, Free Radicals, and GSH. Oxidative Med. Cell. Longev. 2020, 2020, 3970860. [Google Scholar] [CrossRef]
- Gong, S.; Lan, T.; Zeng, L.; Luo, H.; Yang, X.; Li, N.; Chen, X.; Liu, Z.; Li, R.; Win, S.; et al. Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice. J. Hepatol. 2018, 69, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Knox, S.J.; Armstrong, J.S.; Steinauer, K.K.; Hornung, B.; Irish, J.M.; Lecane, P.; Birrell, G.W.; Peehl, D.M. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. 2002, 9, 252–263. [Google Scholar]
- Di, C.; Baj, J.; Garruti, G.; Celano, G.; De, A.; Wang, H.; Di, P.; Bonfrate, L.; Wang, D.; Portincasa, P. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk. J. Clin. Med. 2020, 9, 2648. [Google Scholar] [CrossRef]
- Qiu, P.; Dong, Y.; Zhu, T.; Luo, Y.; Kang, X.; Pang, M.; Li, H.; Xu, H.; Gu, C.; Pan, S.; et al. Semen hoveniae extract ameliorates alcohol-induced chronic liver damage in rats via modulation of the abnormalities of gut-liver axis. Phytomed. Int. J. Phytother. Phytopharm. 2019, 52, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Liu, C.; Huang, C.; Luo, F.; Zhu, X. Ursolic Acid Improves Intestinal Damage and Bacterial Dysbiosis in Liver Fibrosis Mice. Front. Pharmacol. 2019, 10, 1321. [Google Scholar] [CrossRef]
- Li, H.; Shi, J.; Zhao, L.; Guan, J.; Liu, F.; Huo, G.; Li, B. Lactobacillus plantarum KLDS1.0344 and KLDS1.0901 Mixture Prevents Chronic Alcoholic Liver Injury in Mice by Protecting the Intestinal Barrier and Regulating Gut Microbiota and Liver-Related Pathways. J. Agric. Food Chem. 2021, 69, 183–197. [Google Scholar] [CrossRef]
- Nakamoto, N.; Kanai, T. Role of toll-like receptors in immune activation and tolerance in the liver. Front. Immunol. 2014, 5, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiest, R.; Lawson, M.; Geuking, M. Pathological bacterial translocation in liver cirrhosis. J. Hepatol. 2014, 60, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Sorribas, M.; Jakob, M.; Yilmaz, B.; Li, H.; Stutz, D.; Noser, Y.; de Gottardi, A.; Moghadamrad, S.; Hassan, M.; Albillos, A.; et al. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J. Hepatol. 2019, 71, 1126–1140. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Hesketh, J.; Huang, D.; Gan, F.; Hao, S.; Tang, S.; Guo, Y.; Huang, K. Protective effects of selenium-glutathione-enriched probiotics on CCl-induced liver fibrosis. J. Nutr. Biochem. 2018, 58, 138–149. [Google Scholar] [CrossRef]
- Yao, F.; Jia, R.; Huang, H.; Yu, Y.; Mei, L.; Bai, L.; Ding, Y.; Zheng, P. Lactobacillus paracaseiEffect of N1115 and fructooligosaccharides in nonalcoholic fatty liver disease. Arch. Med. Sci. 2019, 15, 1336–1344. [Google Scholar] [CrossRef]
- Ko, I.; Jin, J.; Hwang, L.; Kim, S.; Kim, C.; Han, J.; Lee, S.; Kim, H.; Shin, H.; Jeon, J. Polydeoxyribonucleotide Exerts Protective Effect Against CCl-Induced Acute Liver Injury Through Inactivation of NF-κB/MAPK Signaling Pathway in Mice. Int. J. Mol. Sci. 2020, 21, 7894. [Google Scholar] [CrossRef]
- Kinnally, K.; Antonsson, B. A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis 2007, 12, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, Z.; Duan, C.; Wang, C.; Zhao, Y.; Yang, G.; Gao, L.; Niu, C.; Xu, J.; Li, S. Lactobacillus plantarum C88 protects against aflatoxin B-induced liver injury in mice via inhibition of NF-κB-mediated inflammatory responses and excessive apoptosis. BMC Microbiol. 2019, 19, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, C.; Xiao, X.; Li, D.; Tun, S.; Wang, Y.; Velkov, T.; Tang, S. Chloroquine ameliorates carbon tetrachloride-induced acute liver injury in mice via the concomitant inhibition of inflammation and induction of apoptosis. Cell Death Dis. 2018, 9, 1164. [Google Scholar] [CrossRef]
- Bai, F.; Huang, Q.; Nie, J.; Lu, S.; Lu, C.; Zhu, X.; Wang, Y.; Zhuo, L.; Lu, Z.; Lin, X. Trolline Ameliorates Liver Fibrosis by Inhibiting the NF-kappaB Pathway, Promoting HSC Apoptosis and Suppressing Autophagy. Cell Physiol. Biochem. 2017, 44, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.; Merves, J.; Giroux, V.; Tanaka, K.; Guo, A.; Chandramouleeswaran, P.; Benitez, A.; Dods, K.; Que, J.; Masterson, J.; et al. Autophagy mediates epithelial cytoprotection in eosinophilic oesophagitis. Gut 2017, 66, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Huo, Y.; Zhang, Q.; Chen, S.; Lv, H.; Peng, L.; Wei, H.; Wan, C. Protective Effect of Lactiplantibacillus plantarum 1201 Combined with Galactooligosaccharide on Carbon Tetrachloride-Induced Acute Liver Injury in Mice. Nutrients 2021, 13, 4441. https://doi.org/10.3390/nu13124441
Ren Z, Huo Y, Zhang Q, Chen S, Lv H, Peng L, Wei H, Wan C. Protective Effect of Lactiplantibacillus plantarum 1201 Combined with Galactooligosaccharide on Carbon Tetrachloride-Induced Acute Liver Injury in Mice. Nutrients. 2021; 13(12):4441. https://doi.org/10.3390/nu13124441
Chicago/Turabian StyleRen, Zhongyue, Yalan Huo, Qimeng Zhang, Shufang Chen, Huihui Lv, Lingling Peng, Hua Wei, and Cuixiang Wan. 2021. "Protective Effect of Lactiplantibacillus plantarum 1201 Combined with Galactooligosaccharide on Carbon Tetrachloride-Induced Acute Liver Injury in Mice" Nutrients 13, no. 12: 4441. https://doi.org/10.3390/nu13124441
APA StyleRen, Z., Huo, Y., Zhang, Q., Chen, S., Lv, H., Peng, L., Wei, H., & Wan, C. (2021). Protective Effect of Lactiplantibacillus plantarum 1201 Combined with Galactooligosaccharide on Carbon Tetrachloride-Induced Acute Liver Injury in Mice. Nutrients, 13(12), 4441. https://doi.org/10.3390/nu13124441