Predictors of Post-Exercise Energy Intake in Adolescents Ranging in Weight Status from Overweight to Severe Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Measures
2.2.1. Anthropometrics and Body Composition
2.2.2. Exercise Testing
2.2.3. Trait Measures
2.2.4. State Measures
2.2.5. Ad Libitum Test-Meal
2.3. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Energy Intake Reliability
3.3. Baseline Predictors of Energy Intake (n = 30)
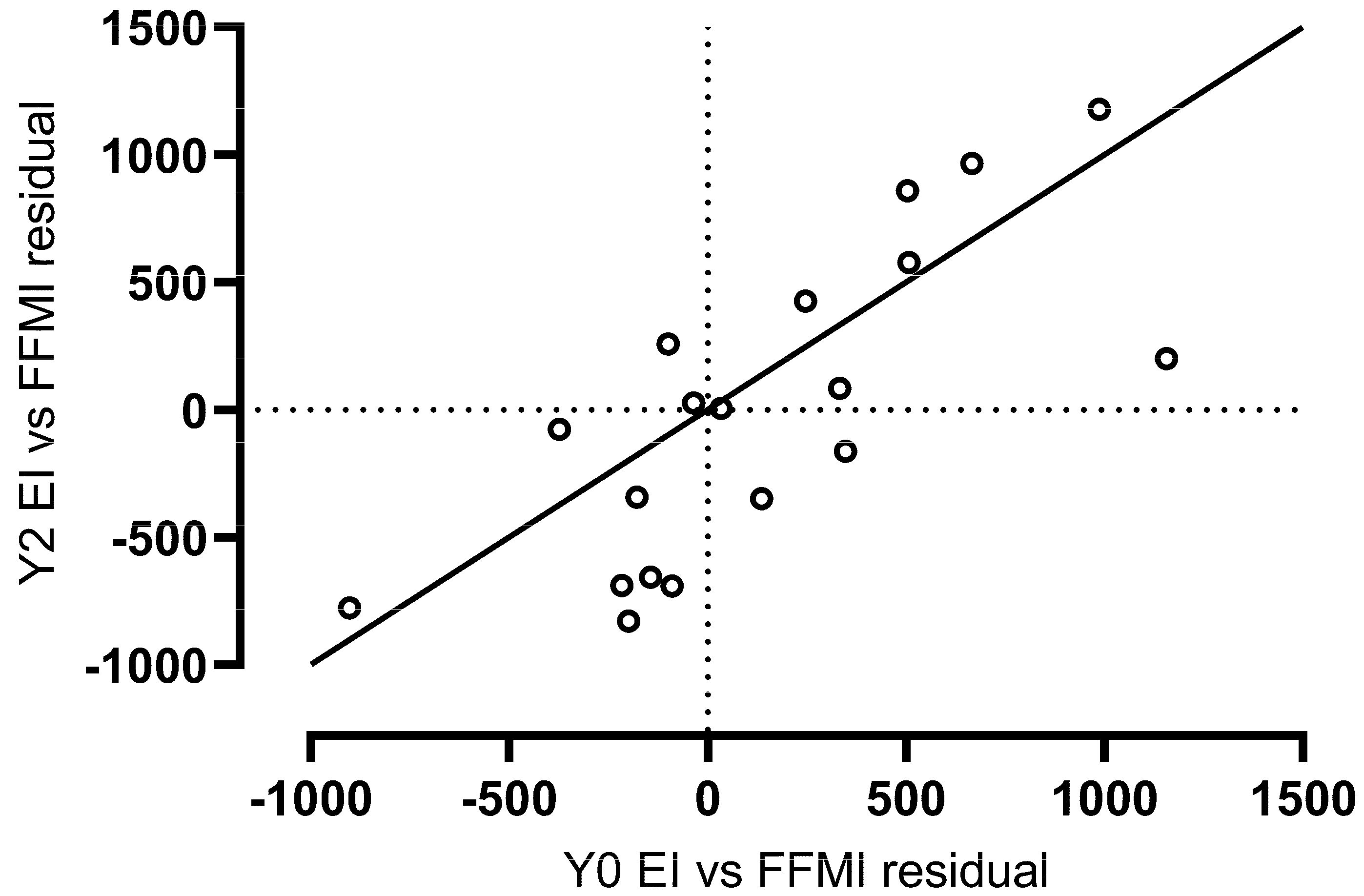
3.4. Year 2 Follow-Up Predictors of Energy Intake (n = 19)
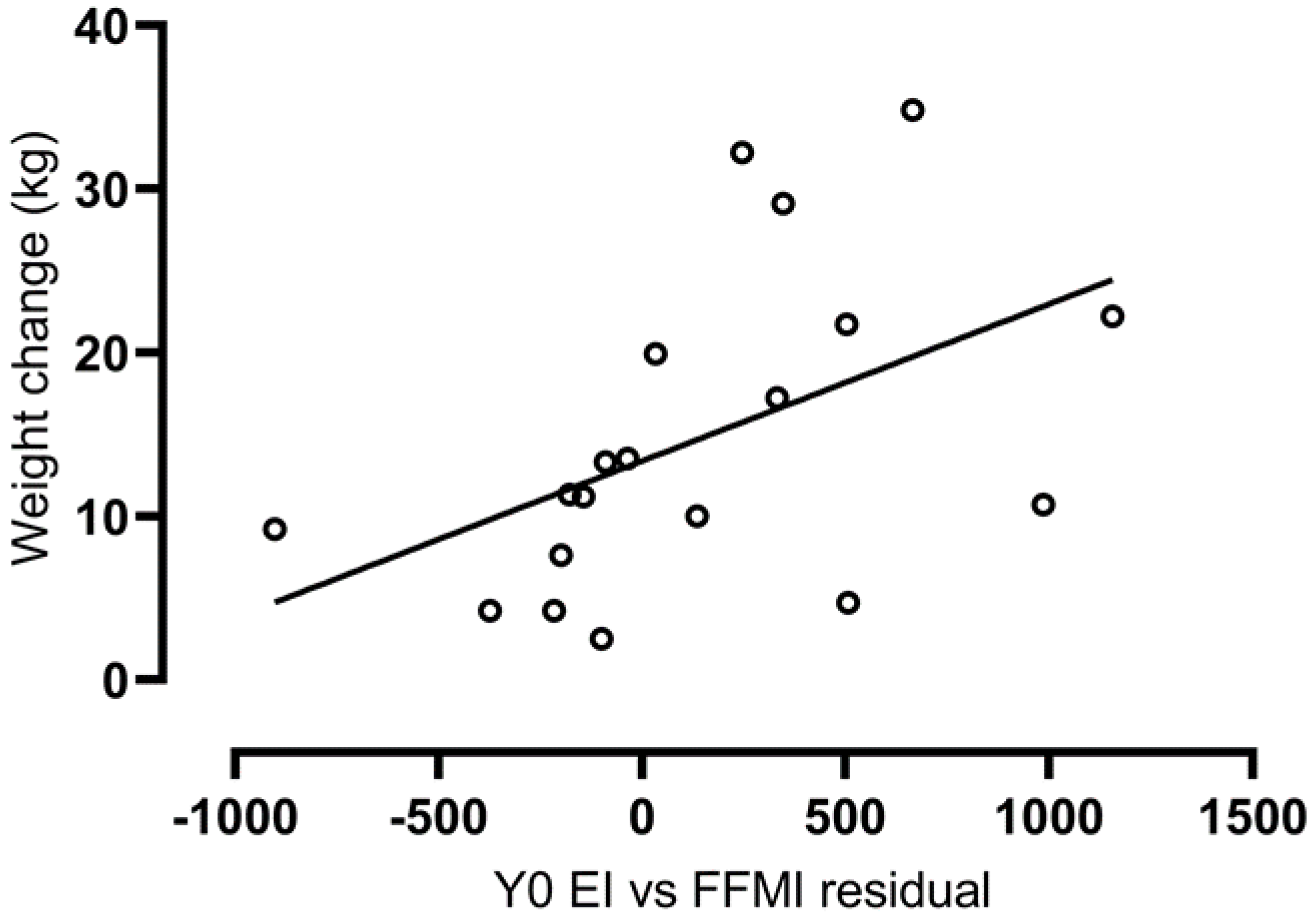
3.5. Associations with Weight and Fat Mass Change over Two Years
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keller, K.L.; Kling, S.M.R.; Fuchs, B.; Pearce, A.L.; Reigh, N.A.; Masterson, T.; Hickok, K. A Biopsychosocial Model of Sex Differences in Children’s Eating Behaviors. Nutrients 2019, 11, 682. [Google Scholar] [CrossRef] [Green Version]
- Russell, C.G.; Russell, A. A biopsychosocial approach to processes and pathways in the development of overweight and obesity in childhood: Insights from developmental theory and research. Obes. Rev. 2019, 20, 725–749. [Google Scholar] [CrossRef]
- Kininmonth, A.; Smith, A.; Carnell, S.; Steinsbekk, S.; Fildes, A.; Llewellyn, C. The association between childhood adiposity and appetite assessed using the Child Eating Behavior Questionnaire and Baby Eating Behavior Questionnaire: A systematic review and meta-analysis. Obes. Rev. 2021, 22, 13169. [Google Scholar] [CrossRef]
- Batterink, L.; Yokum, S.; Stice, E. Body mass correlates inversely with inhibitory control in response to food among ad-olescent girls: An fMRI study. Neuroimage 2010, 52, 1696–1703. [Google Scholar] [CrossRef] [Green Version]
- Price, M.; Lee, M.; Higgs, S. Food-specific response inhibition, dietary restraint and snack intake in lean and over-weight/obese adults: A moderated-mediation model. Int. J. Obes. 2016, 40, 877–882. [Google Scholar] [CrossRef] [Green Version]
- Vainik, U.; Garcia, I.G.; Dagher, A. Uncontrolled eating: A unifying heritable trait linked with obesity, overeating, personality and the brain. Eur. J. Neurosci. 2019, 50, 2430–2445. [Google Scholar] [CrossRef] [Green Version]
- Brunner, E.J.; Maruyama, K.; Shipley, M.; Cable, N.; Iso, H.; Hiyoshi, A.; Stallone, D.; Kumari, M.; Tabak, A.; Singh-Manoux, A.; et al. Appetite disinhibition rather than hunger explains genetic effects on adult BMI trajectory. Int. J. Obes. 2021, 45, 758–765. [Google Scholar] [CrossRef]
- Garcia-Garcia, I.; Neseliler, S.; Morys, F.; Dadar, M.; Yau, Y.H.C.; Scala, S.G.; Zeighami, Y.; Sun, N.; Collins, D.L.; Vainik, U.; et al. Relationship between impulsivity, uncontrolled eating and body mass index: A hierarchical model. Int. J. Obes. 2021, 46, 129–136. [Google Scholar] [CrossRef]
- de Lauzon-Guillain, B.; Basdevant, A.; Romon, M.; Karlsson, J.; Borys, J.M.; Charles, M.A. FLVS Study Group Is restrained eating a risk factor for weight gain in a general population? Am. J. Clin. Nutr. 2006, 83, 132–138. [Google Scholar] [CrossRef]
- Appelhans, B.M.; Woolf, K.; Pagoto, S.; Schneider, K.L.; Whited, M.C.; Liebman, R. Inhibiting Food Reward: Delay Discounting, Food Reward Sensitivity, and Palatable Food Intake in Overweight and Obese Women. Obesity 2011, 19, 2175–2182. [Google Scholar] [CrossRef]
- Epstein, L.H.; Yokum, S.; Feda, D.M.; Stice, E. Food reinforcement and parental obesity predict future weight gain in non-obese adolescents. Appetite 2014, 82, 138–142. [Google Scholar] [CrossRef] [Green Version]
- Rollins, B.Y.; Loken, E.; Savage, J.S.; Birch, L.L. Measurement of food reinforcement in preschool children. Associations with food intake, BMI, and reward sensitivity. Appetite 2014, 72, 21–27. [Google Scholar] [CrossRef] [Green Version]
- French, S.A.; Epstein, L.H.; Jeffery, R.W.; Blundell, J.E.; Wardle, J. Eating behavior dimensions. Associations with energy intake and body weight. A review. Appetite 2012, 59, 541–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feig, E.H.; Piers, A.D.; Kral, T.V.; Lowe, M.R. Eating in the absence of hunger is related to loss-of-control eating, hedonic hunger, and short-term weight gain in normal-weight women. Appetite 2018, 123, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Sadoul, B.C.; Schuring, E.A.; Mela, D.J.; Peters, H.P. The relationship between appetite scores and subsequent energy intake: An analysis based on 23 ran-domized controlled studies. Appetite 2014, 83, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, R.J.; Hughes, D.A.; Johnstone, A.M.; Rowley, E.; Reid, C.; Elia, M.; Stratton, R.; Delargy, H.; King, N.; Blundell, J.E. The use of visual analogue scales to assess motivation to eat in human subjects: A review of their reliabil-ity and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br. J. Nutr. 2000, 84, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Ruddick-Collins, L.C.; Byrne, N.; King, N.A. Assessing the influence of fasted and postprandial states on day-to-day variability of appetite and food preferences. Physiol. Behav. 2018, 199, 219–228. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. 2000, 24, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Smethers, A.D.; Roe, L.S.; Sanchez, C.E.; Zuraikat, F.M.; Keller, K.L.; Kling, S.M.R.; Rolls, B.J. Portion size has sustained effects over 5 days in preschool children: A randomized trial. Am. J. Clin. Nutr. 2019, 109, 1361–1372. [Google Scholar] [CrossRef]
- Kling, S.M.; Roe, L.S.; Keller, K.L.; Rolls, B.J. Double trouble: Portion size and energy density combine to increase preschool children’s lunch intake. Physiol. Behav. 2016, 162, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Boutelle, K.N.; Manzano, M.A.; Eichen, D.M. Appetitive traits as targets for weight loss: The role of food cue respon-siveness and satiety responsiveness. Physiol. Behav. 2020, 224, 113018. [Google Scholar] [CrossRef]
- Dalton, M.; Hollingworth, S.; Blundell, J.; Finlayson, G. Weak Satiety Responsiveness Is a Reliable Trait Associated with Hedonic Risk Factors for Overeating among Women. Nutrients 2015, 7, 7421–7436. [Google Scholar] [CrossRef] [Green Version]
- Edholm, O.G. Energy expenditure in relation to nutrition. Proc. Nutr. Soc. 1956, 15, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Roy, P.; Mitra, K.P. Relation between caloric intake, body weight, and physical work: Studies in an industrial male population in West Bengal. Am. J. Clin. Nutr. 1956, 4, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Caudwell, P.; Gibbons, C.; Hopkins, M.; Näslund, E.; King, N.A.; Finlayson, G. Body composition and appetite: Fat-free mass (but not fat mass or BMI) is positively associated with self-determined meal size and daily energy intake in humans. Br. J. Nutr. 2012, 107, 445–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caudwell, P.; Finlayson, G.; Gibbons, C.; Hopkins, M.; King, N.; Näslund, E.; Blundell, J.E. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. Am. J. Clin. Nutr. 2012, 97, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Weise, C.M.; Hohenadel, M.G.; Krakoff, J.; Votruba, S.B. Body composition and energy expenditure predict ad-libitum food and macronutrient intake in humans. Int. J. Obes. 2013, 38, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, M.; Finlayson, G.; Duarte, C.; Whybrow, S.; Ritz, P.; Horgan, G.W.; Blundell, J.E.; Stubbs, R.J. Modelling the associations between fat-free mass, resting metabolic rate and energy intake in the con-text of total energy balance. Int. J. Obes. 2015, 40, 312–318. [Google Scholar] [CrossRef] [Green Version]
- Cameron, J.D.; Sigal, R.J.; Kenny, G.P.; Alberga, A.S.; Prud’Homme, D.; Phillips, P.; Doucette, S.; Goldfield, G. Body composition and energy intake—Skeletal muscle mass is the strongest predictor of food intake in obese adolescents: The HEARTY trial. Appl. Physiol. Nutr. Metab. 2016, 41, 611–617. [Google Scholar] [CrossRef] [Green Version]
- Fearnbach, S.N.; Masterson, T.D.; Schlechter, H.A.; Loken, E.; Downs, D.S.; Thivel, D.; Keller, K.L. Perceived Exertion during Exercise Is Associated with Children’s Energy Intake. Med. Sci. Sports Exerc. 2017, 49, 785–792. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, M.; Finlayson, G.; Duarte, C.; Gibbons, C.; Johnstone, A.; Whybrow, S.; Horgan, G.W.; Blundell, J.E.; Stubbs, R.J. Biological and psychological mediators of the relationships between fat mass, fat-free mass and energy intake. Int. J. Obes. 2018, 43, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Kracht, C.; Champagne, C.M.; Hsia, D.S.; Martin, C.K.; Newton, R.L.; Katzmarzyk, P.T.; Staiano, A.E. Association Between Meeting Physical Activity, Sleep, and Dietary Guidelines and Cardiometabolic Risk Factors and Adiposity in Adolescents. J. Adolesc. Health 2020, 66, 733–739. [Google Scholar] [CrossRef]
- Kracht, C.L.; Chaput, J.-P.; Martin, C.K.; Champagne, C.M.; Katzmarzyk, P.T.; Staiano, A.E. Associations of Sleep with Food Cravings, Diet, and Obesity in Adolescence. Nutrients 2019, 11, 2899. [Google Scholar] [CrossRef] [Green Version]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2018; 472p. [Google Scholar]
- Kuczmarski, R.; Ogden, C.; Guo, S.; Grummer-Strawn, L.M.; Flegal, K.; Mei, Z.; Wei, R.; Curtin, L.; Roche, A.; Johnson, C. 2000 CDC Growth Charts for the United States: Methods and Development; Department of Health and Human Services: Washington, DC, USA, 2002. [Google Scholar]
- VanItallie, T.B.; Yang, M.U.; Heymsfield, S.B.; Funk, R.C.; Boileau, R.A. Height-normalized indices of the body’s fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am. J. Clin. Nutr. 1990, 52, 953–959. [Google Scholar] [CrossRef] [Green Version]
- Fearnbach, S.N.; Johannsen, N.M.; Martin, C.K.; Katzmarzyk, P.T.; Beyl, R.A.; Hsia, D.; Carmichael, O.T.; Staiano, A.E. A Pilot Study of Cardiorespiratory Fitness, Adiposity, and Cardiometabolic Health in Youth with Overweight and Obesity. Pediatr. Exerc. Sci. 2020, 32, 124–131. [Google Scholar] [CrossRef]
- Karlsson, J.; Persson, L.-O.; Sjöström, L.; Sullivan, M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int. J. Obes. 2000, 24, 1715–1725. [Google Scholar] [CrossRef] [Green Version]
- Cappelleri, J.C.; Bushmakin, A.G.; Gerber, R.A.; Leidy, N.K.; Sexton, C.C.; Lowe, M.R.; Karlsson, J. Psychometric analysis of the Three-Factor Eating Questionnaire-R21: Results from a large diverse sample of obese and non-obese participants. Int. J. Obes. 2009, 33, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Grammer, J.K.; Carrasco, M.; Gehring, W.J.; Morrison, F.J. Age-related changes in error processing in young children: A school-based investigation. Dev. Cogn. Neurosci. 2014, 9, 93–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanofsky-Kraff, M.; Yanovski, J.; Ba, N.A.S.; Olsen, C.H.; Bs, J.G.; Yanovski, J.A. A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. Int. J. Eat. Disord. 2009, 42, 26–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravussin, E. Energy Metabolism in Obesity: Studies in the Pima Indians. Diabetes Care 1993, 16, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.J.; Kompaniyets, L.; Freedman, D.S.; Kraus, E.M.; Porter, R.; Blanck, H.M.; Goodman, A.B. Dnp3 Longitudinal Trends in Body Mass Index before and during the COVID-19 Pandemic among Persons Aged 2–19 Years—United States, 2018–2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Calvo, D.; Galioto, R.; Gunstad, J.; Spitznagel, M.B. Uncontrolled eating is associated with reduced executive functioning. Clin. Obes. 2014, 4, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Thivel, D.; Rumbold, P.; King, N.A.; Pereira, B.; Blundell, J.E.; Mathieu, M.-E. Acute post-exercise energy and macronutrient intake in lean and obese youth: A systematic review and meta-analysis. Int. J. Obes. 2016, 40, 1469–1479. [Google Scholar] [CrossRef] [Green Version]
- Hazell, T.J.; Islam, H.; Townsend, L.K.; Schmale, M.S.; Copeland, J.L. Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: Potential mechanisms. Appetite 2016, 98, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Ouerghi, N.; Feki, M.; Bragazzi, N.L.; Knechtle, B.; Hill, L.; Nikolaidis, P.T.; Bouassida, A. Ghrelin Response to Acute and Chronic Exercise: Insights and Implications from a Systematic Review of the Literature. Sports Med. 2021, 51, 2389–2410. [Google Scholar] [CrossRef] [PubMed]

| Baseline (n = 30) | Year 2 (n = 19) | Change | |||
|---|---|---|---|---|---|
| Sex | |||||
| Male | 15 | (50%) | 10 | (53%) | |
| Female | 15 | (50%) | 9 | (47%) | |
| Race/ethnicity | |||||
| Black or African-American | 14 | (47%) | 12 | (63%) | |
| Non-Hispanic White | 13 | (43%) | 6 | (32%) | |
| Hispanic | 3 | (10%) | 1 | (5%) | |
| Weight status | |||||
| Healthy weight | 0 | 2 | (11%) | ||
| Overweight | 5 | (17%) | 2 | (11%) | |
| Obesity, not severe | 14 | (47%) | 8 | (42%) | |
| Obesity, severe | 11 | (37%) | 7 | (37% | |
| Age (year) | 12.9 | (1.9) | 14.6 | (2.0) | 1.7 |
| Weight (kg) | 78.7 | (20.5) | 91.8 | (24.5) | 13.1 |
| Fat mass (kg) | 32.4 | (11.5) | 38.4 | (16.9) | 6.0 |
| Fat-free mass index (kg/m2) | 16.7 | (2.5) | 19.0 | (2.7) | 2.3 |
| Test-meal energy intake (kcal) | 1078 | (524) | 1252 | (610) | 174 |
| TFEQ-R18v2 | |||||
| Uncontrolled Eating | 34 | (22) | 38 | (18) | 4 |
| Cognitive Restraint | 36 | (22) | 31 | (26) | −5 |
| Emotional Eating | 24 | (23) | 24 | (22) | 0 |
| Baseline Energy Intake (n = 30) | Year 2 Energy Intake (n = 19) | |
|---|---|---|
| Trait Measures | ||
| TFEQ-R18v2 | ||
| Uncontrolled Eating | 0.40 * | 0.22 |
| Cognitive Restraint | 0.04 | −0.02 |
| Emotional Eating | 0.27 | −0.12 |
| Go/no-go Task | ||
| False alarms (%) | 0.23 | 0.27 |
| Error rates (%) | 0.13 | 0.26 |
| State Measures | ||
| Pre-meal VAS | ||
| Hunger | 0.39 * | 0.55 * |
| Fullness | −0.42 * | −0.47 * |
| Desire to eat | 0.26 | 0.61 ** |
| Prospective food consumption | 0.50 ** | 0.64 ** |
| Satisfaction | −0.31 | −0.59 ** |
| Pre-meal explicit wanting | 0.35 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fearnbach, N.; Staiano, A.E.; Johannsen, N.M.; Hsia, D.S.; Beyl, R.A.; Carmichael, O.T.; Martin, C.K. Predictors of Post-Exercise Energy Intake in Adolescents Ranging in Weight Status from Overweight to Severe Obesity. Nutrients 2022, 14, 223. https://doi.org/10.3390/nu14010223
Fearnbach N, Staiano AE, Johannsen NM, Hsia DS, Beyl RA, Carmichael OT, Martin CK. Predictors of Post-Exercise Energy Intake in Adolescents Ranging in Weight Status from Overweight to Severe Obesity. Nutrients. 2022; 14(1):223. https://doi.org/10.3390/nu14010223
Chicago/Turabian StyleFearnbach, Nicole, Amanda E. Staiano, Neil M. Johannsen, Daniel S. Hsia, Robbie A. Beyl, Owen T. Carmichael, and Corby K. Martin. 2022. "Predictors of Post-Exercise Energy Intake in Adolescents Ranging in Weight Status from Overweight to Severe Obesity" Nutrients 14, no. 1: 223. https://doi.org/10.3390/nu14010223
APA StyleFearnbach, N., Staiano, A. E., Johannsen, N. M., Hsia, D. S., Beyl, R. A., Carmichael, O. T., & Martin, C. K. (2022). Predictors of Post-Exercise Energy Intake in Adolescents Ranging in Weight Status from Overweight to Severe Obesity. Nutrients, 14(1), 223. https://doi.org/10.3390/nu14010223







