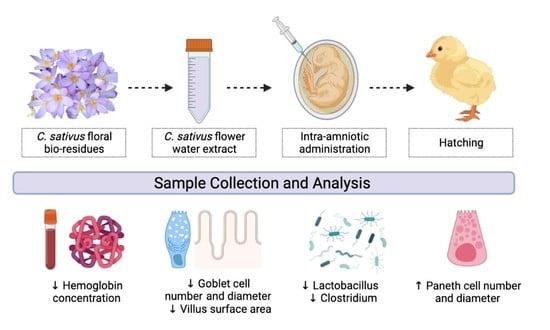
Saffron (Crocus sativus L.) Flower Water Extract Disrupts the Cecal Microbiome, Brush Border Membrane Functionality, and Morphology In Vivo (Gallus gallus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extract Preparation
2.2. Analysis of Polyphenols
2.2.1. Polyphenol Extraction
2.2.2. Liquid Chromatography-Mass Spectroscopy (LC-MS)
2.3. Animals and Study Design
2.4. Blood Analysis and Hb Measurements
2.5. Gene Expression Analysis
2.5.1. Isolation of Total RNA from the Duodenum
2.5.2. Real Time Polymerase Chain Reaction (RT-PCR)
2.5.3. Primer Design
2.5.4. RT-PCR Design
2.6. Morphological Examination
2.7. Cecal Sample Collection and DNA Purification
2.8. Primers Design and PCR Amplification of Bacterial 16S rDNA Analysis
2.9. Statistical Analysis
3. Results
3.1. Polyphenol Profile
3.2. Body Weight and Blood Hemoglobin Concentration
3.3. Duodenal Gene Expression
3.3.1. Fe-Related Protein Gene Expression
3.3.2. Zn-Related Protein Gene Expression
3.3.3. Inflammatory Cytokine Gene Expression
3.3.4. BBM Functionality Related Protein Gene Expression
3.4. Duodenal Morphological Measurement
3.4.1. Gallus gallus Duodenum Cross-Section Images
3.4.2. Goblet Cell Number and Type (Villi and Crypt)
3.4.3. Paneth Cell Number, Diameter, and Crypt Depth
3.4.4. Average Villi Surface Area and Goblet Cell Diameter
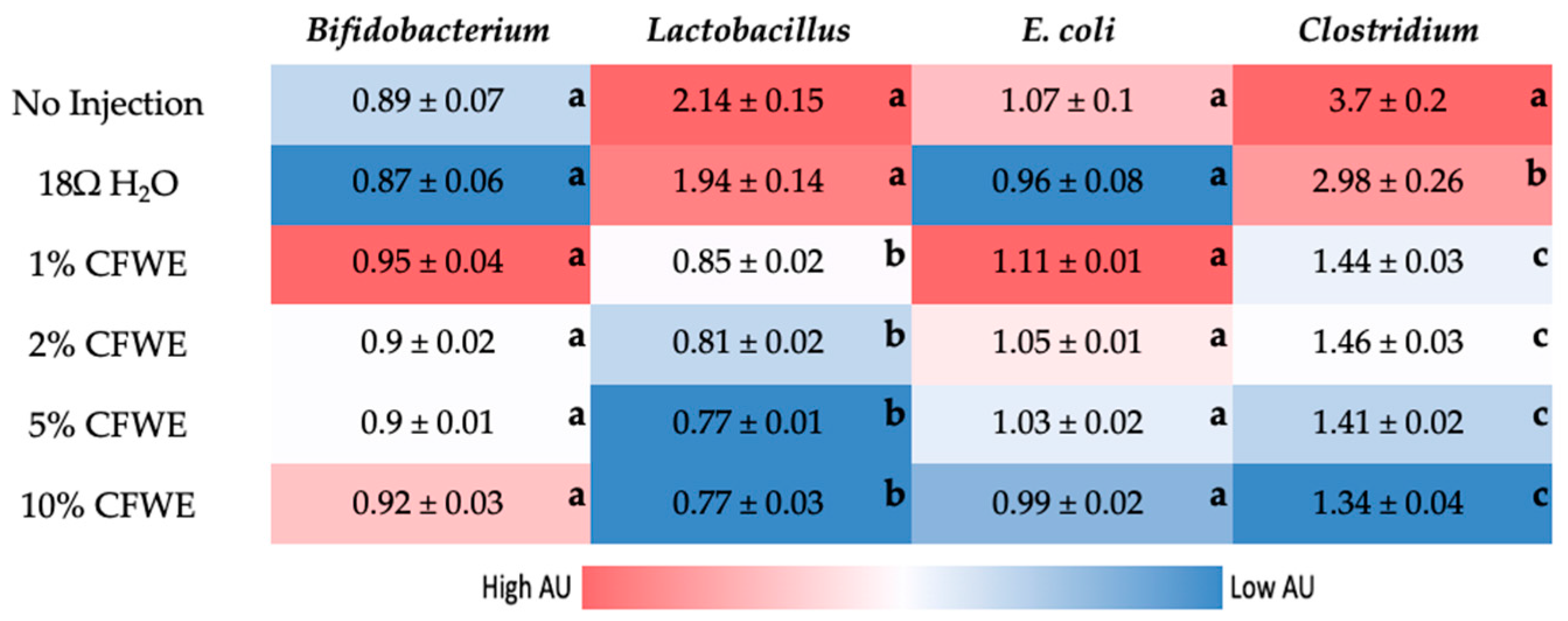
3.5. Gut Microbiome Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Caser, M.; Demasi, S.; Stelluti, S.; Donno, D.; Scariot, V. Crocus sativus L. Cultivation in Alpine Environments: Stigmas and Tepals as Source of Bioactive Compounds. Agronomy 2020, 10, 1473. [Google Scholar] [CrossRef]
- Kianmehr, M.; Khazdair, M.R. Possible Therapeutic Effects of Crocus sativus Stigma and Its Petal Flavonoid, Kaempferol, on Respiratory Disorders. Pharm. Biol. 2020, 58, 1140–1149. [Google Scholar] [CrossRef]
- Serrano-Díaz, J.; Sánchez, A.M.; Martínez-Tomé, M.; Winterhalter, P.; Alonso, G.L. A Contribution to Nutritional Studies on Crocus Sativus Flowers and Their Value as Food. J. Food Compos. Anal. 2013, 31, 101–108. [Google Scholar] [CrossRef]
- Moratalla-López, N.; Bagur, M.J.; Lorenzo, C.; Martínez-Navarro, M.E.; Rosario Salinas, M.; Alonso, G.L. Bioactivity and Bioavailability of the Major Metabolites of Crocus sativus L. Flower. Molecules 2019, 24, 2827. [Google Scholar] [CrossRef] [Green Version]
- Rahaiee, S.; Moini, S.; Hashemi, M.; Shojaosadati, S.A. Evaluation of Antioxidant Activities of Bioactive Compounds and Various Extracts Obtained from Saffron (Crocus sativus L.): A Review. J. Food Sci. Technol. 2015, 52, 1881–1888. [Google Scholar] [CrossRef] [Green Version]
- Zeinali, M.; Zirak, M.R.; Rezaee, S.A.; Karimi, G.; Hosseinzadeh, H. Immunoregulatory and Anti-Inflammatory Properties of Crocus sativus (Saffron) and Its Main Active Constituents: A Review. Iran. J. Basic Med. Sci. 2019, 22, 334–344. [Google Scholar] [CrossRef]
- Saleem, N.; Ahmad, M.; Kamran, S.; Riaz, H.; Mehmood, Y.; Raza, S. Hepatoprotective Effect of Crocus Sativus on Amiodarone-Induced Liver Toxicity. Br. J. Pharm. Res. 2016, 12, 1–11. [Google Scholar] [CrossRef]
- Linardaki, Z.I.; Orkoula, M.G.; Kokkosis, A.G.; Lamari, F.N.; Margarity, M. Investigation of the Neuroprotective Action of Saffron (Crocus sativus L.) in Aluminum-Exposed Adult Mice through Behavioral and Neurobiochemical Assessment. Food Chem. Toxicol. 2013, 52, 163–170. [Google Scholar] [CrossRef]
- Siddiqui, M.; Saleh, M.M.; Basharuddin, S.B.B.; Zamri, S.B.; Mohd Najib, M.B.; Che Ibrahim, M.; Binti Mohd Noor, N.; Binti Mazha, H.; Mohd Hassan, N.; Khatib, A. Saffron (Crocus sativus L.): As an Antidepressant. J. Pharm. Bioallied Sci. 2018, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Mashmoul, M.; Azlan, A.; Khaza’Ai, H.; Yusof, B.N.M.; Noor, S.M. Saffron: A Natural Potent Antioxidant as a Promising Anti-Obesity Drug. Antioxidants 2013, 2, 293–308. [Google Scholar] [CrossRef] [Green Version]
- Zeka, K.; Ruparelia, K.C.; Continenza, M.A.; Stagos, D.; Vegliò, F.; Arroo, R.R.J. Petals of Crocus sativus L. as a Potential Source of the Antioxidants Crocin and Kaempferol. Fitoterapia 2015, 107, 128–134. [Google Scholar] [CrossRef]
- Serrano-Díaz, J.; Sánchez, A.M.; Maggi, L.; Martínez-Tomé, M.; García-Diz, L.; Murcia, M.A.; Alonso, G.L. Increasing the Applications of Crocus sativus Flowers as Natural Antioxidants. J. Food Sci. 2012, 77, C1162–C1168. [Google Scholar] [CrossRef] [PubMed]
- Stelluti, S.; Caser, M.; Demasi, S.; Scariot, V. Sustainable Processing of Floral Bio-Residues of Saffron (Crocus sativus L.) for Valuable Biorefinery Products. Plants 2021, 10, 523. [Google Scholar] [CrossRef]
- Verediano, T.A.; Stampini Duarte Martino, H.; Dias Paes, M.C.; Tako, E. Effects of Anthocyanin on Intestinal Health: A Systematic Review. Nutrients 2021, 13, 1331. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Ji, G.E.; Sung, M.K. Dietary Kaempferol Suppresses Inflammation of Dextran Sulfate Sodium-Induced Colitis in Mice. Dig. Dis. Sci. 2012, 57, 355–363. [Google Scholar] [CrossRef]
- Bian, Y.; Dong, Y.; Sun, J.; Sun, M.; Hou, Q.; Lai, Y.; Zhang, B. Protective Effect of Kaempferol on LPS-Induced Inflammation and Barrier Dysfunction in a Coculture Model of Intestinal Epithelial Cells and Intestinal Microvascular Endothelial Cells. J. Agric. Food Chem. 2020, 68, 160–167. [Google Scholar] [CrossRef]
- Kumar, V.; Bhat, Z.A.; Kumar, D.; Shah, M.Y.; Chashoo, I.A.; Khan, N.A. Physicochemical and Preliminary Phytochemical Studies on Petals of Crocus sativus “Cashmerianus”. Pharmacogn. J. 2011, 3, 46–49. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Saffron (Crocus sativus) Petal as a New Pharmacological Target: A Review. Iran. J. Basic Med. Sci. 2018, 21, 1091–1099. [Google Scholar]
- Zeka, K.; Marrazzo, P.; Micucci, M.; Ruparelia, K.C.; Arroo, R.R.J.; Macchiarelli, G.; Nottola, S.A.; Continenza, M.A.; Chiarini, A.; Angeloni, C.; et al. Activity of Antioxidants from Crocus sativus l. Petals: Potential Preventive Effects towards Cardiovascular System. Antioxidants 2020, 9, 1102. [Google Scholar] [CrossRef]
- Omidi, A.; Riahinia, N.; Bagher, M.; Torbati, M.; Behdani, M.-A. Hepatoprotective Effect of Crocus sativus (Saffron) Petals Extract against Acetaminophen Toxicity in Male Wistar Rats Protective Effects of Saffron Petals in Acetaminophen-Induced Liver Damages. Avicenna J. Phytomed. 2014, 4, 330–336. [Google Scholar]
- Montoro, P.; Maldini, M.; Luciani, L.; Tuberoso, C.I.G.; Congiu, F.; Pizza, C. Radical Scavenging Activity and LC-MS Metabolic Profiling of Petals, Stamens, and Flowers of Crocus sativus L. J. Food Sci. 2012, 77. [Google Scholar] [CrossRef]
- Mohaqiq, Z.; Moossavi, M.; Hemmati, M.; Kazemi, T.; Mehrpour, O. Antioxidant Properties of Saffron Stigma and Petals: A Potential Therapeutic Approach for Insulin Resistance through an Insulin-Sensitizing Adipocytokine in High-Calorie Diet Rats. Int. J. Prev. Med. 2020, 11, 184. [Google Scholar]
- He, Y.; Peng, H.; Zhang, H.; Liu, Y.; Sun, H. Structural Characteristics and Immunopotentiation Activity of Two Polysaccharides from the Petal of Crocus sativus. Int. J. Biol. Macromol. 2021, 180, 129–142. [Google Scholar] [CrossRef]
- Azizi, M.; Abbasi, N.; Mohamadpour, M.; Bakhtiyari, S.; Asadi, S.; Shirzadpour, E.; Aidy, A.; Mohamadpour, M.; Amraei, M. Investigating the Effect of Crocus sativus l. Petal Hydroalcoholic Extract on Inflammatory and Enzymatic Indices Resulting from Alcohol Use in Kidney and Liver of Male Rats. J. Inflamm. Res. 2019, 12, 269–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedimanesh, N.; Motlagh, B.; Abedimanesh, S.; Bathaie, S.Z.; Separham, A.; Ostadrahimi, A. Effects of Crocin and Saffron Aqueous Extract on Gene Expression of SIRT1, AMPK, LOX1, NF-ΚB, and MCP-1 in Patients with Coronary Artery Disease: A Randomized Placebo-Controlled Clinical Trial. Phytother. Res. 2020, 34, 1114–1122. [Google Scholar] [CrossRef]
- Alipour, F.; Vakili, A.; Mesgaran, M.D.; Ebrahimi, H. The Effect of Adding Ethanolic Saffron Petal Extract and Vitamin E on Growth Performance, Blood Metabolites and Antioxidant Status in Baluchi Male Lambs. Asian-Australas. J. Anim. Sci. 2019, 32, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Mottaghipisheh, J.; Mahmoodi Sourestani, M.; Kiss, T.; Horváth, A.; Tóth, B.; Ayanmanesh, M.; Khamushi, A.; Csupor, D. Comprehensive Chemotaxonomic Analysis of Saffron Crocus Tepal and Stamen Samples, as Raw Materials with Potential Antidepressant Activity. J. Pharm. Biomed. Anal. 2020, 184, 113183. [Google Scholar] [CrossRef]
- Ghaderi, A.; Rasouli-Azad, M.; Vahed, N.; Banafshe, H.R.; Soleimani, A.; Omidi, A.; Ghoreishi, F.S.; Asemi, Z. Clinical and Metabolic Responses to Crocin in Patients under Methadone Maintenance Treatment: A Randomized Clinical Trial. Phytother. Res. 2019, 33, 2714–2725. [Google Scholar] [CrossRef]
- Khalatbari-Mohseni, A.; Banafshe, H.R.; Mirhosseini, N.; Asemi, Z.; Ghaderi, A.; Omidi, A. The Effects of Crocin on Psychological Parameters in Patients under Methadone Maintenance Treatment: A Randomized Clinical Trial. Subst. Abus. Treat. Prev. Policy 2019, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Mukherjee, S. Evolving Interplay Between Dietary Polyphenols and Gut Microbiota—An Emerging Importance in Healthcare. Front. Nutr. 2021, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Hair, R.; Sakaki, J.R.; Chun, O.K. Anthocyanins, Microbiome and Health Benefits in Aging. Molecules 2021, 26, 537. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [Green Version]
- Hou, T.; Tako, E. The in Ovo Feeding Administration (Gallus gallus)—An Emerging in Vivo Approach to Assess Bioactive Compounds with Potential Nutritional Benefits. Nutrients 2018, 10, 418. [Google Scholar] [CrossRef] [PubMed]
- Tako, E.; Rutzke, M.A.; Glahn, R.P. Using the Domestic Chicken (Gallus gallus) as an in Vivo Model for Iron Bioavailability. Poult. Sci. 2010, 89, 514–521. [Google Scholar] [CrossRef]
- Beasley, J.T.; Johnson, A.A.T.; Kolba, N.; Bonneau, J.P.; Glahn, R.P.; Ozeri, L.; Koren, O.; Tako, E. Nicotianamine-Chelated Iron Positively Affects Iron Status, Intestinal Morphology and Microbial Populations in Vivo (Gallus gallus). Sci. Rep. 2020, 10, 2297. [Google Scholar] [CrossRef] [Green Version]
- Reed, S.; Knez, M.; Uzan, A.; Stangoulis, J.C.R.; Glahn, R.P.; Koren, O.; Tako, E. Alterations in the Gut (Gallus gallus) Microbiota Following the Consumption of Zinc Biofortified Wheat (Triticum aestivum)-Based Diet. J. Agric. Food Chem. 2018, 66, 6291–6299. [Google Scholar] [CrossRef]
- Martino, H.S.D.; Kolba, N.; Tako, E. Yacon (Smallanthus sonchifolius) Flour Soluble Extract Improve Intestinal Bacterial Populations, Brush Border Membrane Functionality and Morphology in Vivo (Gallus gallus). Food Res. Int. 2020, 137. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kolba, N.; Liang, J.; Tako, E. Alterations in Gut Microflora Populations and Brush Border Functionality Following Intra-Amniotic Administration (Gallus gallus) of Wheat Bran Prebiotic Extracts. Food Funct. 2019, 10, 4834–4843. [Google Scholar] [CrossRef]
- Da Silva, B.P.; Kolba, N.; Martino, H.S.D.; Hart, J.; Tako, E. Soluble Extracts from Chia Seed (Salvia hispanica L.) Affect Brush Border Membrane Functionality, Morphology and Intestinal Bacterial Populations in Vivo (Gallus gallus). Nutrients 2019, 11, 2457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carboni, J.; Reed, S.; Kolba, N.; Eshel, A.; Koren, O.; Tako, E. Alterations in the Intestinal Morphology, Gut Microbiota, and Trace Mineral Status Following Intra-Amniotic Administration (Gallus gallus) of Teff (Eragrostis tef) Seed Extracts. Nutrients 2020, 12, 3020. [Google Scholar] [CrossRef]
- Dias, D.M.; Kolba, N.; Hart, J.J.; Ma, M.; Sha, S.T.; Lakshmanan, N.; Nutti, M.R.; Martino, H.S.D.; Glahn, R.P.; Tako, E. Soluble Extracts from Carioca Beans (Phaseolus vulgaris L.) Affect the Gut Microbiota and Iron Related Brush Border Membrane Protein Expression in Vivo (Gallus gallus). Food Res. Int. 2019, 123, 172–180. [Google Scholar] [CrossRef]
- Hou, T.; Kolba, N.; Glahn, R.P.; Tako, E. Intra-Amniotic Administration (Gallus gallus) of Cicer Arietinum and Lens Culinaris Prebiotics Extracts and Duck Egg White Peptides Affects Calcium Status and Intestinal Functionality. Nutrients 2017, 9, 785. [Google Scholar] [CrossRef] [Green Version]
- Tako, E.; Bar, H.; Glahn, R.P. The Combined Application of the Caco-2 Cell Bioassay Coupled with in Vivo (Gallus gallus) Feeding Trial Represents an Effective Approach to Predicting Fe Bioavailability in Humans. Nutrients 2016, 8, 732. [Google Scholar] [CrossRef]
- Warkentin, T.; Kolba, N.; Tako, E. Low Phytate Peas (Pisum sativum L.) Improve Iron Status, Gut Microbiome, and Brush Border Membrane Functionality in Vivo (Gallus gallus). Nutrients 2020, 12, 2563. [Google Scholar] [CrossRef]
- Tako, E.; Glahn, R.P.; Knez, M.; Stangoulis, J.C. The Effect of Wheat Prebiotics on the Gut Bacterial Population and Iron Status of Iron Deficient Broiler Chickens. Nutr. J. 2014, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Tako, E.; Beebe, S.E.; Reed, S.; Hart, J.J.; Glahn, R.P. Polyphenolic Compounds Appear to Limit the Nutritional Benefit of Biofortified Higher Iron Black Bean (Phaseolus vulgaris L.). Nutr. J. 2014, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Zhong, T.; Pandya, Y.; Joerger, R.D. 16S RRNA-Based Analysis of Microbiota from the Cecum of Broiler Chickens. Appl. Environ. Microbiol. 2002, 68, 124–137. [Google Scholar] [CrossRef] [Green Version]
- Amit-Romach, E.; Sklan, D.; Uni, Z. Microflora Ecology of the Chicken Intestine Using 16S Ribosomal DNA Primers. Poult. Sci. 2004, 83, 1093–1098. [Google Scholar] [CrossRef]
- Langendijk, P.S.; Schut, F.; Jansen, G.J.; Raangs, G.C.; Kamphuis, G.R.; Wilkinson, M.H.F.; Welling, G.W. Quantitative Fluorescence in Situ Hybridization of Bifidobacterium Spp. with Genus-Specific 16S RRNA-Targeted Probes and Its Application in Fecal Samples. Appl. Environ. Microbiol. 1995, 61, 3069. [Google Scholar] [CrossRef] [Green Version]
- Tako, E.; Glahn, R.P.; Welch, R.M.; Lei, X.; Yasuda, K.; Miller, D.D. Dietary Inulin Affects the Expression of Intestinal Enterocyte Iron Transporters, Receptors and Storage Protein and Alters the Microbiota in the Pig Intestine. Br. J. Nutr. 2008, 99, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Sharp, P.; Srai, S.K. Molecular Mechanisms Involved in Intestinal Iron Absorption. World J. Gastroenterol. 2007, 13, 4716–4724. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Hoque, R.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.S.; Sharp, P.A. Quercetin Inhibits Intestinal Iron Absorption and Ferroportin Transporter Expression In Vivo and In Vitro. PLoS ONE 2014, 9, e102900. [Google Scholar] [CrossRef] [Green Version]
- Lesjak, M.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.S. Quercetin Inhibits Intestinal Non-Haem Iron Absorption by Regulating Iron Metabolism Genes in the Tissues. Eur. J. Nutr. 2018, 58, 743–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petry, N.; Egli, I.; Zeder, C.; Walczyk, T.; Hurrell, R. Polyphenols and Phytic Acid Contribute to the Low Iron Bioavailability from Common Beans in Young Women. J. Nutr. 2010, 140, 1977–1982. [Google Scholar] [CrossRef]
- Ma, Q.; Kim, E.-Y.; Han, O. Bioactive Dietary Polyphenols Decrease Heme Iron Absorption by Decreasing Basolateral Iron Release in Human Intestinal Caco-2 Cells. J. Nutr. 2010, 140, 1117–1121. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Reddy, M.; Cook, J.D. Inhibition of Non-Haem Iron Absorption in Man by Polyphenolic-Containing Beverages. Br. J. Nutr. 1999, 81, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Layrisse, M.; García-Casal, M.N.; Solano, L.; Barón, M.A.; Arguello, F.; Llovera, D.; Ramírez, J.; Leets, I.; Tropper, E. Iron Bioavailability in Humans from Breakfasts Enriched with Iron Bis-Glycine Chelate, Phytates and Polyphenols. J. Nutr. 2000, 130, 2195–2199. [Google Scholar] [CrossRef]
- Gomes, M.J.C.; Kolba, N.; Agarwal, N.; Kim, D.; Eshel, A.; Koren, O.; Tako, E. Modifications in the Intestinal Functionality, Morphology and Microbiome Following Intra-Amniotic Administration (Gallus gallus) of Grape (Vitis vinifera) Stilbenes (Resveratrol and Pterostilbene). Nutrients 2021, 13, 3247. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, Z.; Heller, L.I.; Krasnoff, S.B.; Glahn, R.P.; Welch, R.M. Kaempferol Red Pinto Bean Seed (Phaseolus vulgaris L.) Coats Inhibits Iron Bioavailability Using an in Vitro Digestion/Human Caco-2 Cell Model. J. Agric. Food Chem. 2006, 54, 9254–9261. [Google Scholar] [CrossRef]
- Babaei, A.; Arshami, J.; Haghparast, A.; Mesgaran, M.D. Effects of Saffron (Crocus sativus) Petal Ethanolic Extract on Hematology, Antibody Response, and Spleen Histology in Rats. Avicenna J. Phytomedicine 2014, 4, 103. [Google Scholar]
- Pan, P.; Lam, V.; Salzman, N.; Huang, Y.W.; Yu, J.; Zhang, J.; Wang, L.S. Black Raspberries and Their Anthocyanin and Fiber Fractions Alter the Composition and Diversity of Gut Microbiota in F-344 Rats. Nutr. Cancer 2017, 69, 943. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Thomas-Ahner, J.M.; Riedl, K.M.; Bailey, M.T.; Vodovotz, Y.; Schwartz, S.J.; Clinton, S.K. Dietary Black Raspberries Impact the Colonic Microbiome and Phytochemical Metabolites in Mice. Mol. Nutr. Food Res. 2019, 63, 1800636. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. BioMed Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez Rodriguez, N.R.; Eloi, M.D.; Huynh, A.; Dominguez, T.; Lam, A.H.C.; Carcamo-Molina, D. Expansion of Paneth Cell Population in Response to Enteric Salmonella Enterica Serovar Typhimurium Infection. Infect. Immun. 2012, 80, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Gassler, N. Paneth Cells in Intestinal Physiology and Pathophysiology. World J. Gastrointest. Pathophysiol. 2017, 8, 150. [Google Scholar] [CrossRef]
- Damiano, S.; Sasso, A.; de Felice, B.; di Gregorio, I.; la Rosa, G.; Lupoli, G.A.; Belfiore, A.; Mondola, P.; Santillo, M. Quercetin Increases MUC2 and MUC5AC Gene Expression and Secretion in Intestinal Goblet Cell-Like LS174T via PLC/PKCα/ERK1-2 Pathway. Front. Physiol. 2018, 9, 357. [Google Scholar] [CrossRef] [Green Version]


| Analyte | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Base Pair | GI Identifier |
|---|---|---|---|---|
| Iron Metabolism | ||||
| DcytB | CATGTGCATTCTCTTCCAAAGTC | CTCCTTGGTGACCGCATTAT | 103 | 20,380,692 |
| DMT1 | TTGATTCAGAGCCTCCCATTAG | GCGAGGAGTAGGCTTGTATTT | 101 | 206,597,489 |
| Ferroportin | CTCAGCAATCACTGGCATCA | ACTGGGCAACTCCAGAAATAAG | 98 | 61,098,365 |
| Zinc Metabolism | ||||
| ZIP1 | TGCCTCAGTTTCCCTCAC | GGCTCTTAAGGGCACTTCT | 144 | 107,055,139 |
| ZnT1 | GGTAACAGAGCTGCCTTAACT | GGTAACAGAGCTGCCTTAACT | 105 | 54,109,718 |
| Inflammatory Response | ||||
| NF-κβ | CACAGCTGGAGGGAAGTAAAT | TTGAGTAAGGAAGTGAGGTTGAG | 100 | 2,130,627 |
| TNF-α | GACAGCCTATGCCAACAAGTA | TTACAGGAAGGGCAACTCATC | 109 | 53,854,909 |
| IL8 | TCATCCATCCCAAGTTCATTCA | GACACACTTCTCTGCCATCTT | 105 | 395,872 |
| BBM functionality | ||||
| SGLT1 | GCATCCTTACTCTGTGGTACTG | TATCCGCACATCACACATCC | 106 | 8,346,783 |
| SI | CCAGCAATGCCAGCATATTG | CGGTTTCTCCTTACCACTTCTT | 95 | 2,246,388 |
| MUC2 | CCTGCTGCAAGGAAGTAGAA | GGAAGATCAGAGTGGTGCATAG | 155 | 423,101 |
| Polyphenolic Compounds | % * |
|---|---|
| Malvidin 3,5-di-O-glucoside | 0.03 |
| Kaempferol-3-O-glucoside | 7.90 |
| Quercetin-3-O-gluside | 0.37 |
| Kaempferol-3-O-sophoroside | 91.70 |
| Treatment Group | Average Hemoglobin (g/dL) | Average Body Weight (g) |
|---|---|---|
| No Injection | 12.52 ± 0.91 a | 42.06 ± 1.35 a |
| 18 Ω H2O | 10.13 ± 0.71 a,b | 42.16 ± 1.28 a |
| 1% CFWE | 12.26 ± 0.72 a | 42.96 ± 0.91 a |
| 2% CFWE | 11.15 ± 0.56 a | 42.13 ± 0.68 a |
| 5% CFWE | 10.24 ± 0.73 a | 42.89 ± 0.77 a |
| 10% CFWE | 9.62 ± 1.21 b | 42.88 ± 0.96 a |
| Treatment Group | Average Goblet Cell Number Per Villi | Total Villi Goblet Number | Total Crypt Goblet Number | ||
|---|---|---|---|---|---|
| Acidic | Neutral | Mixture | |||
| No Injection | 36.76 ± 0.85 a | 0.02 ± 0.01 a | 0.3 ± 0.06 a | 37.08 ± 0.86 a | 8.33 ± 0.25 a |
| 18 Ω H2O | 32.92 ± 0.68 b | 0 ± 0 a | 0.05 ± 0.03 b | 32.97 ± 0.68 b | 7.44 ± 0.22 b |
| 1% CFWE | 29.28 ± 0.86 c | 0.03 ± 0.02 a | 0.04 ± 0.02 a | 29.34 ± 0.87 c | 6.73 ± 0.22 c |
| 10% CFWE | 22.49 ± 0.5 d | 0.01 ± 0.01 a | 0.01 ± 0.01 b | 22.51 ± 0.5 d | 6.68 ± 0.22 c |
| Treatment Group | Paneth Cell Number | Paneth Cell Diameter (µM) | Crypt Depth (µM) |
|---|---|---|---|
| No Injection | 2.24 ± 0.08 b | 1.56 ± 0.03 c | 45.77 ± 1.32 b |
| 18 Ω H2O | 1.89 ± 0.07 c | 1.89 ± 0.05 b | 50.73 ± 1.1 a |
| 1% CFWE | 1.93 ± 0.07 c | 1.67 ± 0.03 c | 32.81 ± 0.73 d |
| 10% CFWE | 2.56 ± 0.08 a | 2.47 ± 0.06 a | 37.75 ± 0.8 c |
| Treatment Group | Average Surface Area (mm2) | Villi Goblet Cell Diameter (µM) | Crypt Goblet Cell Diameter (µM) |
|---|---|---|---|
| No Injection | 168.19 ± 3.72 a | 4.65 ± 0.06 b | 2.98 ± 0.06 c |
| 18 Ω H2O | 171.45 ± 4.2 a | 5.13 ± 0.06 a | 3.32 ± 0.07 a |
| 1% CFWE | 137.91 ± 3.4 b | 4.18 ± 0.06 c | 3.11 ± 0.06 b,c |
| 10% CFWE | 116.41 ± 2.71 c | 3.95 ± 0.06 d | 3.17 ± 0.06 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarwal, N.; Kolba, N.; Jung, Y.; Cheng, J.; Tako, E. Saffron (Crocus sativus L.) Flower Water Extract Disrupts the Cecal Microbiome, Brush Border Membrane Functionality, and Morphology In Vivo (Gallus gallus). Nutrients 2022, 14, 220. https://doi.org/10.3390/nu14010220
Agarwal N, Kolba N, Jung Y, Cheng J, Tako E. Saffron (Crocus sativus L.) Flower Water Extract Disrupts the Cecal Microbiome, Brush Border Membrane Functionality, and Morphology In Vivo (Gallus gallus). Nutrients. 2022; 14(1):220. https://doi.org/10.3390/nu14010220
Chicago/Turabian StyleAgarwal, Nikita, Nikolai Kolba, YeonJin Jung, Jacquelyn Cheng, and Elad Tako. 2022. "Saffron (Crocus sativus L.) Flower Water Extract Disrupts the Cecal Microbiome, Brush Border Membrane Functionality, and Morphology In Vivo (Gallus gallus)" Nutrients 14, no. 1: 220. https://doi.org/10.3390/nu14010220
APA StyleAgarwal, N., Kolba, N., Jung, Y., Cheng, J., & Tako, E. (2022). Saffron (Crocus sativus L.) Flower Water Extract Disrupts the Cecal Microbiome, Brush Border Membrane Functionality, and Morphology In Vivo (Gallus gallus). Nutrients, 14(1), 220. https://doi.org/10.3390/nu14010220