Significance of the Modified NUTRIC Score for Predicting Clinical Outcomes in Patients with Severe Community-Acquired Pneumonia
Abstract
1. Introduction
2. Material and Methods
2.1. Setting and Study Design
2.2. Definition
2.3. Data Record and Evaluation
2.4. Ethics
2.5. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Comparison of Predictive Accuracy for Hospital Mortality
3.3. Comparison of Predictive Accuracy for Treatment Outcome
3.4. Predictors for Hospital Mortality and Treatment Outcome
3.5. Relevance between Clinical Factors and Clinical Prediction Rules
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leoni, D.; Rello, J. Severe community-acquired pneumonia: Optimal management. Curr. Opin. Infect. Dis. 2017, 30, 240–247. [Google Scholar] [CrossRef] [PubMed]
- El Bcheraoui, C.; Mokdad, A.H.; Dwyer-Lindgren, L.; Bertozzi-Villa, A.; Stubbs, R.W.; Morozoff, C.; Shirude, S.; Naghavi, M.; Murray, C.J.L. Trends and Patterns of Differences in Infectious Disease Mortality among US Counties, 1980–2014. JAMA 2018, 319, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Welte, T. Managing CAP patients at risk of clinical failure. Respir. Med. 2015, 109, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Niederman, M.S.; Bass, J.B., Jr.; Campbell, G.D.; Fein, A.M.; Grossman, R.F.; Mandell, L.A.; Marrie, T.J.; Sarosi, G.A.; Torres, A.; Yu, V.L. Guidelines for the initial management of adults with community-acquired pneumonia: Diagnosis, assessment of severity, and initial antimicrobial therapy. American Thoracic Society. Medical Section of the American Lung Association. Am. Rev. Respir. Dis. 1993, 148, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Niederman, M.S.; Mandell, L.A.; Anzueto, A.; Bass, J.B.; Broughton, W.A.; Campbell, G.D.; Dean, N.; File, T.; Fine, M.J.; Gross, P.A.; et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am. J. Respir. Crit. Care Med. 2001, 163, 1730–1754. [Google Scholar] [CrossRef]
- Lim, W.S.; van der Eerden, M.M.; Laing, R.; Boersma, W.G.; Karalus, N.; Town, G.I.; Lewis, S.A.; Macfarlane, J.T. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 2003, 58, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Fine, M.J.; Auble, T.E.; Yealy, D.M.; Hanusa, B.H.; Weissfeld, L.A.; Singer, D.E.; Coley, C.M.; Marrie, T.J.; Kapoor, W.N. A prediction rule to identify low-risk patients with community-acquired pneumonia. N. Engl. J. Med. 1997, 336, 243–250. [Google Scholar] [CrossRef]
- Ma, C.M.; Wang, N.; Su, Q.W.; Yan, Y.; Yin, F.Z. The Performance of CURB-65 and PSI for Predicting in-hospital Mortality of Community-Acquired Pneumonia in Patients with Type 2 Diabetes Compared with the Non-Diabetic Population. Diabetes Metab. Syndr. Obes. 2021, 14, 1359–1366. [Google Scholar] [CrossRef]
- Richards, G.; Levy, H.; Laterre, P.F.; Feldman, C.; Woodward, B.; Bates, B.M.; Qualy, R.L. CURB-65, PSI, and APACHE II to assess mortality risk in patients with severe sepsis and community acquired pneumonia in PROWESS. J. Intensive Care Med. 2011, 26, 34–40. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Ferreira, F.L.; Bota, D.P.; Bross, A.; Melot, C.; Vincent, J.L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001, 286, 1754–1758. [Google Scholar] [CrossRef]
- Raith, E.P.; Udy, A.A.; Bailey, M.; McGloughlin, S.; MacIsaac, C.; Bellomo, R.; Pilcher, D.V.; For the Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcomes and Resource Evaluation (CORE). Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality among Adults with Suspected Infection Admitted to the Intensive Care Unit. JAMA 2017, 317, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Zimmerman, J.E.; Wagner, D.P.; Draper, E.A.; Lawrence, D.E. APACHE-acute physiology and chronic health. evaluation: A physiologically based classification system. Crit. Care Med. 1981, 9, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Gursel, G.; Demirtas, S. Value of APACHE II, SOFA and CPIS scores in predicting prognosis in patients with ventilator-associated pneumonia. Respir. Int. Rev. Thorac. Dis. 2006, 73, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Travierso, C.; Cilloniz, C.; Gabarrus, A.; Ranzani, O.T.; Polverino, E.; Liapikou, A.; Blasi, F.; Torres, A. Severe community-acquired pneumonia: Characteristics and prognostic factors in ventilated and non-ventilated patients. PLoS ONE 2018, 13, e0191721. [Google Scholar] [CrossRef] [PubMed]
- Chourdakis, M.; Grammatikopoulou, M.G.; Day, A.G.; Bouras, E.; Heyland, D.K. Are all low-NUTRIC-score patients the same? Analysis of a multi-center observational study to determine the relationship between nutrition intake and outcome. Clin. Nutr. 2019, 38, 2783–2789. [Google Scholar] [CrossRef]
- De Vries, M.C.; Koekkoek, W.K.; Opdam, M.H.; van Blokland, D.; van Zanten, A.R. Nutritional assessment of critically ill patients: Validation of the modified NUTRIC score. Eur. J. Clin. Nutr. 2018, 72, 428–435. [Google Scholar] [CrossRef]
- Wang, W.N.; Wang, C.Y.; Hsu, C.Y.; Fu, P.K. Comparison of Feeding Efficiency and Hospital Mortality between Small Bowel and Nasogastric Tube Feeding in Critically Ill Patients at High Nutritional Risk. Nutrients 2020, 12, 2009. [Google Scholar] [CrossRef]
- Jeong, D.H.; Hong, S.B.; Lim, C.M.; Koh, Y.; Seo, J.; Kim, Y.; Min, J.Y.; Huh, J.W. Comparison of Accuracy of NUTRIC and Modified NUTRIC Scores in Predicting 28-Day Mortality in Patients with Sepsis: A Single Center Retrospective Study. Nutrients 2018, 10, 911. [Google Scholar] [CrossRef]
- Acehan, S.; Gulen, M.; Isikber, C.; Unlu, N.; Sumbul, H.E.; Gulumsek, E.; Satar, S. mNUTRIC tool is capable to predict nutritional needs and mortality early in patients suffering from severe pneumonia. Clin. Nutr. ESPEN 2021, 45, 184–191. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Lin, C.Y.; Chen, Y.M.; Chang, Y.P.; Hung, K.Y.; Chang, Y.C.; Chen, H.C.; Huang, K.T.; Chen, Y.C.; Wang, Y.H.; et al. Impact of Body Mass Index on the Survival of Patients with Sepsis with Different Modified NUTRIC Scores. Nutrients 2021, 13, 1873. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.L.; Heyland, D.K.; Silva, F.M.; Rabito, E.I.; Rosa, M.; Tarnowski, M.D.S.; Fernandes, D.; Marcadenti, A. Complementarity of modified NUTRIC score with or without C-reactive protein and subjective global assessment in predicting mortality in critically ill patients. Rev. Bras. Ter. Intensiva 2019, 31, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Ata Ur-Rehman, H.M.; Ishtiaq, W.; Yousaf, M.; Bano, S.; Mujahid, A.M.; Akhtar, A. Modified Nutrition Risk in Critically Ill (mNUTRIC) Score to Assess Nutritional Risk in Mechanically Ventilated Patients: A Prospective Observational Study from the Pakistani Population. Cureus 2018, 10, e3786. [Google Scholar] [CrossRef] [PubMed]
- Brascher, J.M.M.; Peres, W.A.F.; Padilha, P.C. Use of the modified “Nutrition Risk in the critically ill” score and its association with the death of critically ill patients. Clin. Nutr. ESPEN 2020, 35, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Tu, C.Y.; Chen, W.C.; Kuo, L.K.; Wang, Y.T.; Fu, P.K.; Ku, S.C.; Fang, W.F.; Chen, C.M.; Lai, C.C. Clinical Efficacy of Cefoperazone-Sulbactam versus Piperacillin-Tazobactam in the Treatment of Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia. Infect. Drug Resist. 2021, 14, 2251–2258. [Google Scholar] [CrossRef]
- Huang, C.T.; Chen, C.H.; Chen, W.C.; Wang, Y.T.; Lai, C.C.; Fu, P.K.; Kuo, L.K.; Chen, C.M.; Fang, W.F.; Tu, C.Y.; et al. Clinical effectiveness of cefoperazone-sulbactam versus piperacillin-tazobactam for the treatment of pneumonia in the elderly population. Int. J. Antimicrob. Agents 2021, 106491. [Google Scholar] [CrossRef]
- Rajamanickam, A.; Munisankar, S.; Dolla, C.K.; Thiruvengadam, K.; Babu, S. Impact of malnutrition on systemic immune and metabolic profiles in type 2 diabetes. BMC Endocr. Disord. 2020, 20, 168. [Google Scholar] [CrossRef]
- Hung, K.Y.; Tsai, Y.H.; Lin, C.Y.; Chang, Y.C.; Wang, Y.H.; Lin, M.C.; Fang, W.F. Application of Peak Glucose Range and Diabetes Status in Mortality Risk Stratification in Critically Ill Patients with Sepsis. Diagnostics 2021, 11, 1798. [Google Scholar] [CrossRef]
- Ghitea, T.C.; Aleya, L.; Tit, D.M.; Behl, T.; Stoicescu, M.; Sava, C.; Iovan, C.; El-Kharoubi, A.; Uivarosan, D.; Pallag, A.; et al. Influence of diet and sport on the risk of sleep apnea in patients with metabolic syndrome associated with hypothyroidism—A 4-year survey. Environ. Sci. Pollut. Res. Int. 2021. [Google Scholar] [CrossRef]
- Bondar, A.; Popa, A.R.; Papanas, N.; Popoviciu, M.; Vesa, C.M.; Sabau, M.; Daina, C.; Stoica, R.A.; Katsiki, N.; Stoian, A.P. Diabetic neuropathy: A narrative review of risk factors, classification, screening and current pathogenic treatment options (Review). Exp. Ther. Med. 2021, 22, 690. [Google Scholar] [CrossRef]
- Harvey, S.E.; Parrott, F.; Harrison, D.A.; Bear, D.E.; Segaran, E.; Beale, R.; Bellingan, G.; Leonard, R.; Mythen, M.G.; Rowan, K.M.; et al. Trial of the route of early nutritional support in critically ill adults. N. Engl. J. Med. 2014, 371, 1673–1684. [Google Scholar] [CrossRef]
- Ramamurthy, M. Trial of the route of early nutritional support in critically ill adults. N. Engl. J. Med. 2015, 372, 488. [Google Scholar]
- Mukhopadhyay, A.; Henry, J.; Ong, V.; Leong, C.S.; Teh, A.L.; van Dam, R.M.; Kowitlawakul, Y. Association of modified NUTRIC score with 28-day mortality in critically ill patients. Clin. Nutr. 2017, 36, 1143–1148. [Google Scholar] [CrossRef]
- Jung, Y.T.; Park, J.Y.; Jeon, J.; Kim, M.J.; Lee, S.H.; Lee, J.G. Association of Inadequate Caloric Supplementation with 30-Day Mortality in Critically Ill Postoperative Patients with High Modified NUTRIC Score. Nutrients 2018, 10, 1589. [Google Scholar] [CrossRef] [PubMed]
- Marti, C.; Garin, N.; Grosgurin, O.; Poncet, A.; Combescure, C.; Carballo, S.; Perrier, A. Prediction of severe community-acquired pneumonia: A systematic review and meta-analysis. Crit. Care 2012, 16, R141. [Google Scholar] [CrossRef]
- Renaud, B.; Coma, E.; Labarere, J.; Hayon, J.; Roy, P.M.; Boureaux, H.; Moritz, F.; Cibien, J.F.; Guerin, T.; Carre, E.; et al. Routine use of the Pneumonia Severity Index for guiding the site-of-treatment decision of patients with pneumonia in the emergency department: A multicenter, prospective, observational, controlled cohort study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2007, 44, 41–49. [Google Scholar] [CrossRef]
- Aydogdu, M.; Ozyilmaz, E.; Aksoy, H.; Gursel, G.; Ekim, N. Mortality prediction in community-acquired pneumonia requiring mechanical ventilation; values of pneumonia and intensive care unit severity scores. Tuberk. Toraks 2010, 58, 25–34. [Google Scholar]
- Yoshimoto, A.; Nakamura, H.; Fujimura, M.; Nakao, S. Severe community-acquired pneumonia in an intensive care unit: Risk factors for mortality. Intern. Med. 2005, 44, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Leroy, O.; Devos, P.; Guery, B.; Georges, H.; Vandenbussche, C.; Coffinier, C.; Thevenin, D.; Beaucaire, G. Simplified prediction rule for prognosis of patients with severe community-acquired pneumonia in ICUs. Chest 1999, 116, 157–165. [Google Scholar] [CrossRef]
- Almirall, J.; Mesalles, E.; Klamburg, J.; Parra, O.; Agudo, A. Prognostic factors of pneumonia requiring admission to the intensive care unit. Chest 1995, 107, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; Marrie, T.J.; Obrosky, D.S.; Clermont, G.; Dremsizov, T.T.; Coley, C.; Fine, M.J.; Singer, D.E.; Kapoor, W.N. Severe community-acquired pneumonia: Use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am. J. Respir. Crit. Care Med. 2002, 166, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Valencia, M.; Badia, J.R.; Cavalcanti, M.; Ferrer, M.; Agusti, C.; Angrill, J.; Garcia, E.; Mensa, J.; Niederman, M.S.; Torres, A. Pneumonia severity index class v patients with community-acquired pneumonia: Characteristics, outcomes, and value of severity scores. Chest 2007, 132, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Buising, K.L.; Thursky, K.A.; Black, J.F.; MacGregor, L.; Street, A.C.; Kennedy, M.P.; Brown, G.V. A prospective comparison of severity scores for identifying patients with severe community acquired pneumonia: Reconsidering what is meant by severe pneumonia. Thorax 2006, 61, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Leroy, O.; Georges, H.; Beuscart, C.; Guery, B.; Coffinier, C.; Vandenbussche, C.; Thevenin, D.; Beaucaire, G. Severe community-acquired pneumonia in ICUs: Prospective validation of a prognostic score. Intensive Care Med. 1996, 22, 1307–1314. [Google Scholar] [CrossRef]
- Ewig, S.; de Roux, A.; Bauer, T.; Garcia, E.; Mensa, J.; Niederman, M.; Torres, A. Validation of predictive rules and indices of severity for community acquired pneumonia. Thorax 2004, 59, 421–427. [Google Scholar] [CrossRef]
- Capelastegui, A.; Espana, P.P.; Quintana, J.M.; Areitio, I.; Gorordo, I.; Egurrola, M.; Bilbao, A. Validation of a predictive rule for the management of community-acquired pneumonia. Eur. Respir. J. 2006, 27, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.T.; Knaus, W.A. Predicting outcome in critical care: The current status of the APACHE prognostic scoring system. Can. J. Anaesth. 1991, 38, 374–383. [Google Scholar] [CrossRef]
- Charlson, M.E.; Charlson, R.E.; Peterson, J.C.; Marinopoulos, S.S.; Briggs, W.M.; Hollenberg, J.P. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J. Clin. Epidemiol. 2008, 61, 1234–1240. [Google Scholar] [CrossRef]
- Tessier, A.; Finch, L.; Daskalopoulou, S.S.; Mayo, N.E. Validation of the Charlson Comorbidity Index for predicting functional outcome of stroke. Arch. Phys. Med. Rehabil. 2008, 89, 1276–1283. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Marti, S.; Munoz, X.; Rios, J.; Morell, F.; Ferrer, J. Body weight and comorbidity predict mortality in COPD patients treated with oxygen therapy. Eur. Respir. J. 2006, 27, 689–696. [Google Scholar] [CrossRef]
- Olsson, T.; Terent, A.; Lind, L. Charlson Comorbidity Index can add prognostic information to Rapid Emergency Medicine Score as a predictor of long-term mortality. Eur. J. Emerg. Med. Off. J. Eur. Soc. Emerg. Med. 2005, 12, 220–224. [Google Scholar] [CrossRef]
- Torres, O.H.; Munoz, J.; Ruiz, D.; Ris, J.; Gich, I.; Coma, E.; Gurgui, M.; Vazquez, G. Outcome predictors of pneumonia in elderly patients: Importance of functional assessment. J. Am. Geriatr. Soc. 2004, 52, 1603–1609. [Google Scholar] [CrossRef]
- Wang, N.; Wang, M.P.; Jiang, L.; Du, B.; Zhu, B.; Xi, X.M. Association between the modified Nutrition Risk in Critically Ill (mNUTRIC) score and clinical outcomes in the intensive care unit: A secondary analysis of a large prospective observational study. BMC Anesthesiol. 2021, 21, 220. [Google Scholar] [CrossRef]
- Molnar, J.A.; Underdown, M.J.; Clark, W.A. Nutrition and Chronic Wounds. Adv. Wound Care 2014, 3, 663–681. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Kontopidis, I.; Gkegkes, I.D.; Rafailidis, P.I.; Falagas, M.E. Incidence, characteristics, and outcomes of patients with bone and joint infections due to community-associated methicillin-resistant Staphylococcus aureus: A systematic review. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Singh, W.; Raj, V.S.; Pokhrel, B.M.; Mohapatra, T.M. High prevalence of Panton-Valentine leukocidin (PVL) genes in nosocomial-acquired Staphylococcus aureus isolated from tertiary care hospitals in Nepal. BioMed Res. Int. 2014, 2014, 790350. [Google Scholar] [CrossRef] [PubMed]
- Chastre, J.; Wolff, M.; Fagon, J.Y.; Chevret, S.; Thomas, F.; Wermert, D.; Clementi, E.; Gonzalez, J.; Jusserand, D.; Asfar, P.; et al. Comparison of 8 vs. 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: A randomized trial. JAMA 2003, 290, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
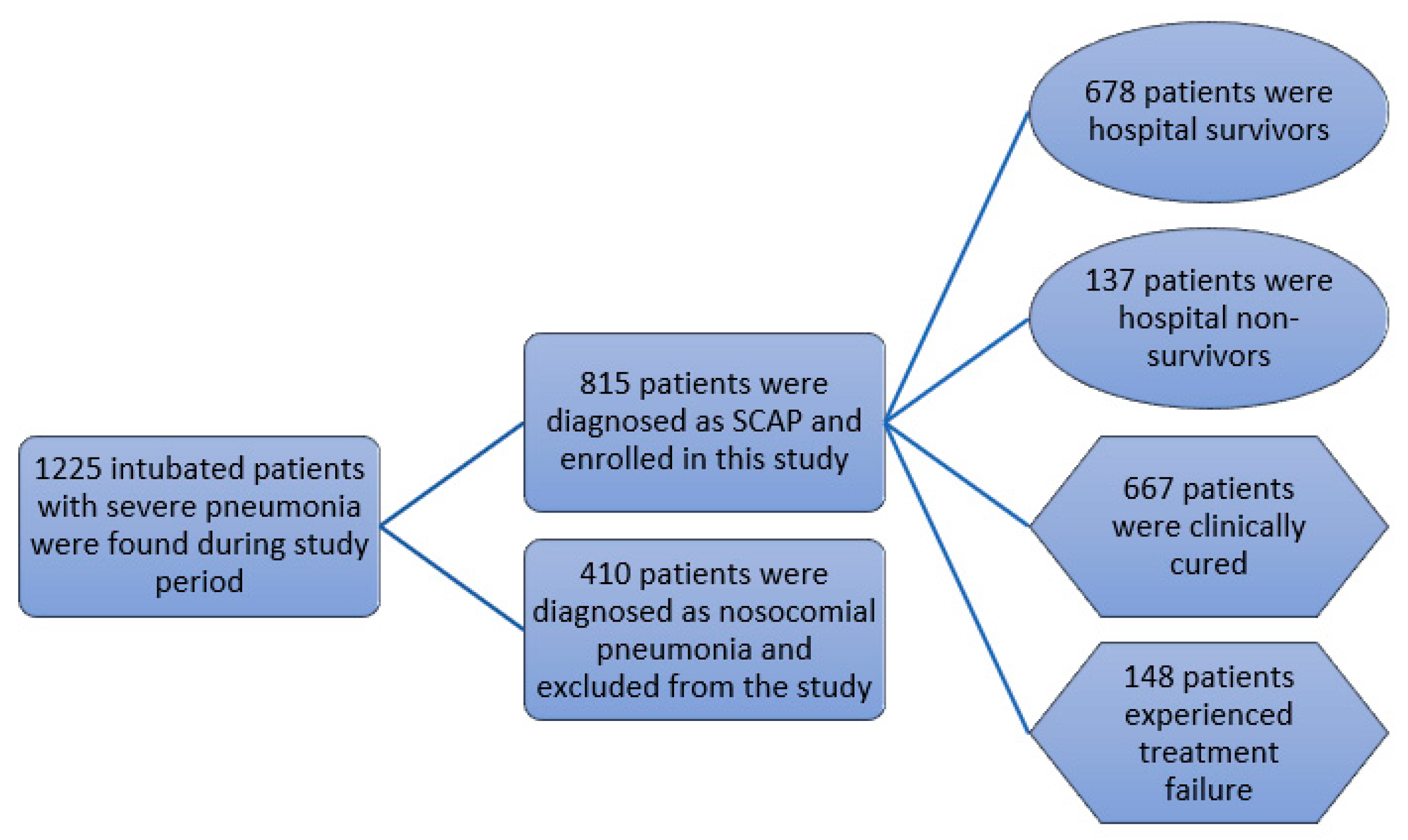
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| • Age ≥18 years • Newly developed or progressive radiographic lung infiltration/consolidation (confirmed by radiologist) • At least two of the following symptoms of lower respiratory tract infection Cough Purulent expectoration Fever ≥38.3 °C or hypothermia <35 °C Pathological lung auscultation | • Pneumonia developed 48 h after admission or intubation; • Pneumonia developed at a nursing home; • Hospitalization in the last 30 days; • Immunosuppressed state (i.e., treatment with steroids, cytotoxic agents, and/or immunosuppressive agents for longer than 1 month). |
| Survivors (n = 678) | Non-Survivors (n = 137) | p-Value | |
|---|---|---|---|
| Age | 76.72 ± 14.25 | 76.48 ± 16.14 | 0.860 |
| Sex Male Female | 496 (73.16%) 182 (26.85%) | 85 (62.04%) 52 (37.96%) | 0.007 ** |
| mNUTRIC score | 4.78 ± 1.20 | 6.87 ± 1.72 | <0.001 *** |
| CCI | 5.76 ± 2.54 | 6.72 ± 3.25 | <0.001 *** |
| PSI score | 142.92 ± 42.61 | 165.56 ± 46.11 | <0.001 *** |
| CURB-65 score | 2.55 ± 1.25 | 2.99 ± 1.31 | <0.001 *** |
| APACHE II score | 20.70 ± 6.86 | 25.89 ± 7.10 | <0.001 *** |
| SOFA | 6.36 ± 3.45 | 7.86 ± 4.10 | 0.031 * |
| qSOFA | 1.79 ± 0.64 | 1.75 ± 0.86 | 0.844 |
| Treatment duration | 9.03 ± 3.36 | 10.12 ± 4.78 | 0.011 * |
| Length of hospital stay | 29.00 ± 13.09 | 28.86 ± 20.24 | 0.977 |
| Bacteremia Yes No | 76 (11.21%) 602 (88.79%) | 29 (21.17%) 108 (78.83%) | 0.001 ** |
| Pathogens of blood culture Streptococcus pneumonia Haemophilus influenzae Moraxella catarrhalis Serratia marcescens Escherichia coli Klebsiella pneumonia Staphylococcus aureus | 14 (2.1%) 5 (0.7%) 6 (0.9%) 10 (1.5%) 16 (2.4%) 18 (2.7%) 7 (1.0%) | 5 (3.6%) 1 (0.7%) 1 (0.7%) 3 (2.2%) 5 (3.6%) 6 (4.4%) 8 (5.8%) | 0.345 0.734 0.666 0.467 0.376 0.270 0.001 ** |
| Sputum culture Growth No Growth | 322 (47.49%) 356 (52.51%) | 65 (47.45%) 72 (52.55%) | 0.508 |
| Pathogens of sputum culture Streptococcus pneumonia Haemophilus influenza Moraxella catarrhalis Serratia marcescens Escherichia coli Klebsiella pneumonia Staphylococcus aureus | 91(13.4%) 40 (5.9%) 24 (3.5%) 36 (5.3%) 31 (4.6%) 62 (9.2%) 38 (5.6%) | 19 (13.9%) 4 (2.9%) 4 (2.9%) 8 (5.8%) 5 (3.6%) 6 (4.4%) 19 (13.9%) | 0.490 0.110 0.479 0.466 0.418 0.088 † 0.001 ** |
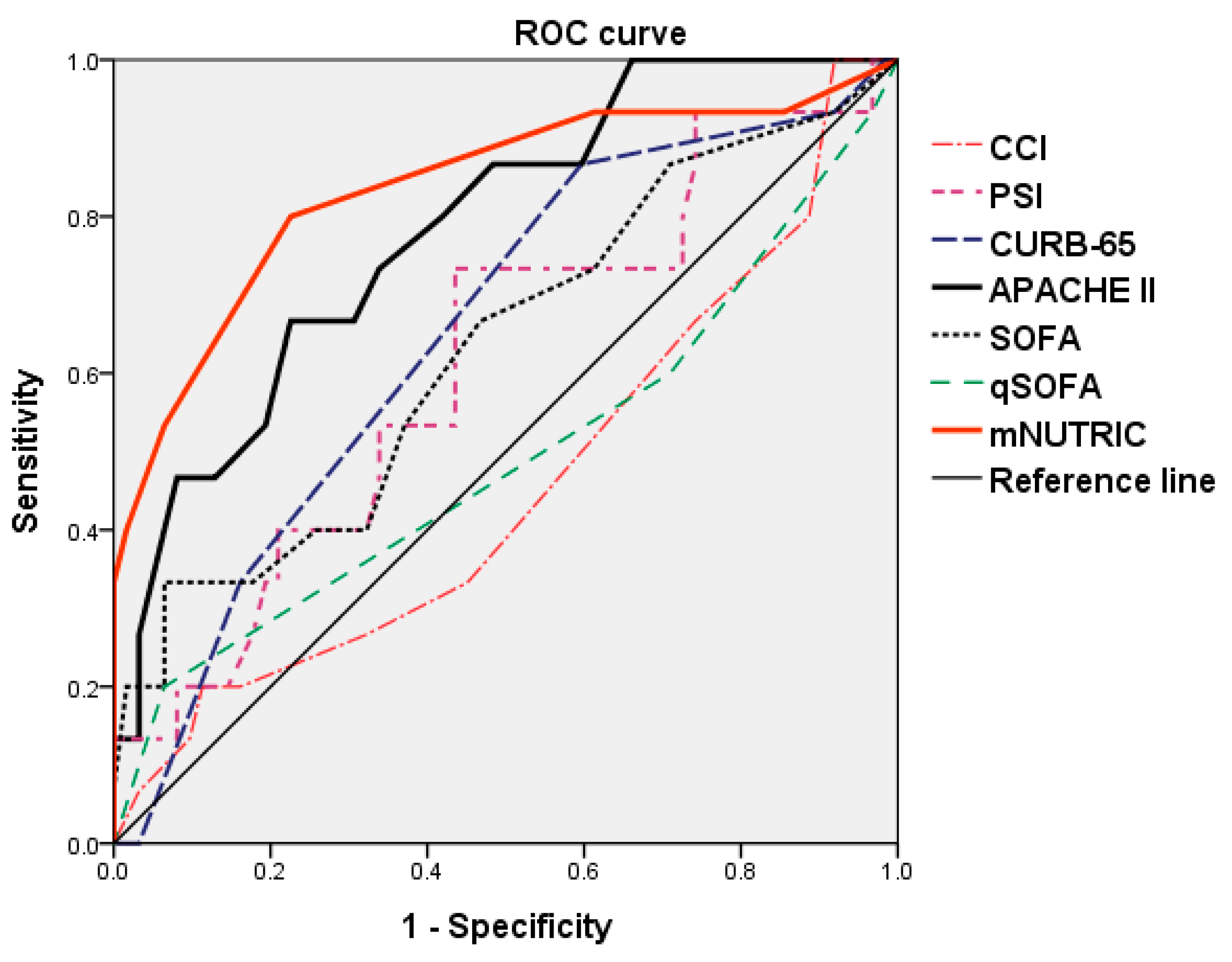
| Scoring Factors | Sensitivity | Specificity | Cut-Off Value | AUC | p-Value |
|---|---|---|---|---|---|
| CCI | 0.200 | 0.887 | 9.5 | 0.458 | 0.611 |
| PSI | 0.733 | 0.565 | 143 | 0.615 | 0.171 |
| CURB-65 | 0.867 | 0.403 | 2.5 | 0.652 | 0.070 † |
| APACHE II | 0.667 | 0.774 | 23.5 | 0.785 | 0.001 ** |
| SOFA | 0.667 | 0.532 | 4.5 | 0.631 | 0.117 |
| qSOFA | 0.200 | 0.935 | 2.5 | 0.494 | 0.938 |
| mNUTRIC | 0.800 | 0.774 | 5.5 | 0.838 | <0.001 *** |
| Clinical Cure (n = 667) | Failure (n = 148) | p-Value | |
|---|---|---|---|
| Age | 76.86 ± 14.45 | 76.54 ± 15.60 | 0.824 |
| Sex Male Female | 490 (73.46%) 177 (26.54%) | 91 (61.49%) 57 (38.51%) | 0.006 ** |
| mNUTRIC score | 4.93 ± 1.38 | 6.03 ± 1.78 | <0.001 *** |
| CCI | 5.79 ± 2.59 | 6.40 ± 2.95 | 0.018 * |
| PSI score | 141.46 ± 41.64 | 176.64 ± 45.25 | <0.001 *** |
| CURB-65 score | 2.50 ± 1.22 | 3.41 ± 1.24 | <0.001 *** |
| APACHE II score | 20.72 ± 6.98 | 25.44 ± 6.80 | 0.001 ** |
| SOFA | 6.25 ± 3.58 | 7.73 ± 3.60 | 0.040 * |
| qSOFA | 1.78 ± 0. 67 | 1.77 ± 0.83 | 0.943 |
| Treatment duration | 9.03 ± 3.33 | 9.81 ± 4.64 | 0.074 † |
| Length of hospital stay | 26.19 ± 11.25 | 32.00 ± 19.48 | 0.161 |
| Bacteremia Yes No | 77 (11.54%) 590 (88.46%) | 28 (18.92%) 120 (81.08%) | 0.069 † |
| Pathogens of blood culture Streptococcus pneumonia Haemophilus influenza Moraxella catarrhalis Serratia marcescens Escherichia coli Klebsiella pneumonia Staphylococcus aureus | 15 (2.2%) 5 (0.7%) 6 (0.9%) 9 (1.3%) 17 (2.5%) 18 (2.7%) 7 (1.0%) | 4 (2.7%) 1 (0.7%) 1 (0.7%) 4 (2.7%) 4 (2.7%) 6 (4.1%) 8 (5.4%) | 0.763 0.701 0.628 0.269 0.548 0.417 0.010 * |
| Sputum culture Growth No Growth | 312 (46.78%) 355 (53.22%) | 75 (50.68%) 73 (49.32%) | 0.441 |
| Pathogens of sputum culture Streptococcus pneumonia Haemophilus influenza Moraxella catarrhalis Serratia marcescens Escherichia coli Klebsiella pneumonia Staphylococcus aureus | 90 (13.5%) 35 (5.2%) 21 (3.1%) 34 (5.1%) 30 (4.5%) 59 (8.8%) 43 (6.4%) | 20 (13.5%) 9 (6.1%) 7 (4.7%) 10 (6.8%) 6 (4.1%) 9 (6.1%) 14 (9.5%) | 0.542 0.688 0.323 0.422 0.511 0.533 0.212 |
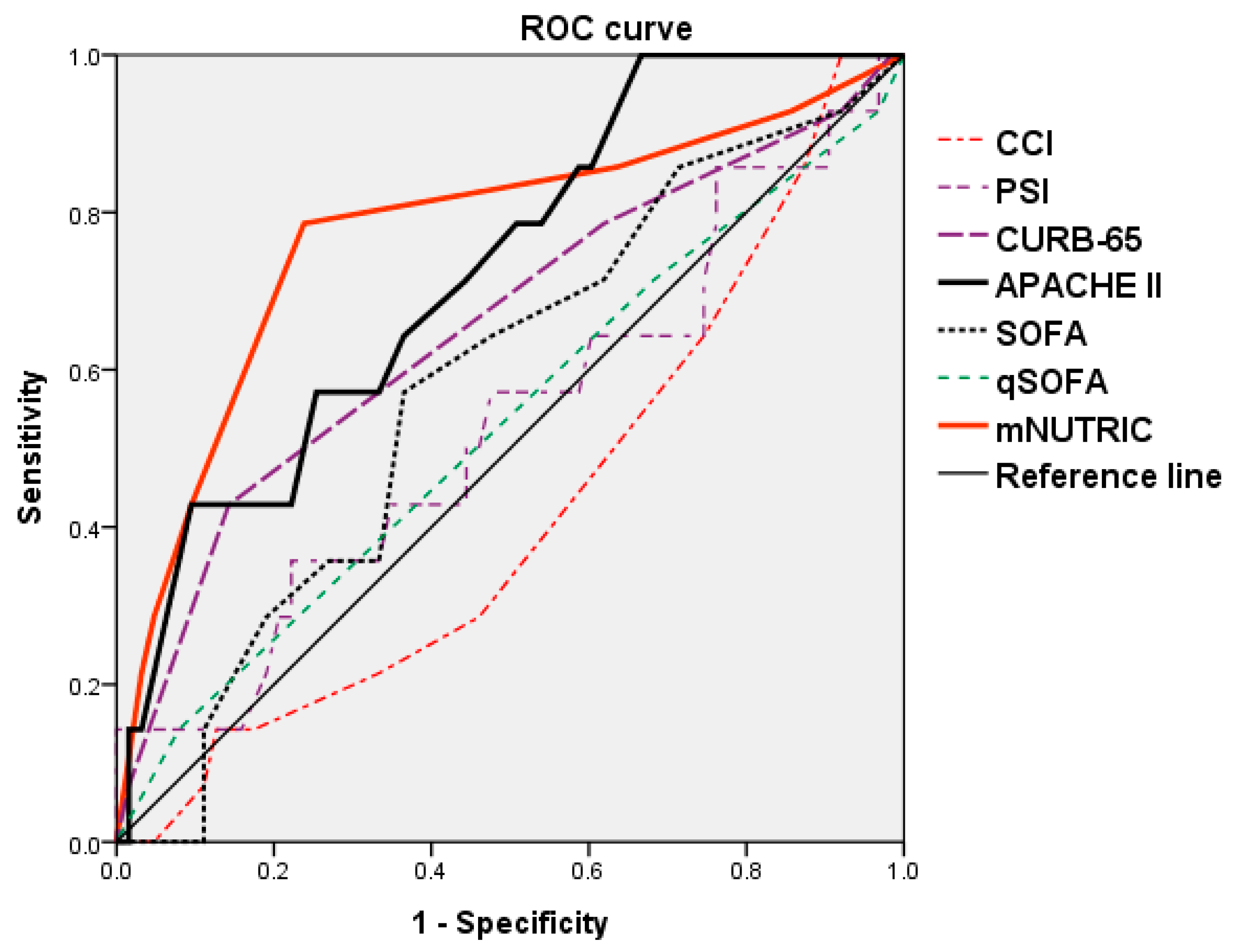
| Scoring Factors | Sensitivity | Specificity | Cut-Off Value | AUC | p-Value |
|---|---|---|---|---|---|
| CCI | 0.143 | 0.873 | 9.5 | 0.422 | 0.366 |
| PSI | 0.143 | 1.000 | 219 | 0.530 | 0.726 |
| CURB-65 | 0.429 | 0.857 | 3.5 | 0.657 | 0.067 † |
| APACHE II | 0.571 | 0.746 | 23.5 | 0.727 | 0.008 ** |
| SOFA | 0.571 | 0.635 | 5.5 | 0.580 | 0.352 |
| qSOFA | 0.143 | 0.921 | 2.5 | 0.529 | 0.731 |
| mNUTRIC | 0.786 | 0.762 | 5.5 | 0.773 | 0.001 ** |
| Hospital Mortality AOR (95% CI) | p-Value | Treatment Outcome AOR (95% CI) | p-Value | |
|---|---|---|---|---|
| CCI | 1.194 (0.813–1.753) | 0.365 | 0.924 (0.623–1.371) | 0.695 |
| PSI score | 1.018 (0.982–1.056) | 0.324 | 0.985 (0.955–1.016) | 0.344 |
| CURB-65 score | 1.410 (0.371–5.353) | 0.614 | 2.257 (0.702–7.259) | 0.172 |
| APACHE II score | 1.240 (0.995–1.544) | 0.056 † | 1.197 (1.012–1.416) | 0.036 * |
| SOFA score | 0.856 (0.611–1.200) | 0.368 | 0. 762 (0.555–1.047) | 0.093 |
| qSOFA score | 0.800 (0.158–4.054) | 0.788 | 1.569 (0.438–5.622) | 0.489 |
| mNUTRIC score | 2.954 (1.457–5.991) | 0.003 ** | 1.848 (1.107–3.086) | 0.019 * |
| Bacteremia | 0.065 (0.006–0.772) | 0.030 * | 0.604 (0.065–5.655) | 0.659 |
| Treatment duration | 1.246 (0.977–1.588) | 0.076 † | 1.100 (0.996–1.215) | 0.047 * |
| mNUTRIC Score | p-Value | APACHE II Score | p-Value | |||
|---|---|---|---|---|---|---|
| >5 N = 333 | ≤5 N = 481 | >23.5 N = 347 | ≤23.5 N = 467 | |||
| Bacteremia Yes No | 70 (21.02%) 263 (78.98%) | 35 (7.27%) 446 (92.72%) | <0.001 *** | 72 (20.75%) 275 (79.25%) | 33 (7.07%) 434 (92.93%) | <0.001 *** |
| Treatment duration | 10.53 ± 4.53 | 8.30 ± 2.53 | <0.001 *** | 9.88 ± 4.37 | 8.72 ± 2.94 | <0.001 *** |
| Length of hospital stay | 28.71 ± 15.68 | 29.71 ± 16.88 | 0.838 | 29.24 ± 16.00 | 27.50 ± 15.71 | 0.754 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, C.-C.; Tu, C.-Y.; Chen, C.-H.; Wang, Y.-T.; Chen, W.-C.; Fu, P.-K.; Chen, C.-M.; Lai, C.-C.; Kuo, L.-K.; Ku, S.-C.; et al. Significance of the Modified NUTRIC Score for Predicting Clinical Outcomes in Patients with Severe Community-Acquired Pneumonia. Nutrients 2022, 14, 198. https://doi.org/10.3390/nu14010198
Tseng C-C, Tu C-Y, Chen C-H, Wang Y-T, Chen W-C, Fu P-K, Chen C-M, Lai C-C, Kuo L-K, Ku S-C, et al. Significance of the Modified NUTRIC Score for Predicting Clinical Outcomes in Patients with Severe Community-Acquired Pneumonia. Nutrients. 2022; 14(1):198. https://doi.org/10.3390/nu14010198
Chicago/Turabian StyleTseng, Chia-Cheng, Chih-Yen Tu, Chia-Hung Chen, Yao-Tung Wang, Wei-Chih Chen, Pin-Kuei Fu, Chin-Ming Chen, Chih-Cheng Lai, Li-Kuo Kuo, Shih-Chi Ku, and et al. 2022. "Significance of the Modified NUTRIC Score for Predicting Clinical Outcomes in Patients with Severe Community-Acquired Pneumonia" Nutrients 14, no. 1: 198. https://doi.org/10.3390/nu14010198
APA StyleTseng, C.-C., Tu, C.-Y., Chen, C.-H., Wang, Y.-T., Chen, W.-C., Fu, P.-K., Chen, C.-M., Lai, C.-C., Kuo, L.-K., Ku, S.-C., & Fang, W.-F. (2022). Significance of the Modified NUTRIC Score for Predicting Clinical Outcomes in Patients with Severe Community-Acquired Pneumonia. Nutrients, 14(1), 198. https://doi.org/10.3390/nu14010198