Long-Term Space Nutrition: A Scoping Review
Abstract
1. Introduction
1.1. Backgrounds
1.2. Rationale and Objectives
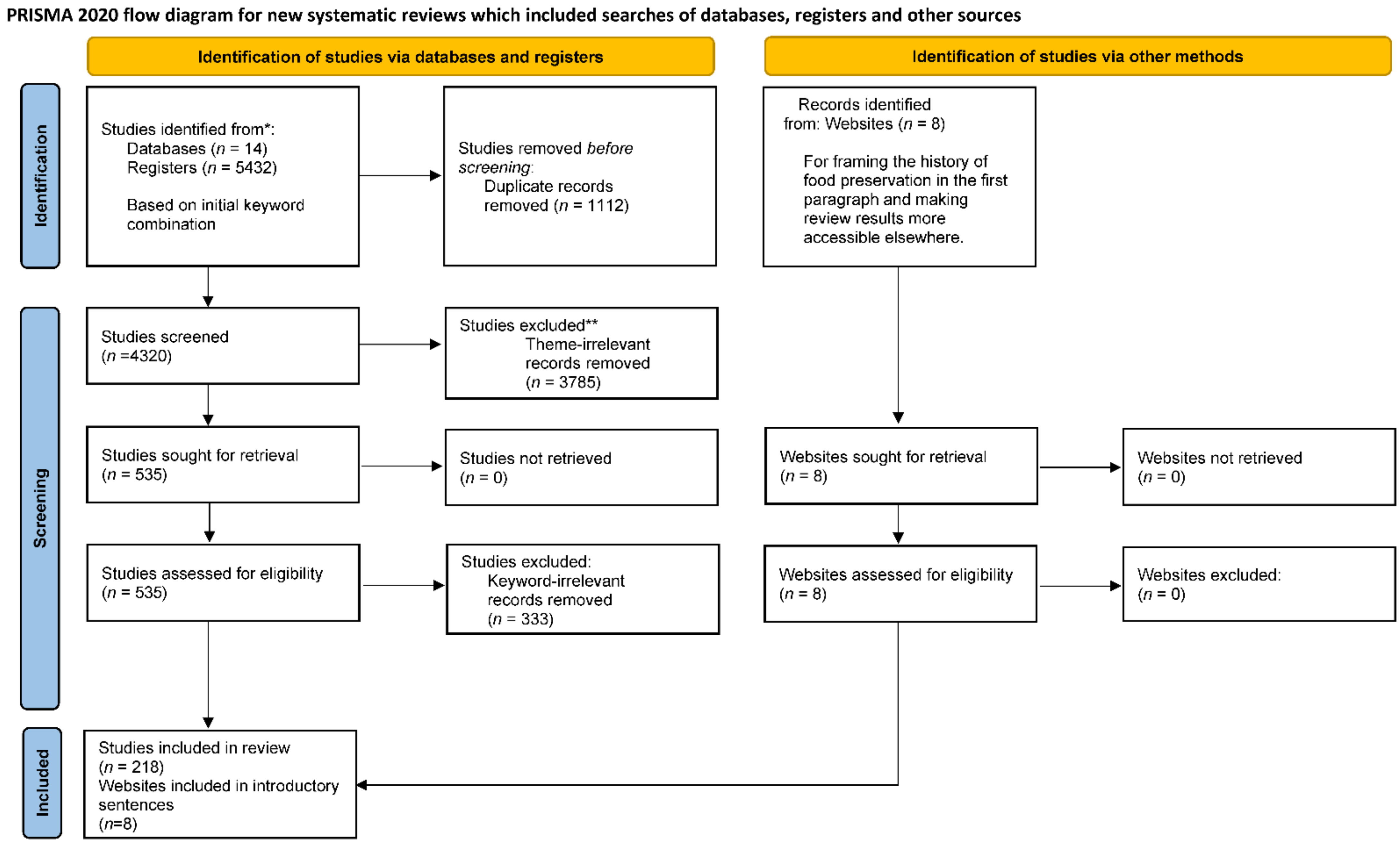
2. Materials and Methods
2.1. Protocol and Registration Information Sources
2.2. Search Strategies and Eligibility Criteria
2.3. Information Sources
2.4. Search and Selection of Sources of Evidence
3. Results
3.1. Current State of Space Nutrition
3.1.1. Key Components of Space Nutrition
3.1.2. The Evolution of Space Nutrition
3.1.3. Space Food Categories
3.1.4. Space Food Menus
3.2. Limitations of Existing Space Nutrition
3.2.1. Dominance of Processed over Fresh Food
3.2.2. No Quality Advantage for Resource-Intensive Refrigerated and Frozen Food
3.2.3. Space Food Supply Is Restricted by Limited Transportation and Storage Space
3.2.4. Long-Term Space Nutrition Requirements for Food Storage and Cooking Methods
3.2.5. Diet Menu Fatigue
3.2.6. Lack of Nutrients to Cope with Extreme Conditions of Space
3.3. The Influence of Adverse Space Environment on Astronauts’ Diet and Health
3.3.1. Less Energy Intake and Weight Loss
3.3.2. Effect of Microgravity
3.3.3. Long-Term Radiation
3.3.4. Metabolic Stress
3.3.5. Changes in Physical Condition
3.3.6. Intestinal Microecology Disorder
3.3.7. Vision Damage
3.3.8. Fluid and Electrolyte Imbalance
4. Discussion
4.1. Nutritional Measures to Cope with Reduced Intake
4.1.1. Increase Palatability through Fresh Food with Distinctive Flavors
4.1.2. Boost Energy Intake through Dietary Culture and Food Production Activities
4.1.3. Enhance Caloric Intake through Nutrient-Dense and Fresh Foods
4.1.4. Counterbalance the Effects of Space Environment on Leptin Secretion with Nutrition
4.1.5. Improve Immunity with Nutritional Measures
4.2. Nutritional Countermeasures for the Effects of Microgravity
4.2.1. Mitigate Bone Loss with Nutritional Measures
4.2.2. Reduce Sodium Intake
4.2.3. Increase Intake of Vegetable Protein, Potassium, and Bisphosphonates
4.2.4. Increase Vitamin D Intake
4.2.5. Increase Vitamin K Intake
4.2.6. Increase Calcium Intake
4.2.7. Increase Unsaturated Fatty Acids and Decrease Saturated Fatty Acids
4.2.8. Increase Protein Intake to Counteract Muscle Atrophy
4.2.9. Combat Intestinal Microecology Disorder with Foods Rich in Calcium and Probiotics
4.3. Nutritional Countermeasures for the Effects of Radiation
4.4. Brief Summary of Nutritional Countermeasures to the Adverse Effects of Space Environment
4.5. Nutrient Loss during Food Processing and Storage
4.6. Hazards of Food Packaging and Additives
4.6.1. Toxicity of Packaging Materials
4.6.2. Health Threats from Food Additives
4.6.3. Challenges Associated with New Packaging Technology
4.7. Development of Space Food Systems
4.7.1. Aseptic Food Production Systems for Transit Space Habitats
4.7.2. Long-Term Food Production Systems as Closed-Loop Life-Support Systems
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | Active Packaging |
| BPA | Bisphenol A |
| DBP | Di-butyl phthalate |
| BBP | Butyl Benzyl Phthalate |
| DENP | Diethyl p-nitrophenyl phosphate |
| DHA | Docosahexaenoic Acid |
| EPA | Eicosapentaenoic acid |
| EMMIHS-II | EuroMoonMars IMA HI-SEAS II |
| HI-SEAS | Hawaii Space Exploration Analog and Simulation |
| IP | Intelligent Packaging |
| ISS | International Space Station |
| LLDPE | Linear Low-Density Polyethylene |
| MAP | Modified Air Packaging |
| NASA | National Aeronautics and Space Administration |
| NY | Nylon |
| OPP | Oriented Polypropylene (Film) |
| PONDS | Passive Orbital Nutrient Delivery System |
| PE | Polyethylene |
| PET | Polyethylene Terephthalate |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews |
| ROS | Reactive Oxygen Species |
| VA | Vitamin A |
| VB1 | Vitamin B1 |
| VB12 | Vitamin B12 |
| VB2 | Vitamin B2 |
| VB6 | Vitamin B6 |
| VC | Vitamins C |
| VD | Vitamin D |
| VE | Vitamin E |
| VPS | Vegetable Production System |
| VK | Vitamin K |
| VP | Vitamin P |
| WHO | World Health Organization |
References
- Hsu, J. Diet of the Ancient Mariner: An Unprecedented Archaeology Experiment is Putting Historical Shipboard Food and Drink to Test. Hakai Magazine, 14 March 2018. Available online: https://www.hakaimagazine.com/news/diet-of-the-ancient-mariner/ (accessed on 1 August 2020).
- Kittredge, J. Food Preservation through the Ages. The Natural Farmer: The Newspaper of the Northeast Organic Farming Association. 2019. Available online: https://thenaturalfarmer.org/article/food-preservation-through-the-ages/ (accessed on 1 August 2020).
- Fictum, D. Salt Pork, Ship’s Biscuit, and Burgoo: Sea Provisions for Common Sailors and Pirates. Colon. Ships Pirat, 24 January 2016. Available online: https://csphistorical.com/2016/01/24/salt-pork-ships-biscuit-and-burgoo-sea-provisions-for-common-sailors-and-pirates-part-1/ (accessed on 1 August 2020).
- Wilson, C.A. Preserving Food to Preserve Life: The Response to Glut and Famine from Early Times to the End of the Middle Ages. In Waste Not, Want Not: Food Preservation from Early Times to the Present; Wilson, C.A., Ed.; Edinburgh University Press: Edinburgh, Scotland, 1991; pp. 5–31. [Google Scholar]
- Shephard, S. Pickled, Potted, and Canned: How the Art and Science of Food Preserving Changed the World; Simon & Schuster: Manhattan, NY, USA, 2001; p. 336. [Google Scholar]
- Nummer, B.A. Historical Origins of Food Preservation; National Cener for Home Food Preservation, University of Georgia: Athens, GA, USA, 2002; Available online: https://nchfp.uga.edu/publications/nchfp/factsheets/food_pres_hist.html (accessed on 1 August 2020).
- Kourkoutas, Y.; Proestos, C. Food Preservation: Challenges and Efforts for the Future. Foods 2020, 9, 391. [Google Scholar] [CrossRef] [PubMed]
- Nordion, Food Irradiation. 2011. Available online: https://www.nordion.com/wp-content/uploads/2014/10/GT_History-of-Food-Irradiation.pdf (accessed on 1 August 2020).
- Witze, A. NASA rethinks approach to Mars exploration. Nature 2016, 538, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A. Review of NASA approach to space radiation risk assessments for Mars exploration. Health Phys. 2015, 108, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Messina, P.; Vennemann, D. The European space exploration programme: Current status of ESA’s plans for Moon and Mars exploration. Acta Astronaut. 2005, 57, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.P.; Fellows, A.M.; Binsted, K.A.; Hegel, M.T.; Buckey, J.C. Autonomous, computer-based behavioral health countermeasure evaluation at HI-SEAS Mars analog. Aerosp. Med. Hum. Perform. 2016, 87, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Grosse, J.; Wehland, M.; Mann, V.; Reseland, J.E.; Sundaresan, A.; Corydon, T.J. The impact of microgravity on bone in humans. Bone 2016, 87, 44–56. [Google Scholar] [CrossRef]
- Stein, T.P. Weight, muscle and bone loss during space flight: Another perspective. Eur. J. Appl. Physiol. 2013, 113, 2171–2181. [Google Scholar] [CrossRef]
- Bullard, R.W. Physiological problems of space travel. Annu. Rev. Physiol. 1972, 34, 205–234. [Google Scholar] [CrossRef]
- Narici, M.V.; De Boer, M.D. Disuse of the musculo-skeletal system in space and on earth. Eur. J. Appl. Physiol. 2011, 111, 403–420. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Liu, Z. Effects of realand simulated weightlessness on the cardiac and peripheral vascular functions of humans: A review. Int. J. Occup. Med. Environ. Health 2015, 28, 793–802. [Google Scholar] [CrossRef]
- Graveline, D.E. Cardiovascular deconditioning: Role of blood volume and sympathetic neurohormones. Life Sci. Space Res. 1964, 2, 287–298. [Google Scholar]
- Coupe, M.; Fortrat, J.O.; Larina, I.; Gauquelin-Koch, G.; Gharib, C.; Custaud, M.A. Cardiovascular deconditioning: From autonomic nervous system to microvascular dysfunctions. Respir. Physiol. Neurobiol. 2009, 169, S10–S12. [Google Scholar] [CrossRef]
- Samsonov, N.M.; Bobe, L.S.; Gavrilov, L.I.; Novikov, V.M.; Farafonov, N.S.; Grigoriev, J.I.; Zaitsev, E.N.; Romanov, S.J.; Grogoriev, A.I.; Sinjak, J.E. Long-duration space mission regenerative life support. Acta Astronautica. 2000, 47, 129–138. [Google Scholar] [CrossRef]
- Schreckenghost, D.; Thronesbery, C.; Bonasso, P.; Kortenkamp, D.; Martin, C. Intelligent control of life support for space missions. IEEE Intell. Syst. 2002, 17, 24–31. [Google Scholar] [CrossRef]
- Tamponnet, C. Life support systems for lunar missions. Adv. Space Res. 1996, 18, 103–110. [Google Scholar] [CrossRef]
- Monje, O.; Stutte, G.W.; Goins, G.D.; Porterfield, D.M.; Bingham, G.E. Farming in space: Environmental and biophysical concerns. Adv. Space Res. 2003, 31, 151–167. [Google Scholar] [CrossRef]
- Rygalov, V.Y. Cultivation of plants in space: Their contribution to stabilizing atmospheric composition in closed ecological systems. Adv. Space Res. 1996, 18, 165–176. [Google Scholar] [CrossRef]
- Volk, T. Considerations in miniaturizing simplified agro-ecosystems for advanced life support. Ecol. Eng. 1996, 6, 99–108. [Google Scholar] [CrossRef]
- Bluem, V.; Paris, F. Aquatic food production modules in bioregenerative life support systems based on higher plants. Adv. Space Res. 2001, 27, 1513–1522. [Google Scholar] [CrossRef]
- Ivanova, T.N.; Kostov, P.T.; Sapunova, S.M.; Dandolov, I.W.; Salisbury, F.B.; Bingham, G.E.; Sytchov, V.N.; Levinskikh, M.A.; Podolski, I.G.; Bubenheim, D.B. Six-month space greenhouse experiments—A step to creation of future biological life support systems. Acta Astronaut. 1998, 42, 11–23. [Google Scholar] [CrossRef]
- Ferl, R.; Wheeler, R.; Levine, H.G.; Paul, A.L. Plants in space. Curr. Opin. Plant Biol. 2002, 5, 258–263. [Google Scholar] [CrossRef]
- Musgrave, M.E. Seeds in space. Seed Sci. Res. 2002, 12, 1–17. [Google Scholar] [CrossRef]
- Bergouignan, A.; Stein, T.P.; Habold, C.; Coxam, V.; Gorman, D.O.; Blanc, S. Towards human exploration of space: The THESEUS review series on nutrition and metabolism research priorities. NPJ Microgravity 2016, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.; Douglas, G.; Perchonok, M. Developing the NASA Food System for Long-Duration Missions. Food Sci. 2011, 76, 40–48. [Google Scholar] [CrossRef]
- Lupton, J.R.; Brooks, J.A.; Butte, N.F.; Caballero, B.; Flatt, J.P.; Fried, S.K. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids; Institute of Medicine, The National Academy Press: Washington, DC, USA, 2002; Volume 5, pp. 589–768. [Google Scholar]
- Kamman, J.F.; Labuza, T.P.; Warthesen, J.J. Kinetics of thiamin and riboflavin loss in pasta asa function of constant and variable storage conditions. J. Food Sci. 1981, 46, 1457–1461. [Google Scholar] [CrossRef]
- Bourland, C.T. The development of food systems for space. Trends Food Sci. Technol. 1993, 4, 271–276. [Google Scholar] [CrossRef]
- Perchonok, M.; Bourland, C. NASA food systems: Past, present and future. Nutrition 2002, 18, 913–920. [Google Scholar] [CrossRef]
- Perchonok, M.H. NASA packaged food systems. In The World of Food Science [Internet]; Institute of Food Technologists and the International Union of Food Science and Technology: Oakville, ON, Canada. Available online: http://www.worldfoodscience.org/cms/?pid= (accessed on 30 August 2021).
- Lane, H.W.; Bourland, C.; Barrett, A.; Heer, M.; Smith, S.M. The Role of Nutritional Research in the Success of Human Space Flight, ASN 2013 annual meeting symposium summary, @2013 American Society for Nutrition. Adv. Nutr. 2013, 4, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Ball, N.; Hogan, J.; Hindupur, A.; Kagawa, H.; Levri, J.; Sims, K. BioNutrients-1: Development of an On-Demand Nutrient Production System for Long-Duration Missions. International Conference on Envionmental Systems. 2020. Available online: https://ttu-ir.tdl.org/bitstream/handle/2346/86343/ICES-2020-119.pdf?sequence=1&isAllowed=y (accessed on 30 August 2021).
- Sun, G.S.; Tou, J.C.; Yu, D.; Gorten, B.; Cohen, J. The past, present, and future of National Aeronautics and Space Administration spaceflight diet in support of microgravity rodent experiments. Nutrition 2014, 30, 125–130. [Google Scholar] [CrossRef]
- Song, B.S.; Park, J.G.; Park, J.N.; Han, I.J.; Choi, J.I.; Kim, J.H.; Byun, M.W.; Kang, S.W.; Choi, G.H.; Lee, J.W. High-dose processing and application to Korean space foods. Radiat. Phys. Chem. 2009, 78, 671–674. [Google Scholar] [CrossRef]
- Song, B.S.; Park, J.G.; Park, J.N.; Han, I.J.; Choi, J.I.; Kim, J.H.; Choi, G.H.; Byun, M.W.; Lee, J.W. Korean space food development: Ready-to-eat Kimchi, a traditional Korean fermented vegetable, sterilized with high-dose gamma irradiation. Adv. Space Res. 2009, 44, 162. [Google Scholar] [CrossRef]
- Gomar-Serrano, J.A.; Castillo, S.D.; Bilbao-Cerc, S.F.L. Food in manned spaceflight: From Gemini Program to the ISS/Shuttle programs. Rev. Esp. Nutr. Hum. Diet. 2015, 19, 116. [Google Scholar] [CrossRef]
- Smith, C.M. An adaptive paradigm for human space settlement. Acta Astronaut. 2016, 119, 207–217. [Google Scholar] [CrossRef]
- Buckley, N.D.; Champagne, C.P.; Masotti, A.I.; Wagar, L.E.; Tompkins, T.A.; Green-Johnson, J.M. Harnessing functional food strategies for the health challenges of space travel: Fermented soy for astronaut nutrition. Acta Astronaut. 2011, 68, 731–738. [Google Scholar] [CrossRef]
- Schoeller, D.A.; Gretebeck, R.E. Energy utilization and exercise in space flight. In Nutrition in Spaceflight and Weightlessness Models; Lane, H.W., Schoeller, D.A., Eds.; CRC Press: New York, NY, USA, 2000. [Google Scholar]
- Šikl, R.; Šimeček, M. Confinement has no effect on visual space perception: The results of the Mars-500 experiment. Atten. Percept. Psychophys. 2014, 76, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.; Hardiman, G. Nutritional challenges and counermeasures for space travel. Nutr. Bull. 2020, 45, 98–105. [Google Scholar] [CrossRef]
- Bumgarner, N.R.; Scheerens, J.C.; Yield, M.D.K. Nutritional yield: A proposed index for fresh food improvement illustrated with leafy vegetable data. Plant Foods Hum. Nutr. 2012, 67, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.S.; Wall, K.R.; Kerth, C.R.; Pillai, S.D. Benchmarking the minimum Electron Beam (eBeam) dose required for the sterilization of space food. Radiat. Phys. Chem. 2018, 143, 72–78. [Google Scholar] [CrossRef]
- Drummer, C.; Hesse, C.; Baisch, F.; Norsk, P.; Elmann-Larsen, B.; Gerzer, R.; Heer, M. Water and sodium balances and their relation to body mass changes in microgravity. Eur. J. Clin. Investig. 2000, 30, 1066–1075. [Google Scholar] [CrossRef]
- Cena, H.; Sculati, M.; Roggi, C. Nutritional concerns and possible countermeasures to nutritional issues related to space flight. Eur. J. Nutr. 2003, 42, 99–110. [Google Scholar] [CrossRef]
- Takahashi, K.; Okumura, H.; Guo, R.; Naruse, K. Effect of Oxidative Stress on Cardiovascular System in Response to Gravity. Int. J. Mol. Sci. 2017, 18, 1426. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Zwart, S.R.; Block, G.; Rice, B.L.; Davis-Street, J.E. The nutritional status of astronauts is altered after long-term space flight aboard the International Space Station. J. Nutr. 2005, 135, 437–443. [Google Scholar] [CrossRef]
- Stein, T.P. Space flight and oxidative stress. Nutrition 2002, 18, 867–871. [Google Scholar] [CrossRef]
- Smith, S.M.; Zwart, S.R. Nutritional biochemistry of spaceflight. Adv. Clin. Chem. 2008, 46, 87–130. [Google Scholar] [CrossRef]
- Stein, T.P.; Schluter, M.D.; Leskiw, M.J. Cortisol, insulin and leptin during space flight and bed rest. J. Gravit. Physiol. 1999, 6, 85–86. [Google Scholar]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Ferrannini, E.; Gastaldelli, A.; Iozzo, P. Pathophysiology of prediabetes. Med. Clin. N. Am. 2011, 95, 327–339. [Google Scholar] [CrossRef]
- Lawler, J.M.; Song, W.; Demaree, S.R. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic. Biol. Med. 2003, 35, 9–16. [Google Scholar] [CrossRef]
- Wang, J.; Jin, Y.; Guo, H.; Chen, L.; Chen, B. The nutrition and health function of tartary buckwheat and its application prospect in space food). China Food Saf. 2019, 176–179. [Google Scholar] [CrossRef]
- Hargens, A.R.; Vico, L. Long-duration bed rest as an analog to microgravity. J. Appl. Physiol. 2016, 120, 891–903. [Google Scholar] [CrossRef]
- Stahn, A.C.; Werner, A.; Opatz, O.; Maggioni, M.A.; Steinach, M.; von Ahlefeld, V.W.; Moore, A.; Crucian, B.E.; Smith, S.M.; Zwart, S.R.; et al. Increased core body temperature in astronauts during long-duration space missions. Sci. Rep. 2017, 7, 16180. [Google Scholar] [CrossRef] [PubMed]
- Fortney, S.M.; Mikhaylov, V.; Lee, S.M.; Kobzev, Y.; Gonzalez, R.R.; Greenleaf, J.E. Body temperature and thermoregulation during submaximal exercise after 115-day spaceflight. Aviat. Space Environ. Med. 1998, 69, 137–141. [Google Scholar] [PubMed]
- Polyakov, V.V.; Lacota, N.G.; Gundel, A. Human thermohomeostasis onboard “Mir” and in simulated microgravity studies. Acta Astronaut. 2001, 49, 137–143. [Google Scholar] [CrossRef]
- Ying, C.; Yan, L.; Min, Y.C. Research progress on effects of simulated weightlessness on biological functions. J. Air Force Gen. Hosp. 2007, 23, 40. [Google Scholar]
- Cucinotta, F.A.; To, K.; Cacao, E. Predictions of space radiation fatality risk for exploration missions. Life Sci. Space Res. 2017, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zwart, S.R.; Gibson, C.R.; Mader, T.H.; Ericson, K.; Ploutz-Snyder, R.; Heer, M.; Smith, S.M. Vision changes after spaceflight are related to alterations in folate- and vitamin B-12-dependent one-carbon metabolism. J. Nutr. 2012, 142, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.; Stowe, R.P.; Mehta, S.; Quiriarte, H.; Pierson, D.; Sams, C. Alterations in adaptive immunity persist during long-duration spaceflight. NPJ Microgravity 2015, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Wade, C.E. Effects of hindlimb suspension and elevated ambient CO2 on rat growth and renal function. Aviat. Space Environ. Med. 2000, 71, 610–618. [Google Scholar]
- Frey, M.A.; Sulzman, F.M.; Oser, H.; Ruyters, G. The effects of moderately elevated ambient carbon dioxide levels on human physiology and performance: A joint NASA-ESA-DARA study–Overview. Aviat. Space Environ. Med. 1998, 69, 282–284. [Google Scholar]
- Varma, M.; Sato, T.; Zhang, L.; Meguid, M.M. Space flight related anorexia. Lancet 2000, 356, 681. [Google Scholar] [CrossRef]
- Navara, K.J.; Nelson, R.J. The dark side of light at night: Physiological, epidemiological, and ecological consequences. J. Pineal Res. 2007, 43, 215–224. [Google Scholar] [CrossRef]
- Wright, K.P., Jr.; McHill, A.W.; Birks, B.R.; Griffin, B.R.; Rusterholz, T.; Chinoy, E.D. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 2013, 23, 1554–1558. [Google Scholar] [CrossRef]
- Kim, T.W.; Jeong, J.H.; Hong, S.C. The impact of sleep and circadian disturbance on hormones and metabolism. Int. J. Endocrinol. 2015, 591729. [Google Scholar] [CrossRef]
- Challet, E. Circadian aspects of adipokine regulation in rodents. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 573–582. [Google Scholar] [CrossRef]
- Laurens, C.; Simon, C.; Vernikos, J.; Gauquelin-Koch, G.; Blanc1, S.; Bergouignan, A. Revisiting the role of exercise countermeasure on the regulation of energy balance during space flight. Front. Physiol. 2019, 10, 321. [Google Scholar] [CrossRef]
- Drummen, M.; Tischmann, L.; Gatta-Cherifi, B.; Fogelholm, M.; Raben, A.; Adam, T.C.; Westerterp-Plantenga, M.S. High compared with moderate protein intake reduces adaptive thermogenesis and induces a negative energy balance during long-term weight-loss maintenance in participants with prediabetes in the postobese state: A Preview study. J. Nutr. 2020, 150, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.P. The relationship between dietary intake, exercise, energy balance and the space craft environment. Pflug. Arch. 2000, 441, R21–R31. [Google Scholar] [CrossRef] [PubMed]
- Wade, C.E.; Miller, M.M.; Baer, L.A.; Moran, M.M.; Steele, M.K.; Stein, T.P. Body mass, energy intake, and water consumption of rats and humans during space flight. Nutrition 2002, 18, 829–836. [Google Scholar] [CrossRef]
- Da Silva, M.S.; Zimmerman, P.M.; Meguid, M.M.; Nandi, J.; Ohinata, K.; Xu, Y.; Chen, C.; Tada, T.; Inui, A. Anorexia in space and possible etiologies: An overview. Nutrition 2002, 18, 805–813. [Google Scholar] [CrossRef]
- Lam, D.D.; Garfield, A.S.; Marston, O.J.; Shaw, J.; Heisler, L.K. Brain serotonin system in the coordination of food intake and body weight. Pharmacol. Biochem. Behav. 2010, 97, 84–91. [Google Scholar] [CrossRef]
- Heer, M.; Boerger, A.; Kamps, N.; Mika, C.; Korr, C.; Drummer, C. Nutrient supply during recent European missions. Pflügers Arch. Eur. J. Physiol. 2000, 441, R8–R14. [Google Scholar] [CrossRef]
- Smith, S.M.; Rice, B.L.; Dlouhy, H.; Zwart, S.R. Assessment of nutritional intake during space flight and space flight analogs. Procedia Food Sci. 2013, 2, 27–34. [Google Scholar] [CrossRef]
- Rudwill, F.; O’Gorman, D.; Lefai, E.; Chery, I.; Zahariev, A.; Normand, S.; Pagano, A.F.; Chopard, A.; Damiot, A.; Laurens, C.; et al. Metabolic inflexibility is an early marker of bed-rest-induced glucose intolerance even when fat mass is stable. J. Clin. Endocrinol. Metab. 2018, 103, 1910–1920. [Google Scholar] [CrossRef]
- Bergouignan, A.; Momken, I.; Schoeller, D.A.; Normand, S.; Zahariev, A.; Lescure, B.; Simon, C.; Blanc, S. Regulation of energy balance during longterm physical inactivity induced by bed rest with and without exercise training. J. Clin. Endocrinol. Metab. 2010, 95, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- White, O.; Clement, G.; Fortrat, J.O.; Pavy-LeTraon, A.; Thonnard, J.L.; Blanc, S.; Wuyts, F.L.; Paloski, W.H. Towards human exploration of space: The THESEUS review series on neurophysiology research priorities. NPJ Microgravity 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Lang, T.; Van Loon, J.; Bloomfield, S.; Vico, L.; Chopard, A.; Rittweger, J.; Kyparos, A.; Blottner, D.; Vuori, I.; Gerzer, R.; et al. Towards human exploration of space: The THESEUS review series on muscle and bone research priorities. NPJ Microgravity 2017, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vernikos, J.; Schneider, V.S. Space, gravity and the physiology of aging: Parallel or convergent disciplines? A mini-review. Gerontology 2010, 56, 157–166. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Durante, M. Cancer risk from exposure to galactic cosmic rays: Implications for space exploration by human beings. Lancet Oncol. 2006, 7, 431–435. [Google Scholar] [CrossRef]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. 2011, 711, 193. [Google Scholar] [CrossRef]
- Wang, Y.; Boerma, M.; Zhou, D. Ionizing radiation-induced endothelial cell senescence and cardiovascular diseases. Radiat. Res. 2016, 186, 153. [Google Scholar] [CrossRef]
- Delp, M.D.; Charvat, J.M.; Limoli, C.L.; Globus, R.K.; Ghosh, P. Apollo lunar astronauts show higher cardiovascular disease mortality: Possible deep space radiation effects on the vascular endothelium. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Hohn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; Konig, J.; Grune, T.; Castro, J.P. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.P.; Leskiw, M.J.; Schluter, M.D. Diet and nitrogen metabolism during spaceflight on the shuttle. J. Appl. Physiol. 1996, 81, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Lane, H.W.; Gretebeck, R.J.; Schoeller, D.A.; Davis-Street, J.; Socki, R.A.; Gibson, E.K. Comparison of ground-based and space flight energy expenditure and water turnover in middle-aged healthy male US astronauts. Am. J. Clin. Nutr. 1997, 65, 4–12. [Google Scholar] [CrossRef]
- Rambaut, P.C.; Leach, C.S.; Leonard, J.I. Observations in energy-balance in man during spaceflight. Am. J. Physiol. 1977, 233, R208–R212. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Storch, K.J.; Stolfi, A.; Mohler, S.R.; Frey, M.A.; and Stein, T.P. Weight loss in humans in space. Aviat. Space Environ. Med. 2011, 82, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Wastney, M.E.; Morukov, B.V.; Larina, I.M.; Nyquist, L.E.; Abrams, S.A.; Taran, E.N.; Shih, C.Y.; Nillen, J.L.; Davis-Street, J.E.; et al. Calcium metabolism before, during, and after a 3-mo spaceflight: Kinetic and biochemical changes. Am. J. Physiol. 1999, 277, R1–R10. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Davis-Street, J.E.; Rice, B.L.; Nillen, J.L.; Gillman, P.L.; Block, G. Nutritional status assessment in semiclosed environments: Ground-based and space flight studies in humans. J. Nutr. 2001, 131, 2053–2061. [Google Scholar] [CrossRef]
- Zwart, S.R.; Launius, R.D.; Coen, G.K.; Morgan, J.L.; Charles, J.B.; Smith, S.M. Body mass changes during long-duration spaceflight. Aviat. Space Environ. Med. 2014, 85, 897–904. [Google Scholar] [CrossRef]
- Bigard, A.X.; Boussif, M.; Chalabi, H.; Guezennec, C.Y. Alterations in muscular performance and orthostatic tolerance during Ramadan. Aviat. Space Envir. Med. 1998, 69, 341–346. [Google Scholar]
- Smith, S.M.; Zwart, S.R.; Kloeris, V.; Heer, M. Nutritional Biochemistry of Space Flight; Nova Science Publishers: New York, NY, USA, 2009. [Google Scholar]
- Wauquier, F.; Leotoing, L.; Coxam, V.; Guicheux, J.; Wittrant, Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009, 15, 468–477. [Google Scholar] [CrossRef]
- Smith, S.M.; Heer, M.; Zwart, S.R. Nutrition and bone health in space. In Nutrition and Bone Health, 2nd ed.; Holick, M., Nieves, J., Eds.; Springer: New York, NY, USA, 2015; pp. 687–705. [Google Scholar] [CrossRef]
- Orwoll, E.S.; Adler, R.A.; Amin, S.; Binkley, N.; Lewiecki, E.M.; Petak, S.M.; Shapses, S.A.; Sinaki, M.; Watts, N.B.; Sibonga, J.D. Skeletal health in long-duration astronauts: Nature, assessment and management recommendations from the NASA bone summit. J. Bone Miner. Res. 2013, 28, 1243–1255. [Google Scholar] [CrossRef]
- Zwart, S.R.; Rice, B.L.; Dlouhy, H.; Shackelford, L.C.; Heer, M.; Koslovsky, M.D.; Smith, S.M. Dietary acid load and bone turnover during long-duration spaceflight and bed rest. Am. J. Clin. Nutr. 2018, 107, 834–844. [Google Scholar] [CrossRef]
- Briguglio, M. Nutritional orthopedics and space nutrition as two sides of the same coin: A scoping review. Nutrients 2021, 13, 483. [Google Scholar] [CrossRef]
- Smith, S.M.; McCoy, T.; Gazda, D.; Morgan, J.L.L.; Heer, M.; Zwart, S.R. Space Flight Calcium: Implications for Astronaut Health, Spacecraft Operations, and Earth. Nutrients 2012, 4, 2047–2068. [Google Scholar] [CrossRef] [PubMed]
- Whiting, S.J.; Boyle, J.L.; Thompson, A.; Mirwald, R.L.; Faulkner, R.A. Dietary protein, phosphorus and potassium are beneficial to bone mineral density in adult men consuming adequate dietary calcium. J. Am. Coll. Nutr. 2002, 21, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Wastney, M.E.; O’Brien, K.O.; Morukov, B.V.; Larina, I.M.; Abrams, S.A.; Davis-Street, J.E.; Oganov, V.; Shackelford, L.C. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the Mir space station. J. Bone Miner. Res. 2005, 20, 208–218. [Google Scholar] [CrossRef]
- Smith, S.M.; Heer, M.A.; Shackelford, L.; Sibonga, J.D.; Ploutz-Snyder, L.; Zwart, S.R. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J. Bone Miner. Res. 2012, 27, 1896–1906. [Google Scholar] [CrossRef]
- Zittermann, A.; Heer, M.; Caillot-Augusso, A.; Rettberg, P.; Scheld, K.; Drummer, C.; Alexandre, C.; Horneck, G.; Vorobiev, D. Microgravity inhibits intestinal calcium absorption as shown by a stable strontium test. Eur. J. Clin. Investig. 2000, 30, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Lutwak, L.; Whedon, G.D.; Lachance, P.A.; Reid, J.M.; Lipscomb, H.S. Mineral, electrolyte and nitrogen balance studies of the Gemini-VII fourteen-day orbital space flight. J. Clin. Endocrinol. Metab. 1969, 29, 1140. [Google Scholar] [CrossRef] [PubMed]
- Whedon, G.D.; Lutwak, L.; Rambaut, P.C.; Whittle, M.W.; Smith, M.C.; Reid, J.; Leach, C.; Stadler, C.R.; Sanford, D.D. Mineral and Nitrogen Metabolic Studies, Experiment M071. In Biomedical Results from Skylab (NASA Sp-377); Johnston, R.S., Dietlein, L.F., Eds.; National Aeronautics and Space Administration: Washington, DC, USA, 1977; pp. 164–174. [Google Scholar]
- Whedon, G.D.; Lutwak, L.; Reid, J.; Rambaut, P.; Whittle, M.; Smith, M.; Leach, C. Mineral and nitrogen balance study: Results of metabolic observations on Skylab II 28-day orbital mission. Acta Astronaut. 1975, 2, 297–309. [Google Scholar] [CrossRef]
- Zwart, S.R.; Hargens, A.R.; Smith, S.M. The ratio of animal protein intake to potassium intake is a predictor of bone resorption in space flight analogues and in ambulatory subjects. Am. J. Clin. Nutr. 2004, 80, 1058–1065. [Google Scholar] [CrossRef]
- Bourland, C. Food systems for space and planetary flights. In Nutrition in Spaceflight and Weightlessness Models; Lane, H.W., Schoeller, D.A., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 19–40. [Google Scholar]
- Cervantes, J.L.; Hong, B.Y. Dysbiosis and immune dysregulation in outer space. Int. Rev. Immunol. 2015, 35, 67–82. [Google Scholar] [CrossRef]
- Zwart, S.R.; Pierson, D.; Mehta, S.; Gonda, S.; Smith, S.M. Capacity of Omega-3 Fatty Acids or Eicosapentaenoic Acid to Counteract Weightlessness-Induced Bone Loss by Inhibiting NF-kB Activation: From Cells to Bed Rest to Astronauts. J. Bone Miner. Res. 2010, 25, 1049–1057. [Google Scholar] [CrossRef]
- Smith, M.C.; Heidelbaugh, N.D.; Rambaut, P.C.; Rapp, R.M.; Wheeler, H.O.; Huber, C.S.; Bourland, C.T. Apollo food technology. In Biomedical Results of Apollo (NASA SP-368); NASA: Washington, DC, USA; NASA Life Sciences Data Archive at Johnson Space Center: Houston, TX, USA, 1975. Available online: http://lsda.jsc.nasa.gov/books/apollo/apollp_toc.cfm (accessed on 30 April 2020).
- Horneck, G.; Facius, R.; Reichert, M.; Rettberg, P.; Seboldt, W.; Manzey, D.; Comet, B.; Maillet, A.; Preiss, H.; Schauer, L.; et al. HUMEX, a study on the survivability and adaptation of humans to long-duration exploratory missions, part I: Lunar missions. Adv. Space Res. 2003, 31, 2389–2401. [Google Scholar] [CrossRef]
- Hart, S.G.; Staveland, L.E. Development of NASA-TLX (Task Load Index): Results of empirical and theoretical research. Adv. Psychol. 1988, 52, 139. [Google Scholar] [CrossRef]
- Bychkov, A.; Reshetnikova, P.; Bychkova, E.; Podgorbunskikh, E.; Koptev, V. The current state and future trends of space nutrition from a perspective of astronauts’ physiology. Int J Gastron Food Sci. 2021, 24, 100324. [Google Scholar] [CrossRef]
- Zwart, S.R.; Booth, S.L.; Peterson, J.W.; Wang, Z.W.; Smith, S.M. Vitamin K status in spaceflight and ground-based models of spaceflight. J. Bone Miner. Res. 2011, 26, 948–954. [Google Scholar] [CrossRef]
- Bakker, G.C.; Van Erk, M.J.; Pellis, L.; Wopereis, S.; Rubingh, C.M.; Cnubben, N.H.; Kooistra, T.; Van Ommen, B.; Hendriks, H.F. An antiinflammatory dietary mix modulates inflammation and oxidative and metabolic stress in overweight men: A nutrigenomics approach. Am. J. Clin. Nutr. 2010, 91, 1044–1059. [Google Scholar] [CrossRef]
- Iwamoto, J.; Takeda, T.; Sato, Y. Interventions to prevent bone loss in astronauts during space flight. Keio J. Med. 2005, 54, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Guo, S.; Xu, C.; Yang, C.; Ai, W.; Tang, Y.; Qin, L. Effects of different carbon dioxide and LED lighting levels on the anti-oxidative capabilities of Gynura bicolor DC. Adv. Space Res. 2014, 53, 353–361. [Google Scholar] [CrossRef]
- Stein, T.P.; Leskiw, M.J.; Schluter, M.D.; Donaldson, M.R.; Larina, I. Protein kinetics during and after long-duration spaceflight on MIR. Am. J. Physiol. 1999, 276, E1014–E1021. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Ciocchi, B.; Lebenstedt, M.; Barazzoni, R.; Zanetti, M.; Platen, P.; Heer, M.; Guarnieri, G. Short-term bed rest impairs amino acid-induced protein anabolism in humans. J. Physiol. 2004, 558, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.P.; Donaldson, M.R.; Leskiw, M.J.; Schluter, M.D.; Baggett, D.W.; Boden, G. Branched-chain amino acid supplementation during bed rest: Effect on recovery. J. Appl. Physiol. 2003, 94, 1345–1352. [Google Scholar] [CrossRef][Green Version]
- Fernandez-Real, J.M.; Bullo, M.; Moreno-Navarrete, J.M.; Ricart, W.; Ros, E.; Estruch, R.; Salas-Salvado, J. A mediterranean diet enriched with olive oil is associated with higher serum total osteocalcin levels in elderly men at high cardiovascular risk. J. Clin. Endocrinol. Metab. 2012, 97, 3792–3798. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Davis-Street, J.; Rice, B.L.; Lane, H.W. Nutrition in space. Nutr. Today 1997, 32, 6–12. [Google Scholar] [CrossRef]
- Arnaud, S.B.; Wolinsky, I.; Fung, P.; Vernikos, J. Dietary salt and urinary calcium excretion in a human bed rest spaceflight model. Aviat. Space Environ. Med. 2000, 71, 1115–1119. [Google Scholar]
- Chan, A.Y.; Poon, P.; Chan, E.L.; Fung, S.L.; Swaminathan, R. The effect of high sodium intake on bone mineral content in rats fed a normal calcium or a low calcium diet. Osteoporos. Int. 1993, 3, 341–344. [Google Scholar] [CrossRef]
- Massey, L.K.; Whiting, S.J. Dietary salt, urinary calcium, and stone risk. Nutr. Rev. 1995, 53, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Heer, M.; Frings-Meuthen, P.; Titze, J.; Boschmann, M.; Frisch, S.; Baecker, N.; Beck, L. Increasing sodium intake from a previous low or high intake affects water, electrolyte and acid-base differently. Br. J. Nutr. 2009, 101, 1286–1294. [Google Scholar] [CrossRef]
- Frings-Meuthen, P.; Baecker, N.; Heer, M. Low-grade metabolic acidosis may be the cause of sodium chloride-induced exaggerated bone resorption. J. Bone Miner. Res. 2008, 23, 517–524. [Google Scholar] [CrossRef]
- Bergouignan, A.; Momken, I.; Schoeller, D.A.; Simon, C.; Blanc, S. Metabolic fate of saturated and monounsaturated dietary fats: The Mediterranean diet revisited from epidemiological evidence to cellular mechanisms. Prog. Lipid Res. 2009, 48, 128–147. [Google Scholar] [CrossRef]
- Bergouignan, A.; Schoeller, D.A.; Normand, S.; Gauquelin-Koch, G.; Laville, M.; Shriver, T.; Desage, M.; Maho, Y.L.; Ohshima, H.; Gharib, C.; et al. Effect of physical inactivity on the oxidation of saturated and monounsaturated dietary fatty acids: Results of a randomized trial. PLoS Clin. Trials 2006, 1, e27. [Google Scholar] [CrossRef]
- Bergouignan, A.; Trudel, G.; Simon, C.; Chopard, A.; Schoeller, D.A.; Momken, I.; Votruba, S.B.; Desage, M.; Burdge, G.C.; Gauquelin-Koch, G.; et al. Physical inactivity differentially alters dietary oleate and palmitate trafficking. Diabetes 2009, 58, 367–376. [Google Scholar] [CrossRef]
- Servais, S.; Letexier, D.; Favier, R.; Duchamp, C.; Desplanches, D. Prevention of unloading-induced atrophy by vitamin E supplementation: Links between oxidative stress and soleus muscle proteolysis? Free Radic. Biol. Med. 2007, 42, 627–635. [Google Scholar] [CrossRef]
- Griel, A.E.; Kris-Etherton, P.M.; Hilpert, K.F.; Zhao, G.; West, S.G.; Corwin, R.L. An increase in dietary n-3 fatty acids decreases a marker of bone resorption in humans. Nutr. J. 2007, 6, 1–8. [Google Scholar] [CrossRef]
- Watkins, B.A.; Li, Y.; Lippman, H.E.; Feng, S. Modulatory effect of omega-3 polyunsaturated fatty acids on osteoblast function and bone metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 387–398. [Google Scholar] [CrossRef]
- Turner, N.D.; Braby, L.A.; Ford, J.; Lupton, J.R. Opportunities for nutritional amelioration of radiation-induced cellular damage. Nutrition 2002, 18, 904–912. [Google Scholar] [CrossRef]
- Davidson, L.A.; Nguyen, D.V.; Hokanson, R.M.; Callaway, E.S.; Isett, R.B.; Turner, N.D.; Dougherty, E.R.; Wang, N.; Lupton, J.R.; Carroll, R.J.; et al. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Res. 2004, 64, 6797–6804. [Google Scholar] [CrossRef] [PubMed]
- Chapkin, R.S.; Davidson, L.A.; Ly, L.; Weeks, B.R.; Lupton, J.R.; McMurray, D.N. Immunomodulatory effects of (n-3) fatty acids: Putative link to inflammation and colon cancer. J. Nutr. 2007, 137, 200S–204S. [Google Scholar] [CrossRef]
- Hong, M.Y.; Bancroft, L.K.; Turner, N.D.; Davidson, L.A.; Murphy, M.E.; Carroll, R.J.; Chapkin, R.S.; Lupton, J.R. Fish oil decreases oxidative DNA damage by enhancing apoptosis in rat colon. Nutr. Cancer 2005, 52, 166–175. [Google Scholar] [CrossRef]
- Sanders, L.M.; Henderson, C.E.; Hong, M.Y.; Sanders, L.M.; Henderson, C.E.; Hong, M.Y.; Barhoumi, R.; Burghardt, R.C.; Wang, N.; Spinka, C.M.; et al. An increase in reactive oxygen species by dietary fish oil coupled with the attenuation of antioxidant defenses by dietary pectin enhances rat colonocyte apoptosis. J. Nutr. 2004, 134, 3233–3238. [Google Scholar] [CrossRef] [PubMed]
- Vanamala, J.; Glagolenko, A.; Yang, P.; Carroll, R.J.; Murphy, M.E.; Newman, R.A.; Ford, J.R.; Braby, L.A.; Chapkin, R.S.; Turner, N.D.; et al. Dietary fish oil and pectin enhance colonocyte apoptosis in part through suppression of PPARd/PGE2 and elevation of PGE. Carcinogenesis 2008, 29, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, J.; Seddon, R. Eating in space: From an astronaut’s perspective. Nutrition 2002, 18, 921–925. [Google Scholar] [CrossRef]
- Zwart, S.R.; Kloeris, V.L.; Perchonok, M.H.; Braby, L.; Smith, S.M. Assessment of nutrient stability in foods from the space food system after long-duration spaceflight on the ISS. J. Food Sci. 2009, 74, H209–H217. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M. Effect of processing and subsequent storage on nutrition [Internet]. NASA Human Research Program Investigators’ Workshop: Houston, TX, USA. Available online: http://www.dsls.usra.edu/meetings/hrp2010/pdf/Friday/Cooper.pdf (accessed on 1 November 2020).
- Beltifa, A.; Feriani, A.; Machreki, M.; Ghorbel5, A.; Ghazouani, L.; di Bella, G.; van Loco, J.; Reyns, T.; Mansour, H.B. Plasticizers and bisphenol A, in packaged foods sold in the Tunisian markets: Study of their acute in vivo toxicity and their environmental fate. Environ. Sci. Pollut. Res. 2017, 24, 22382–22392. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Tayama, S. Metabolism and cytotoxicity of bisphenol A and other bisphenol in isolated rat hepatocytes. Arch. Toxicol. 2000, 74, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Mourad, I.M.; Khadrawy, Y.A. The sensitivity of liver, kidney and testis of rats to oxidative stress induced by different doses of bisphenol. Life. 2012, 50, 19–28. [Google Scholar]
- Moon, M.K.; Kim, M.J.; Jug, I.K.; Koo, Y.D.; Ann, H.Y.; Lee, K.J.; Kim, S.H.; Yoon, Y.C.; Ch, B.J.; Park, K.S.; et al. Bisphenol A impairs mitochondrial function in the liver at doses below the n observed adverse effect level. J. Korean Med. Sci. 2012, 27, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Sangai, N.P.; Verma, R.J.; Trivedi, M.H. Testing the efficacity of quercetin in mitigating bisphenol A toxicity in liver and kidney of mice. Toxicol. Ind. Health 2012, 30, 581–597. [Google Scholar] [CrossRef]
- Kavlock, R.; Boekelheide, K.; Chapin, R.; Cunningham, M.; Faustman, E.; Foster, P.; Golub, M.; Henderson, R.; Hinberg, I.; Little, R.; et al. NTP center for the evaluation of risks to human reproduction: Phthalates expert panel report on the reproductive and developmental toxicity of di-nbutyl phthalate. Reprod. Toxicol. 2002, 16, 489–527. [Google Scholar] [CrossRef]
- Fromme, H.; Gruber, L.; Schlummer, M.; Wolz, G.; Boehmer, S.; Angerer, J.; Mayer, R.; Liebi, B.; Bolte, G. Intake of phthalates and di (2-ethylhexyl) adipate: Results of the integrated exposure assessment survey based on duplicate diet samples and bio monitoring data. Environ. Int. 2007, 33, 1012–1020. [Google Scholar] [CrossRef]
- Goulas, A.E.; Zygoura, P.; Karatapanis, A.; Georgantelis, D.; Kontominas, M.G. Migration of di(2-ethylhexyl) adipate and acetyltributyl citrate plasticizers from food-grade PVC film into sweetened sesame paste (halawa tehineh): Kinetic and penetration study. Food Chem. Toxicol. 2007, 45, 585–591. [Google Scholar] [CrossRef]
- Ming, H.R.; Ping, C.Z.; Yi, X.X.; Ran, C.H.; Hong, Z. The dissolution of zinc, lead, cadmium and arsenic in food packaging aluminum foil was determined by ICP-AES. Chem. Anal. Meterage 2014, 23, 54–56. [Google Scholar] [CrossRef]
- Zhiying, T.; Zhaoyong, H.; Guanglin, C.; Xiaochuan, S. Research progress on food heavy metal contamination. Guangxi Prev. Med. 2003, 9, 5–18. [Google Scholar]
- Singh, P.; Abas Wani, A.; Saengerlaub, S. Active packaging of food products: Recent trends. J. Nutr. Food. Sci. 2011, 41, 249–260. [Google Scholar] [CrossRef]
- Gerez, C.L.; Torres, M.J.; Font de Valdez, G.; Roll’an, G. Control of spoilage fungi by lactic acid bacteria. Biol. Control. 2013, 64, 231–237. [Google Scholar] [CrossRef]
- Pereira de Abreu, D.A.; Cruz, J.M.; Losada, P.P. Active and intelligent packaging for the food industry. Food Rev. Intl. 2012, 28, 146–187. [Google Scholar] [CrossRef]
- Ohlsson, T.; Bengtsson, N. Minimal Processing Technologies in the Food Industry; Woodhead Publishing Ltd.: Cambridge, UK, 2002. [Google Scholar]
- Rodriguez-Aguilera, R.; Oliveira, J.C. Review of design engineering methods and applications of active and modified atmosphere packaging systems. Food Eng. Rev. 2009, 1, 66–83. [Google Scholar] [CrossRef]
- Sandhya. Modified atmosphere packaging of fresh produce: Current status and future needs. LWT-Food Sci. Technol. 2010, 43, 381–392. [Google Scholar] [CrossRef]
- Zhuang, H.; Barth, M.M.; Cisneros-Zevallos, L. Modified atmosphere packaging for fresh fruits and vegetables. In Innovations in Food Packaging, 2nd ed.; Han, J.H., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 445–473. [Google Scholar]
- Yildirim, S. Active packaging for food biopreservation. In Protective Cultures, Antimicrobial Metabolites and Bacteriophages for Food and Beverage Biopreservation; Arvanitoyannis, I.S., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2011; pp. 460–489. [Google Scholar]
- Janjarasskul, T.; Suppakul, P. Active and intelligent packaging: The indication of quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 808–831. [Google Scholar] [CrossRef]
- Dobrucka, R.; Cierpiszewski, R. Active and intelligent packaging food–Research and development—A review. Pol. J. Food Nutr. Sci. 2014, 64, 7–15. [Google Scholar] [CrossRef]
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Kuorwel, K.K.; Cran, M.J.; Orbell, J.D.; Buddhadasa, S.; Bigger, S.W. Review of mechanical properties, migration, and potential applications in active food packaging systems containing nanoclays and nanosilver. Comp. Rev. Food Sci. Food Saf. 2015, 14, 411–430. [Google Scholar] [CrossRef]
- Brockgreitens, J.; Abbas, A. Responsive food packaging: Recent progress and technological prospects. Comp. Rev. Food Sci. Food Saf. 2016, 15, 3–15. [Google Scholar] [CrossRef]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An overview of the intelligent packaging technologies in the food sector. Trends Food Sci. Techol. 2016, 51, 1–11. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent packaging in the food sector: A brief overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Srinivasa Gopal, T.K. Smart packaging systems for food applications: A review. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef]
- Imran, M.; Revol-Junelles, A.-M.; Martyn, A.; Tehrany, E.A.; Jacquot, M.; Linder, M.; Desobry, S. Active food packaging evolution: Transformation from micro- to nanotechnology. Crit. Rev. Food Sci. Nutr. 2010, 50, 799–821. [Google Scholar] [CrossRef]
- Llorens, A.; Lloret, E.; Picouet, P.A.; Trbojevich, R.; Fernandez, A. Metallic-based micro and nanocomposites in food contact materials and active food packaging. Trends Food Sci. Technol. 2012, 24, 19–29. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Reig, C.S.; Lopez, A.D.; Ramos, M.H.; Ballester, V.A.C. Nanomaterials: A map for their selection in food packaging applications. Packag. Technol. Sci. 2014, 27, 839–866. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Choi, J.; Ko, S. Applications of nanomaterials in food packaging. J. Nanosci. Nanotechnol. 2015, 15, 6357–6372. [Google Scholar] [CrossRef]
- Yildirim, S.; Rocker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Inst. Food Technol. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Suppakul, P.; Miltz, J.; Sonneveld, K.; Bigger, S.W. Active packaging technologies with an emphasis on antimicrobial packaging and its applications. J. Food Sci. 2003, 68, 408–420. [Google Scholar] [CrossRef]
- Van Long, N.N.; Joly, C.; Dantigny, P. Active packaging with antifungal activities. Int. J. Food Microbiol. 2016, 220, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Munevar, G. Space exploration and human survival. Space Policy 2014, 30, 197–201. [Google Scholar] [CrossRef]
- Smith, S.M.; Abrams, S.A.; Davis-Street, J.E.; Heer, M.; O’Brien, K.O.; Wastney, M.E.; Zwart, S.R. Fifty years of human space travel: Implications for bone and calcium research. Annu. Rev. Nutr. 2014, 34, 377–400. [Google Scholar] [CrossRef]
- Cucinotta, F.A. Space radiation risks for astronauts on multiple International Space Station missions. PLoS ONE 2014, 9, e96099. [Google Scholar] [CrossRef]
- Koga, K.; Iwasaki, Y. Psychological and physiological effect in humans of touching plant foliage-using the semantic differential method and cerebral activity as indicators. J. Physiol. Anthropol. 2013, 32, 7. [Google Scholar] [CrossRef]
- Douglas, G.L.; Zwart, S.R.; Smith, S.M. Space food for thought: Challenges and considerations for food and nutrition on exploration missions. J. Nutr. 2020, 150, 2242–2244. [Google Scholar] [CrossRef] [PubMed]
- Mauerer, M.; Schubert, D.; Zabel, P.; Bamsey, M.; Kohlberg, E.; Mengedoht, D. Initial survey on fresh fruit and vegetable preferences of Neumayer Station crew members: Input to crop selection and psychological benifits of space-based plant production systems. Open Agric. 2016, 1, 179–188. [Google Scholar] [CrossRef]
- Perchonok, M. Bulk Ingredient-Based Menu Development [Internet]. Washington, D.C.: NASA Advanced Capabilities Division Research & Technology Task Book. Available online: http://taskbook.nasaprs.com/Publication/index.cfm?action=public_query_taskbook_content&TASKID=5438 (accessed on 11 January 2019).
- Xiao, M.; Reddi, L.N.; Steinberg, S.L. Discontinuous pore fluid distribution under microgravity due to particle rearrangement. In Multiscale and Multiphysics Processes in Geomechanics; Springer: Berlin/Heidelberg, Germany, 2011; pp. 65–68. [Google Scholar]
- Montesinos, C.A.; Khalid, R.; Cristea, O.; Greenberger, J.S.; Epperly, M.W.; Lemon, J.A.; Boreham, D.R.; Popov, D.; Gorthi, G.; Ramkumar, N.; et al. Space radiation protection countermeasures in microgravity and planetary exploration. Life 2021, 11, 829. [Google Scholar] [CrossRef] [PubMed]
- Oluwafemi, F.A.; de la Torre, A.; Afolayan, E.M.; Olalekan-Ajayi, B.M.; Dhital, B.; Mora-Almanza, J.G.; Potrivitu, G.; Creech, J.; Rivolta, A. Space Food and Nutrition in a Long-Term Manned Mission. Adv. Astronaut. Sci. Technol. 2018, 1, 1–21. [Google Scholar] [CrossRef]
- Rickard, C.P.; Bode, R.F. Sporobolus airoides as a Pioneer Plant for Lunar Regolith. In Earth and Space; ASCE: Reston, VA, USA, 2021; pp. 211–221. [Google Scholar]
- Voit, D.C.; Santos, M.R.; Singh, R.P. Development of a multipurpose fruit and vegetable processor for a manned mission to Mars. J. Food Eng. 2006, 77, 230–238. [Google Scholar] [CrossRef]
- Ralphs, M.; Franz, B.; Baker, T.; Howe, S. Water extraction on Mars for an expanding human colony. Life Sci. Space Res. 2015, 7, 57–60. [Google Scholar] [CrossRef]
- Kliss, M.; Heyenga, A.G.; Hoehn, A.; Stodieck, L.S. Recent advances in technologies required for a “Salad Machine”. Adv. Space Res. 2000, 26, 263–269. [Google Scholar] [CrossRef]
- Odeh, R.; Guy, C.L. Gardening for therapeutic people-plant interactions during long-duration space missions. Open Agric. 2017, 2, 1–13. [Google Scholar] [CrossRef]
- Carillo, P.; Morrone, B.; Fusco, G.M.; De Pascale, S.; Rouphael, Y. Challenges for a sustainable food production system on board of the international space station: A technical review. Agronomy 2020, 10, 687. [Google Scholar] [CrossRef]
- Khodadad, C.L.; Hummerick, M.E.; Spencer, L.E.; Dixit, A.R.; Richards, J.T.; Romeyn, M.W.; Smith, T.M.; Wheeler, R.M.; Massa, G.D. Microbiological and nutritional analysis of lettuce crops grown on the international space station. Front. Plant Sci. 2020, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Massa, G.D.; Newsham, G.; Hummerick, M.E.; Morrow, R.C.; Wheeler, R.M. Plant pillow preparation for the veggie plant growth system on the international space station. Gravit. Space Res. 2017, 5. [Google Scholar] [CrossRef]
- Massa, G.D.; Dufour, N.F.; Carver, J.A.; Hummerick, M.E.; Wheeler, R.M.; Morrow, R.C.; Smith, T.M. VEG-01: Veggie hardware validation testing on the International Space Station. Open Agric. 2017, 2, 33–41. [Google Scholar] [CrossRef]
- Tibbetts, J.H. Gardening of the Future—From Outer to Urban Space: Moving from freeze-dried ice cream to fresh-picked salad greens. Bio Sci. 2019, 69, 962–968. [Google Scholar] [CrossRef]
- Horneck, G.; Klaus, D.M.; Mancinelli, R.L. Space microbiology. Microbiol. Mol. Biol. Rev. 2010, 74, 121–156. [Google Scholar] [CrossRef] [PubMed]
- Massa, G.; Romeyn, M.; Fritsche, R. Future Food Production System Development Pulling from Space Biology Crop Growth Testing in Veggie. 2017. Available online: https://pdfs.semanticscholar.org/d208/26e86ed6962fa653be89e083099c9e6afad1.pdf (accessed on 1 August 2021).
- Zabel, P.; Bamsey, M.; Schubert, D.; Tajmar, M. Review and analysis of over 40 years of space plant growth systems. Life Sci. Space Res. 2016, 10, 1–16. [Google Scholar] [CrossRef]
- Escobar, C.; Escobar, A. Duckweed: A tiny aquatic plant with enormous potential for bioregenerative life support systems. In Proceedings of the 47th International Conference on Environmental Systems, Charleston, SC, USA, 16–20 July 2017. [Google Scholar]
- Oze, C.; Beisel, J.; Dabsys, E.; Dall, J.; North, G.; Scott, A.; Lopez, A.M.; Holmes, R.; Fendorf, S. Perchlorate and Agriculture on Mars. Soil Syst. 2021, 5, 37. [Google Scholar] [CrossRef]
- Eichler, A.; Hadland, N.; Pickett, D.; Masaitis, D.; Handy, D.; Perez, A.; Batcheldor, D.; Wheeler, B.; Palmer, A. Challenging the agricultural viability of Martian regolith simulants. Icarus 2021, 354, 114022. [Google Scholar] [CrossRef]
- Ming, D.W.; Henninger, D.L. Use of lunar regolith as a substrate for plant growth. Adv. Space Res. 1994, 14, 435–443. [Google Scholar] [CrossRef]
- Biolo, G.; Heer, M.; Narici, M.; Strollo, F. Microgravity as a model of ageing. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 31. [Google Scholar] [CrossRef]
- Millet, C.; Custaud, M.A.; Maillet, A.; Allevard, A.M.; Duvareille, M.; Gauquelin-Koch, G.; Gharib, C.; Fortrat, J.O. Endocrine responses to 7 days of head-down bed rest and orthostatic tests in men and women. Clin. Physiol. 2001, 21, 172–183. [Google Scholar] [CrossRef]
- Marti, O.; Marti, J.; Armario, A. Effects of chronic stress on food intake in rats: Influence of stressor intensity and duration of daily exposure. Physiol. Behav. 1994, 55, 747–753. [Google Scholar] [CrossRef]
- Ans, A.H.; Anjum, I.; Satija, V.; Inayat, A.; Asghar, Z.; Akram, I.; Shrestha, B. Neurohormonal regulation of appetite and its relationship with stress: A mini literature review. Cureus 2018, 10. [Google Scholar] [CrossRef]
- Carbone, L.D.; Bush, A.J.; Barrow, K.D.; Kang, A.H. The relationship of sodium intake to calcium and sodium excretion and bone mineral density of the hip in postmenopausal African-American and Caucasian women. J. Bone Miner. Metab. 2003, 21, 415–420. [Google Scholar] [CrossRef]
- Massey, L.K.; Whiting, S.J. Dietary salt, urinary calcium, and bone loss. J. Bone Miner. Res. 1996, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Catauro, P.M.; Perchonok, M.H. Assessment of the longterm stability of retort pouch foods to support extended duration spaceflight. J. Food Sci. 2012, 77, S29–S39. [Google Scholar] [CrossRef] [PubMed]
- Nura, A. Advances in food packing technology-a review. J. Postharvest. Tecnol. 2018, 6, 55–64. [Google Scholar]
- Wormuth, M.; Scheringer, M.; Vollenweider, M.; Hungerbuchler, K. What are the sources of exposure to eight frequently used phtalic acid esters in Europeans? Risk. Anal. 2006, 26, 803–824. [Google Scholar] [CrossRef] [PubMed]
- Venir, E.; Del Torre, M.; Stecchini, M.L.; Maltini, E.; Di Nardo, P. Preparation of freeze-dried yoghurt as a space food. J. Food Eng. 2007, 80, 402–407. [Google Scholar] [CrossRef]
| Keywords | Reference Numbers |
|---|---|
| Long-term space tasks | [9,10,11,12] |
| Space food systems | [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] |
| Diet menu fatigue | [31,45,48,49,50,51] |
| The impact of space environment on astronauts | [14,15,16,17,19,30,45,47,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76] |
| Dietary and nutrition deficiencies | [30,77,78,79,80,81,82,83,84] |
| Microgravity | [45,85,86,87,88,89] |
| Space radiation | [90,91,92,93,94] |
| Weight loss | [45,53,78,79,80,81,83,95,96,97,98,99,100,101,102] |
| Bone loss | [13,14,15,47,53,99,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117] |
| Nutritional strategies | [30,31,47,53,69,81,83,84,103,107,111,118,119,120,121,122,123,124,125,126,127,128,129,130,131] |
| Reduce sodium intake | [53,55,83,109,132,133,134,135,136,137,138] |
| Fatty acid | [120,139,140,141,142,143,144,145,146,147,148,149,150] |
| Nutrient loss during food processing and storage | [31,38,84,151,152,153] |
| Security threat of packaging materials and food additives | [75,103,154,155,156,157,158,159,160,161,162,163,164,165] |
| New packaging technology | [163,166,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187] |
| Fresh food materials | [42,151,188,189,190,191,192,193,194] |
| Self-sufficient | [20,21,22,31,195,196,197] |
| Space habitats | [197,198,199,200] |
| Space food production | [201,202,203,204,205,206,207,208,209,210,211,212,213,214] |
| Chinese Databases | English Databases | |
|---|---|---|
| Wanfang Medical Network | EBSCO | |
| X-MOL Information Retrieval | Web of Science (SCIE) | |
| CQVIP Chinese Journal | OVID and CAB Plus Full-Text | |
| CQVIP Chinese Biomedical Journal | PROQUEST Agriculture and Biology | |
| CNKI Citation | Springer Link Full-Text | |
| BvD JSTOR The Merk Index | Oxford Journals Collection | |
| Doc88.com Literature Sharing Platform * | Kopernio Chrome | |
| Issues | Nutritional Strategies | Recommended Food and Nutrition | |
|---|---|---|---|
| 1. Nutritional measures to cope with reduced intake | Increase the appeal of space food | Fresh food with a distinctive flavor | |
| Pay attention to space food culture as a source of joy | Participate in the production, harvesting, cooking, and sharing of fresh food materials with peers to build a sense of belonging | ||
| Meet the astronauts’ carbohydrate preferences | Grow fresh vegetables and food in space to meet astronauts’ demand for large amounts of food | ||
| Add foods with high energy density | Nuts | ||
| 2. Nutritional measures to cope with decreased immune function after weight loss | Supplement VB6 | Yeast, wheat bran, malt, liver and kidneys, rice, potatoes, sweet potatoes, vegetables, carrots, bananas, and peanuts | |
| Supplement VB12 | Shellfish, livers, and all foods derived from animals. Fish, shrimp, eggs, milk, and fermented soy products | ||
| Supplement VE | Nuts, lean meat, milk, eggs, vegetable oil. Wheat germ, green leaves, sweet potato, yam, and kiwi | ||
| Supplement VC | Fresh vegetables and fruits | ||
| Supplement Biotin | Yeast, liver, and kidney. Brown rice, peanut coat, beans, fish, and egg yolk | ||
| Supplement Iron element | Liver, clams, seaweed, fish, shrimp, egg yolk, chicken, beans, green leafy vegetables, and fruits | ||
| Supplement Cuprum | Liver, shellfish, fish, meat (especially poultry), fruits, tomatoes, green peas, potatoes, shellfish, laver, cocoa, and chocolate | ||
| Supplement Selenium | Seafood shellfish, animal viscera, kidneys, and wheat germ | ||
| Supplement Protein | Protein and individual amino acids | ||
| 3. Nutritional measures to cope with the effects of microgravity | Mitigate bone loss | Reduce sodium | Reduce sodium chloride intake to replace stored with fresh food. |
| Add vegetable protein | Increase plant protein: rice noodles, and beans Increase potassium citrate, and supplement high potassium ingredient, such as beans, peanuts, mushrooms, seaweed, and kelp | ||
| Supplement VD | Fish, milk, liver, eggs, mushrooms, and beef | ||
| Supplement VK | Yogurt, alfalfa, egg yolks, fish eggs, algae, carrots, and green leafy vegetables | ||
| Supplement calcium | Milk, beans, fish, shrimp, seaweed, black fungus, seaweed, and sea cucumber | ||
| Prioritize unsaturated Omega-3 fatty acids | Fish, flax, peony seed oil, fruits, and vegetables | ||
| Fight muscle atrophy | Increase protein intake | Food containing branched chain amino acids: fish, shrimp, milk, soy, corn, glutinous rice, and cauliflower. | |
| Intestinal microecology disorder | Supplement probiotics | Yogurt rich in calcium and probiotics | |
| 4.Against radiation | Provides antioxidants to human cells | Natural antioxidants (such as procyanidins), omega-3 fatty acids, VE, VC, and beta carotene, VP, selenium, and dietary fiber in addition to antioxidant-rich food, including tomatoes, garlic, nuts, oats, blueberries, broccoli, salmon, wheat, and green tea | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Rising, H.H.; Majji, M.; Brown, R.D. Long-Term Space Nutrition: A Scoping Review. Nutrients 2022, 14, 194. https://doi.org/10.3390/nu14010194
Tang H, Rising HH, Majji M, Brown RD. Long-Term Space Nutrition: A Scoping Review. Nutrients. 2022; 14(1):194. https://doi.org/10.3390/nu14010194
Chicago/Turabian StyleTang, Hong, Hope Hui Rising, Manoranjan Majji, and Robert D. Brown. 2022. "Long-Term Space Nutrition: A Scoping Review" Nutrients 14, no. 1: 194. https://doi.org/10.3390/nu14010194
APA StyleTang, H., Rising, H. H., Majji, M., & Brown, R. D. (2022). Long-Term Space Nutrition: A Scoping Review. Nutrients, 14(1), 194. https://doi.org/10.3390/nu14010194






