Associations between Taste Perception Profiles and Empirically Derived Dietary Patterns: An Exploratory Analysis among Older Adults with Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Anthropometric and Biochemical Parameters
2.3. Taste Perception Assessment
2.4. Taste Perception Profiles
2.5. Dietary Assessment
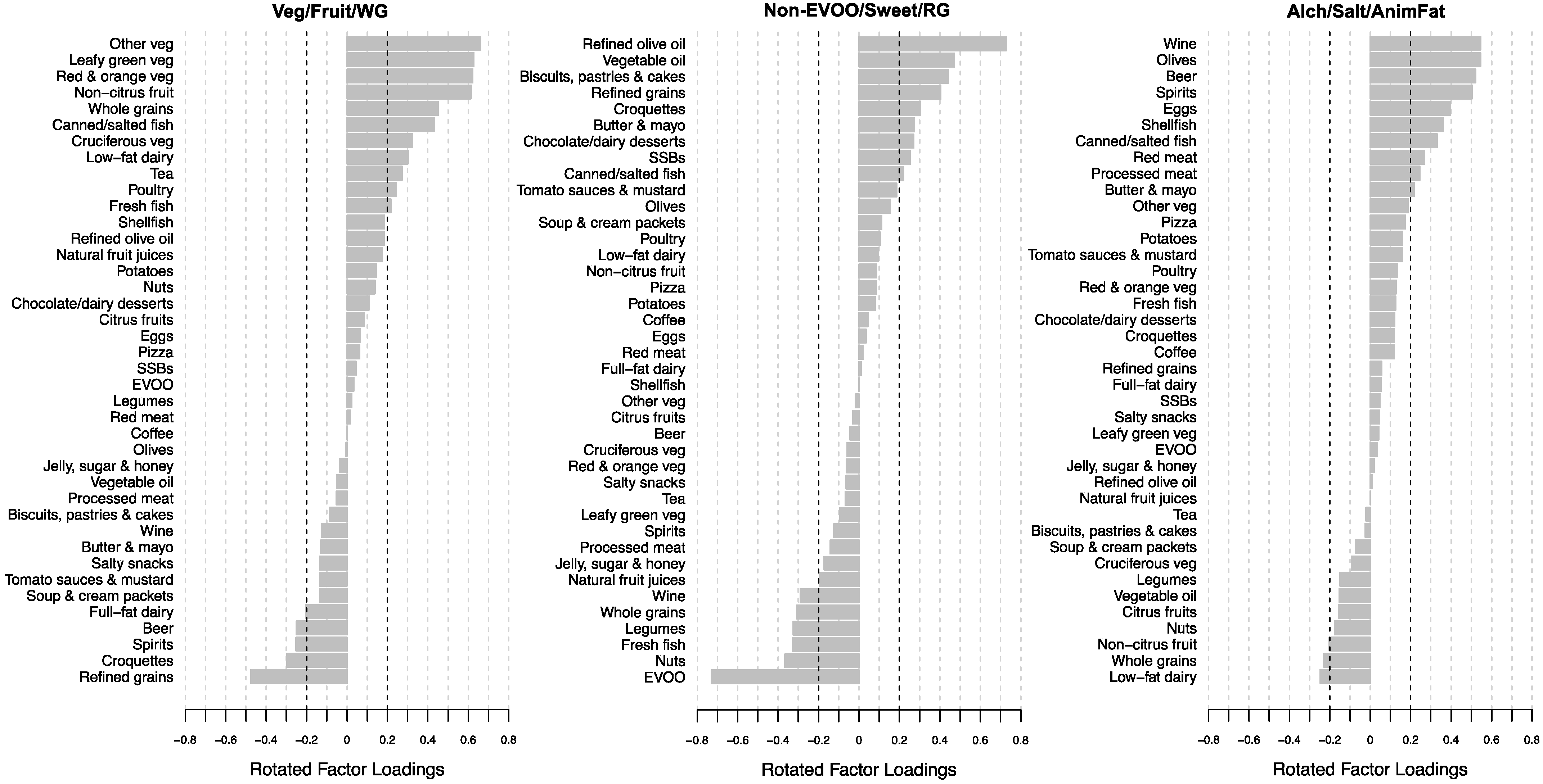
2.6. Empirically Derived Dietary Patterns
2.7. Covariates
2.8. Statistical Analysis
3. Results
3.1. Participant Characteristics by Taste Perception Profile
3.2. Empirically Derived Dietary Patterns
3.3. Participant Characteristics by Level of Adherence to Empirically Derived Dietary Patterns
3.4. Association between Taste Perception Profiles and Empirically Derived Dietary Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Horn, L.; Cornelis, M.C. US Dietary Guidance—Is It Working? JAMA 2019, 322, 1150. [Google Scholar] [CrossRef] [PubMed]
- The US Burden of Disease Collaborators. The State of US Health, 1990–2016: Burden of Diseases, Injuries, and Risk Factors Among US States. JAMA 2018, 319, 1444–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health Effects of Dietary Risks in 195 Countries, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; US Government Publishing Office: Washington, DC, USA, 2020.
- Ordovas, J.M.; Ferguson, L.R.; Tai, E.S.; Mathers, J.C. Personalised Nutrition and Health. BMJ 2018, 361, bmj.k2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bush, C.L.; Blumberg, J.B.; El-Sohemy, A.; Minich, D.M.; Ordovás, J.M.; Reed, D.G.; Behm, V.A.Y. Toward the Definition of Personalized Nutrition: A Proposal by The American Nutrition Association. J. Am. Coll. Nutr. 2020, 39, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Jinnette, R.; Narita, A.; Manning, B.; McNaughton, S.A.; Mathers, J.C.; Livingstone, K.M. Does Personalized Nutrition Advice Improve Dietary Intake in Healthy Adults? A Systematic Review of Randomized Controlled Trials. Adv. Nutr. 2021, 12, 657–669. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celis-Morales, C.; Livingstone, K.M.; Marsaux, C.F.M.; Macready, A.L.; Fallaize, R.; O’Donovan, C.B.; Woolhead, C.; Forster, H.; Walsh, M.C.; Navas-Carretero, S.; et al. Effect of Personalized Nutrition on Health-Related Behaviour Change: Evidence from the Food4me European Randomized Controlled Trial. Int. J. Epidemiol. 2016, 46, 578–588. [Google Scholar] [CrossRef] [Green Version]
- Horne, J.; Madill, J.; O’Connor, C.; Shelley, J.; Gilliland, J. A Systematic Review of Genetic Testing and Lifestyle Behaviour Change: Are We Using High-Quality Genetic Interventions and Considering Behaviour Change Theory? Lifestyle Genom. 2018, 11, 49–63. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Livingstone, K.M.; Moschonis, G. Personalised Nutrition: Updates, Gaps and Next Steps. Nutrients 2019, 11, 1793. [Google Scholar] [CrossRef] [Green Version]
- Breslin, P.A.S. An Evolutionary Perspective on Food Review and Human Taste. Curr. Biol. 2013, 23, R409–R418. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.E.; Feeney, E.L.; Allen, A.L. Do Polymorphisms in Chemosensory Genes Matter for Human Ingestive Behavior? Food Qual. Prefer. 2013, 30, 202–216. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Kennedy, O.B.; Methven, L. Exploring the Effects of Genotypical and Phenotypical Variations in Bitter Taste Sensitivity on Perception, Liking and Intake of Brassica Vegetables in the UK. Food Qual. Prefer. 2016, 50, 71–81. [Google Scholar] [CrossRef]
- Duffy, V.B.; Hayes, J.E.; Davidson, A.C.; Kidd, J.R.; Kidd, K.K.; Bartoshuk, L.M. Vegetable Intake in College-Aged Adults Is Explained by Oral Sensory Phenotypes and TAS2R38 Genotype. Chem. Percept. 2010, 3, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Dinehart, M.E.; Hayes, J.E.; Bartoshuk, L.M.; Lanier, S.L.; Duffy, V.B. Bitter Taste Markers Explain Variability in Vegetable Sweetness, Bitterness, and Intake. Physiol. Behav. 2006, 87, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Shafaie, Y.; Koelliker, Y.; Hoffman, D.J.; Tepper, B.J. Energy Intake and Diet Selection during Buffet Consumption in Women Classified by the 6-n-Propylthiouracil Bitter Taste Phenotype. Am. J. Clin. Nutr. 2013, 98, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, C.; Riso, P.; Laureati, M.; Gargari, G.; Pagliarini, E. Exploring Associations between Interindividual Differences in Taste Perception, Oral Microbiota Composition, and Reported Food Intake. Nutrients 2019, 11, 1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayasinghe, S.N.; Kruger, R.; Walsh, D.C.; Cao, G.; Rivers, S.; Richter, M.; Breier, B.H. Is Sweet Taste Perception Associated with Sweet Food Liking and Intake? Nutrients 2017, 9, 750. [Google Scholar] [CrossRef] [Green Version]
- Veček, N.N.; Mucalo, L.; Dragun, R.; Miličević, T.; Pribisalić, A.; Patarčić, I.; Hayward, C.; Polašek, O.; Kolčić, I. The Association between Salt Taste Perception, Mediterranean Diet and Metabolic Syndrome: A Cross-Sectional Study. Nutrients 2020, 12, 1164. [Google Scholar] [CrossRef]
- Ong, J.-S.; Hwang, L.-D.; Zhong, V.W.; An, J.; Gharahkhani, P.; Breslin, P.A.S.; Wright, M.J.; Lawlor, D.A.; Whitfield, J.; MacGregor, S.; et al. Understanding the Role of Bitter Taste Perception in Coffee, Tea and Alcohol Consumption through Mendelian Randomization. Sci. Rep. 2018, 8, 16414. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.B. Dietary Pattern Analysis: A New Direction in Nutritional Epidemiology. Curr. Opin. Lipidol. 2002, 13, 3. [Google Scholar] [CrossRef]
- Ocké, M.C. Evaluation of Methodologies for Assessing the Overall Diet: Dietary Quality Scores and Dietary Pattern Analysis. Proc. Nutr. Soc. 2013, 72, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Gervis, J.E.; Chui, K.K.H.; Ma, J.; Coltell, O.; Fernández-Carrión, R.; Sorlí, J.V.; Barragán, R.; Fitó, M.; González, J.I.; Corella, D.; et al. Data-Driven Clustering Approach to Derive Taste Perception Profiles from Sweet, Salt, Sour, Bitter, and Umami Perception Scores: An Illustration among Older Adults with Metabolic Syndrome. J. Nutr. 2021, 151, 2843–2851. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J. Toward a Better Understanding of Diet–Taste Relations. J. Nutr. 2021, 151, 2503–2504. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Buil-Cosiales, P.; Corella, D.; Bulló, M.; Fitó, M.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Wärnberg, J.; López-Miranda, J.; et al. Cohort Profile: Design and Methods of the PREDIMED-Plus Randomized Trial. Int. J. Epidemiol. 2019, 48, 387–388o. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coltell, O.; Sorlí, J.V.; Asensio, E.M.; Fernández-Carrión, R.; Barragán, R.; Ortega-Azorín, C.; Estruch, R.; González, J.I.; Salas-Salvadó, J.; Lamon-Fava, S. Association between Taste Perception and Adiposity in Overweight or Obese Older Subjects with Metabolic Syndrome and Identification of Novel Taste-Related Genes. Am. J. Clin. Nutr. 2019, 109, 1709–1723. [Google Scholar] [CrossRef] [PubMed]
- Willett, W. Implications of Total Energy Intake for Epidemiologic Analyses. In Nutritional Epidemiology; Oxford University Press: Oxford, UK, 2012; ISBN 978-0-19-975403-8. [Google Scholar]
- Rosique-Esteban, N.; Díaz-López, A.; Martínez-González, M.A.; Corella, D.; Goday, A.; Martínez, J.A.; Romaguera, D.; Vioque, J.; Arós, F.; Garcia-Rios, A. Leisure-Time Physical Activity, Sedentary Behaviors, Sleep, and Cardiometabolic Risk Factors at Baseline in the PREDIMED-PLUS Intervention Trial: A Cross-Sectional Analysis. PLoS ONE 2017, 12, e0172253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Martín-Moreno, J.M. Relative Validity of a Semi-Quantitative Food-Frequency Questionnaire in an Elderly Mediterranean Population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for Total Energy Intake in Epidemiologic Studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Schwedhelm, C.; Iqbal, K.; Knüppel, S.; Schwingshackl, L.; Boeing, H. Contribution to the Understanding of How Principal Component Analysis–Derived Dietary Patterns Emerge from Habitual Data on Food Consumption. Am. J. Clin. Nutr. 2018, 107, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Thorpe, M.G.; Milte, C.M.; Crawford, D.; McNaughton, S.A. A Comparison of the Dietary Patterns Derived by Principal Component Analysis and Cluster Analysis in Older Australians. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 30. [Google Scholar] [CrossRef] [Green Version]
- Schulze, M.B.; Hoffmann, K.; Kroke, A.; Boeing, H. An Approach to Construct Simplified Measures of Dietary Patterns from Exploratory Factor Analysis. Br. J. Nutr. 2003, 89, 409–418. [Google Scholar] [CrossRef]
- Molina, L.; Sarmiento, M.; Peñafiel, J.; Donaire, D.; Garcia-Aymerich, J.; Gomez, M.; Ble, M.; Ruiz, S.; Frances, A.; Schröder, H. Validation of the Regicor Short Physical Activity Questionnaire for the Adult Population. PLoS ONE 2017, 12, e0168148. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Rimm, E.B.; Spiegelman, D.; Rifai, N.; Tofler, G.H.; Willett, W.C.; Hu, F.B. Association between Dietary Patterns and Plasma Biomarkers of Obesity and Cardiovascular Disease Risk. Am. J. Clin. Nutr. 2001, 73, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Aranceta-Bartrina, J.; Partearroyo, T.; López-Sobaler, A.M.; Ortega, R.M.; Varela-Moreiras, G.; Serra-Majem, L.; Pérez-Rodrigo, C.; Collaborative Group for the Dietary Guidelines for the Spanish Population (SENC). Updating the Food-Based Dietary Guidelines for the Spanish Population: The Spanish Society of Community Nutrition (SENC) Proposal. Nutrients 2019, 11, 2675. [Google Scholar] [CrossRef] [Green Version]
- Puputti, S.; Hoppu, U.; Sandell, M. Taste Sensitivity Is Associated with Food Consumption Behavior but Not with Recalled Pleasantness. Foods 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjöstrand, A.E.; Sjödin, P.; Hegay, T.; Nikolaeva, A.; Shayimkulov, F.; Blum, M.G.; Heyer, E.; Jakobsson, M. Taste Perception and Lifestyle: Insights from Phenotype and Genome Data among Africans and Asians. Eur. J. Hum. Genet. 2020, 29, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Barragán, R.; Coltell, O.; Portolés, O.; Asensio, E.M.; Sorlí, J.V.; Ortega-Azorín, C.; González, J.I.; Sáiz, C.; Fernández-Carrión, R.; Ordovas, J.M.; et al. Bitter, Sweet, Salty, Sour and Umami Taste Perception Decreases with Age: Sex-Specific Analysis, Modulation by Genetic Variants and Taste-Preference Associations in 18 to 80 Year-Old Subjects. Nutrients 2018, 10, 1539. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Perez, C.; San-Cristobal, R.; Guallar-Castillon, P.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Castañer, O.; Martinez, J.A.; Alonso-Gómez, Á.M.; Wärnberg, J.; et al. Use of Different Food Classification Systems to Assess the Association between Ultra-Processed Food Consumption and Cardiometabolic Health in an Elderly Population with Metabolic Syndrome (PREDIMED-Plus Cohort). Nutrients 2021, 13, 2471. [Google Scholar] [CrossRef] [PubMed]
- Blondin, S.A.; Mueller, M.P.; Bakun, P.J.; Choumenkovitch, S.F.; Tucker, K.L.; Economos, C.D. Cross-Sectional Associations between Empirically-Derived Dietary Patterns and Indicators of Disease Risk among University Students. Nutrients 2016, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Northstone, K.; Ness, A.; Emmett, P.; Rogers, I. Adjusting for Energy Intake in Dietary Pattern Investigations Using Principal Components Analysis. Eur. J. Clin. Nutr. 2008, 62, 931–938. [Google Scholar] [CrossRef] [Green Version]
- Markussen, M.S.; Veierød, M.B.; Ursin, G.; Andersen, L.F. The Effect of Under-Reporting of Energy Intake on Dietary Patterns and on the Associations between Dietary Patterns and Self-Reported Chronic Disease in Women Aged 50–69 Years. Br. J. Nutr. 2016, 116, 547–558. [Google Scholar] [CrossRef] [Green Version]
- Low, J.Y.Q.; Lacy, K.E.; McBride, R.; Keast, R.S.J. The Association between Sweet Taste Function, Anthropometry, and Dietary Intake in Adults. Nutrients 2016, 8, 241. [Google Scholar] [CrossRef] [Green Version]
- Mattes, R.D. Reliability of Psychophysical Measures of Gustatory Function. Percept. Psychophys. 1988, 43, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Griep, M.; Borg, E.; Collys, K.; Massart, D. Category Ratio Scale as an Alternative to Magnitude Matching for Age-Related Taste and Odour Perception. Food Qual. Prefer. 1998, 9, 67–72. [Google Scholar] [CrossRef]
- Genick, U.K.; Kutalik, Z.; Ledda, M.; Destito, M.C.; Souza, M.M.; Cirillo, C.A.; Godinot, N.; Martin, N.; Morya, E.; Sameshima, K.; et al. Sensitivity of genome-wide-association signals to phenotyping strategy: The PROP-TAS2R38 taste association as a benchmark. PLoS ONE 2011, 6, e27745, Erratum in PLoS ONE 2015, 10, e0122424. [Google Scholar] [CrossRef] [Green Version]
- Ledda, M.; Kutalik, Z.; Souza Destito, M.C.; Souza, M.M.; Cirillo, C.A.; Zamboni, A.; Martin, N.; Morya, E.; Sameshima, K.; Beckmann, J.S.; et al. GWAS of human bitter taste perception identifies new loci and reveals additional complexity of bitter taste genetics. Hum. Mol. Genet. 2014, 23, 259–267. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Miller, I.J. PTC/PROP Tasting: Anatomy, Psychophysics, and Sex Effects. Physiol. Behav. 1994, 56, 1165–1171. [Google Scholar] [CrossRef]
- Duffy, V.B.; Davidson, A.C.; Kidd, J.R.; Kidd, K.K.; Speed, W.C.; Pakstis, A.J.; Reed, D.R.; Snyder, D.J.; Bartoshuk, L.M. Bitter Receptor Gene (TAS2R38), 6-n-propylthiouracil (PROP) Bitterness and Alcohol Intake. Alcohol. Clin. Exp. Res. 2004, 28, 1629–1637. [Google Scholar] [CrossRef] [Green Version]
- Hansen, J.L.; Reed, D.R.; Wright, M.J.; Martin, N.G.; Breslin, P.A.S. Heritability and Genetic Covariation of Sensitivity to PROP, SOA, Quinine HCl, and Caffeine. Chem. Senses 2006, 31, 403–413. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.E.; Bartoshuk, L.M.; Kidd, J.R.; Duffy, V.B. Supertasting and PROP Bitterness Depends on More than the TAS2R38 Gene. Chem. Senses 2008, 33, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Running, C.A.; Craig, B.A.; Mattes, R.D. Oleogustus: The Unique Taste of Fat. Chem. Senses 2015, 40, 507–516. [Google Scholar] [CrossRef] [Green Version]
- Keast, R.S.; Costanzo, A. Is Fat the Sixth Taste Primary? Evidence and Implications. Flavour 2015, 4, 5. [Google Scholar] [CrossRef] [Green Version]
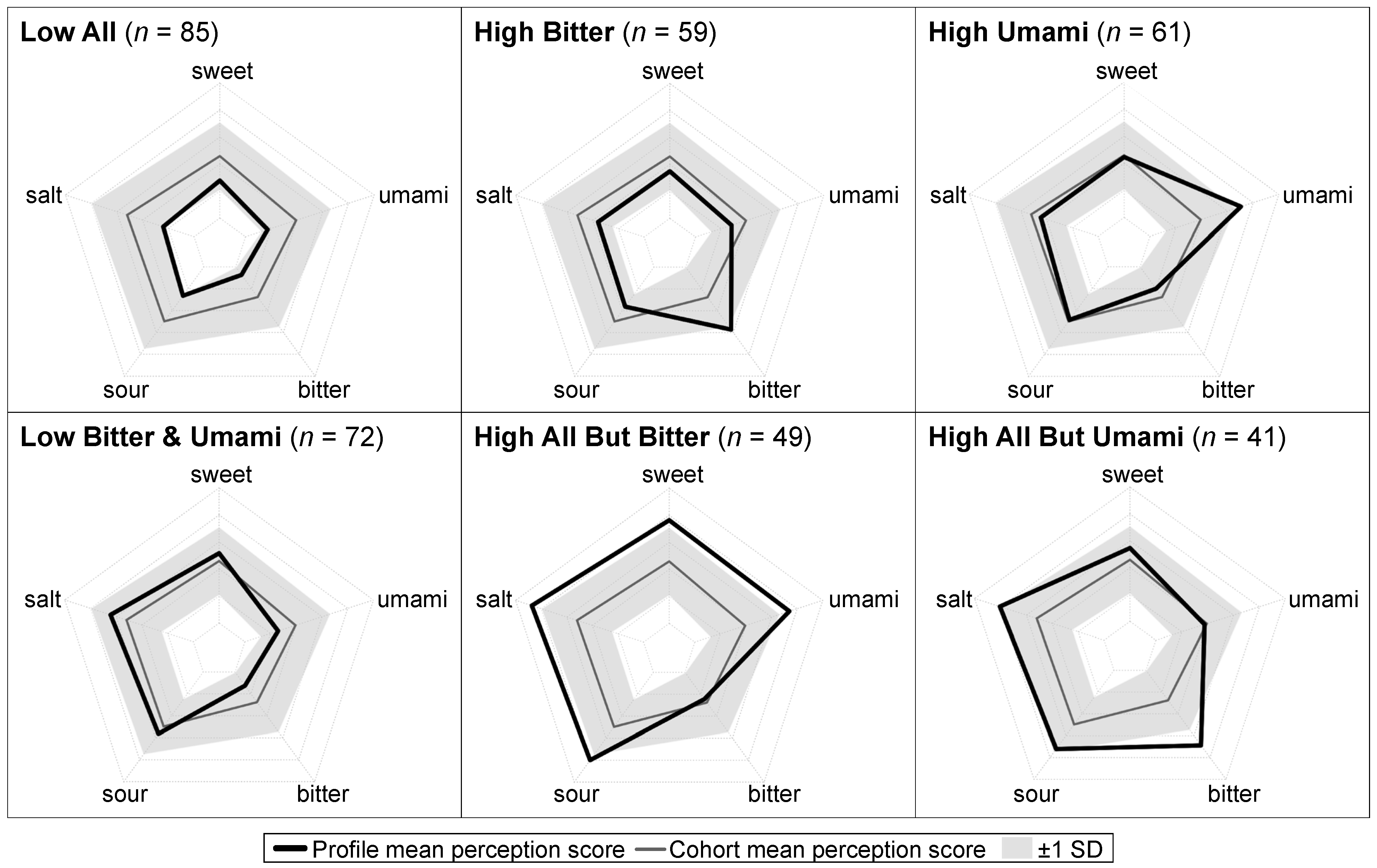

| Overall | Taste Perception Profiles 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low All | High Bitter | High Umami | Low Bitter and Umami | High All But Bitter | High All But Umami | p | |||
| n (%) | 367 | 85 (23) | 59 (16) | 61 (17) | 72 (20) | 49 (13) | 41 (11) | – | |
| Female | 202 (55) | 36 (42) | 30 (51) | 33 (54) | 42 (58) | 34 (69) | 27 (66) | 0.031 | |
| Age (years) | 65 ± 4.7 | 64.5 ± 4.5 | 65.4 ± 4.9 | 64.1 ± 4.6 | 65.2 ± 4.6 | 66.2 ± 4.9 | 65.3 ± 4.5 | 0.217 | |
| BMI (kg/m2) | 32.3 ± 3.6 | 33.2 ± 3.8 | 32 ± 3.3 | 32.4 ± 3.9 | 32.1 ± 3.5 | 31.4 ± 3.2 | 32.4 ± 3.6 | 0.106 | |
| Waist circumference (cm) | |||||||||
| Females | 102 ± 9 | 103 ± 9 | 103 ± 9 | 101 ± 11 | 104 ± 8 | 101 ± 8 | 100 ± 9 | 0.508 | |
| Males | 111 ± 9 | 113 ± 9 | 109 ± 7 | 113 ± 9 | 109 ± 9 | 109 ± 7 | 112 ± 10 | 0.095 | |
| Fasting glucose (mmol/L) 3 | 6.5 ± 1.8 | 6.5 ± 1.3 | 6.3 ± 1.6 | 6.4 ± 1.4 | 6.5 ± 2.5 | 7.0 ± 2.2 | 6.1 ± 1.4 | 0.241 | |
| SBP (mmHg) 3 | 140 ± 17 | 142 ± 17 | 139 ± 17 | 141 ± 19 | 138 ± 11 | 140 ± 19 | 143 ± 18 | 0.457 | |
| DBP (mmHg) | 80 ± 9 | 80 ± 9 | 80 ± 10 | 81 ± 8 | 79 ± 9 | 80 ± 7 | 81 ± 10 | 0.838 | |
| Triglycerides (mmol/L) 3,4 | 9.2 ± 4.6 | 9.2 ± 4.4 | 9.8 ± 5.0 | 9.4 ± 4.5 | 8.9 ± 5.0 | 9.0 ± 4.6 | 8.4 ± 4.0 | 0.383 | |
| Total cholesterol (mmol/L) 3 | 11 ± 2.4 | 11 ± 2.6 ac | 11.4 ± 2. 0 bc | 11.2 ± 2.4 abc | 10.5 ± 2.1 ad | 10.3 ± 2.5 a | 11.9 ± 2.6 b | 0.011 | |
| HDL-c (mmol/L) 3 | 2.7 ± 0.6 | 2.7 ± 0.6 | 2.7 ± 0.5 | 2.6 ± 0.6 | 2.7 ± 0.7 | 2.8 ± 0.6 | 0.5 | 0.305 | |
| LDL-c (mmol/L) 3 | 6.7 ± 2.0 | 6.6 ± 2.2 ab | 7.0 ± 1.7 ac | 6.8 ± 2.0 ab | 6.2 ± 1.8 b | 6.3 ± 1.8 bc | 7.3 ± 2.3 a | 0.037 | |
| Type 2 diabetes | 154 (42) | 46 (54) | 22 (37) | 26 (43) | 29 (40) | 23 (47) | 8 (20) | 0.011 | |
| PA (MET, min/wk) | 1798 ± 1665 | 1661 ± 1522 | 1645 ± 1471 | 1733 ± 1966 | 1804 ± 1466 | 2330 ± 2104 | 1753 ± 1419 | 0.288 | |
| Smoking status & history | 0.031 | ||||||||
| Current/former (<5 yr) | 75 (20) | 17 (20) | 10 (17) | 16 (26) | 18 (25) | 9 (18) | 5 (12) | ||
| Former (>5 yr) | 123 (34) | 39 (46) | 23 (39) | 20 (33) | 20 (28) | 9 (18) | 12 (29) | ||
| Never smoked | 169 (46) | 29 (34) | 26 (44) | 25 (41) | 34 (47) | 31 (63) | 24 (59) | ||
| Glucose medications 5 | 118 (32) | 32 (38) | 20 (34) | 20 (33) | 24 (33) | 17 (35) | 5 (12) | 0.111 | |
| Blood pressure medications | 289 (79) | 66 (78) | 47 (80) | 48 (79) | 56 (78) | 39 (80) | 33 (80) | >0.99 | |
| Cholesterol medications | 240 (65) | 62 (73) | 35 (59) | 37 (61) | 49 (68) | 31 (63) | 26 (63) | 0.535 | |
| Veg/Fruit/WG 2 | Non-EVOO/Sweet/RG 2 | Alch/Salt/AnimFat 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Moderate | High | p2 | Low | Moderate | High | p2 | Low | Moderate | High | p2 | ||
| Dietary pattern score | −1.11 ± 0.60 a | 0.06 ± 0.26 b | 1.06 ± 0.44 c | <0.001 | −1.10 ± 0.49 a | −0.02 ± 0.30 b | 1.13 ± 0.42 c | <0.001 | −1.06 ± 0.66 a | 0.00 ± 0.24 b | 1.07 ± 0.50 c | <0.001 | |
| Female | 49 (40) | 68 (56) | 85 (70) | 0.001 | 60 (49) | 69 (57) | 73 (60) | 0.202 | 91 (74) | 71 (58) | 40 (33) | <0.001 | |
| Age (years) | 64 ± 5 a | 65 ± 5 ab | 66 ± 5 b | 0.018 | 65 ± 5 | 65 ± 4 | 64 ± 5 | 0.172 | 66 ± 5 a | 65 ± 4 ab | 64 ± 5 b | 0.004 | |
| BMI (kg/m2) | 32.6 ± 3.6 | 32.5 ± 3.8 | 31.9 ± 3.3 | 0.206 | 32.2 ± 3.6 | 32.1 ± 3.7 | 32.7 ± 3.5 | 0.327 | 32.6 ± 3.8 | 32.1 ± 3.4 | 32.3 ± 3.6 | 0.551 | |
| Waist circumference (cm) | |||||||||||||
| Females | 104 (9) | 103 (10) | 101 (8) | 0.064 | 102 (8) | 101 (9) | 104 (9) | 0.084 | 103 (9) | 102 (9) | 102 (9) | 0.533 | |
| Males | 111 (9) | 113 (9) | 109 (8) | 0.157 | 110 (8) | 111 (9) | 113 (9) | 0.426 | 111 (9) | 111 (8) | 111 (9) | 0.987 | |
| Fasting glucose (mmol/L) 3 | 6.6 ± 1.8 | 6.3 ± 1.5 | 6.5 ± 2.1 | 0.516 | 6.4 ± 2.0 | 6.5 ± 1.5 | 6.6 ± 1.8 | 0.554 | 6.5 ± 1.9 | 6.6 ± 2.0 | 6.4 ± 1.4 | 0.662 | |
| SBP (mmHg) 3 | 141.9 ± 18 | 140.8 ± 16 | 138.2 ± 17 | 0.209 | 142 (16) | 142 (17) | 137 (17) | 0.054 | 140 (18) | 141 (15) | 140 (17) | 0.922 | |
| DBP (mmHg) | 81.3 ± 10 | 79 ± 10 | 79 ± 7 | 0.142 | 81 (9) | 80 (9) | 79 (9) | 0.398 | 79 (9) a | 79 (9) ab | 82 (9) b | 0.013 | |
| Triglycerides (mmol/L) 3,4 | 10.0 ± 5.3 | 8.9 ± 3.9 | 8.6 ± 4.4 | 0.066 | 8.7 ± 3.9 | 9.5 ± 4.8 | 9.4 5.0 | 0.399 | 8.5 ± 3.2 | 8.9 ± 4.2 | 10.2 ± 5.9 | 0.123 | |
| Total cholesterol (mmol/L) 3 | 11.0 ± 2.6 | 10.7 ± 2.2 | 11.4 ± 2.3 | 0.065 | 10.9 ± 2.5 | 11.4 ± 2.4 | 10.8 ± 2.3 | 0.119 | 10.9 ± 2.2 | 10.7 ± 2.5 | 11.4 ± 2.5 | 0.047 | |
| HDL-C (mmol/L) 3 | 2.7 ± 0.6 | 2.7 ± 0.6 | 2.8 ± 0.6 | 0.460 | 2.8 ± 0.6 | 2.6 ± 0.6 | 2.7 ± 0.7 | 0.105 | 2.7 ± 0.6 | 2.7 ± 0.6 | 2.7 ± 0.6 | 0.917 | |
| LDL-C (mmol/L) 3 | 6.6 ± 2.1 ab | 6.4 ± 1.9 a | 7.0 ± 2.0 b | 0.038 | 6.5 ± 2.0 | 7.0 ± 2.1 | 6.5 ± 1.8 | 0.097 | 6.6 ± 2.0 | 6.5 ± 2.0 | 6.9 ± 2.1 | 0.249 | |
| Diabetes | 51 (41) | 51 (42) | 52 (43) | 0.982 | 39 (32) | 52 (43) | 63 (52) | 0.007 | 50 (41) | 56 (46) | 48 (39) | 0.547 | |
| Energy intake (kcal/d) | 2371 (561) | 2418 (535) | 2392 (472) | 0.776 | 2425 (539) | 2301 (492) | 2455 (528) | 0.052 | 2419 (572) | 2314 (473) | 2448 (513) | 0.111 | |
| PA (MET, min/wk) | 1836(2035) | 1795 (1417) | 1763 (1484) | 0.943 | 2165 (1829) a | 1778 (1454) ab | 1447 (1624) b | 0.003 | 1741 (1797) | 1920 (1527) | 1734 (1666) | 0.615 | |
| Smoking status & history | 0.012 | 0.528 | <0.001 | ||||||||||
| Current/former (<5 yr) | 36 (29) | 17 (14) | 22 (18) | 26 (21) | 20 (16) | 29 (24) | 20 (16) | 20 (16) | 35 (29) | ||||
| Former (>5 yr) | 43 (35) | 44 (36) | 36 (30) | 45 (37) | 41 (34) | 37 (30) | 27 (22) | 47 (39) | 49 (40) | ||||
| Never smoked | 44 (36) | 61 (50) | 64 (52) | 52 (42) | 61 (50) | 56 (46) | 76 (62) | 55 (45) | 38 (31) | ||||
| Glucose medications 5 | 40 (33) | 38 (31) | 40 (33) | 0.958 | 31 (25) | 35 (29) | 52 (43) | 0.009 | 37 (30) | 45 (37) | 36 (30) | 0.390 | |
| Blood pressure medications | 99 (80) | 92 (75) | 98 (80) | 0.544 | 100 (81) | 92 (75) | 97 (80) | 0.513 | 99 (80) | 96 (79) | 94 (77) | 0.805 | |
| Cholesterol medications | 82 (67) | 84 (69) | 74 (61) | 0.378 | 77 (63) | 81 (66) | 82 (67) | 0.720 | 70 (57) | 87 (71) | 83 (68) | 0.046 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gervis, J.E.; Fernández-Carrión, R.; Chui, K.K.H.; Ma, J.; Coltell, O.; Sorli, J.V.; Asensio, E.M.; Ortega-Azorín, C.; Pérez-Fidalgo, J.A.; Portolés, O.; et al. Associations between Taste Perception Profiles and Empirically Derived Dietary Patterns: An Exploratory Analysis among Older Adults with Metabolic Syndrome. Nutrients 2022, 14, 142. https://doi.org/10.3390/nu14010142
Gervis JE, Fernández-Carrión R, Chui KKH, Ma J, Coltell O, Sorli JV, Asensio EM, Ortega-Azorín C, Pérez-Fidalgo JA, Portolés O, et al. Associations between Taste Perception Profiles and Empirically Derived Dietary Patterns: An Exploratory Analysis among Older Adults with Metabolic Syndrome. Nutrients. 2022; 14(1):142. https://doi.org/10.3390/nu14010142
Chicago/Turabian StyleGervis, Julie E., Rebeca Fernández-Carrión, Kenneth K. H. Chui, Jiantao Ma, Oscar Coltell, Jose V. Sorli, Eva M. Asensio, Carolina Ortega-Azorín, José A. Pérez-Fidalgo, Olga Portolés, and et al. 2022. "Associations between Taste Perception Profiles and Empirically Derived Dietary Patterns: An Exploratory Analysis among Older Adults with Metabolic Syndrome" Nutrients 14, no. 1: 142. https://doi.org/10.3390/nu14010142
APA StyleGervis, J. E., Fernández-Carrión, R., Chui, K. K. H., Ma, J., Coltell, O., Sorli, J. V., Asensio, E. M., Ortega-Azorín, C., Pérez-Fidalgo, J. A., Portolés, O., Lichtenstein, A. H., & Corella, D. (2022). Associations between Taste Perception Profiles and Empirically Derived Dietary Patterns: An Exploratory Analysis among Older Adults with Metabolic Syndrome. Nutrients, 14(1), 142. https://doi.org/10.3390/nu14010142









