From Diospyros kaki L. (Persimmon) Phytochemical Profile and Health Impact to New Product Perspectives and Waste Valorization
Abstract
1. Introduction
1.1. Persimmon Composition
1.1.1. Nutritional Characterization
1.1.2. Phenolic Compounds
1.2. Persimmon Biological Activity
1.2.1. Proanthocyanidins (PACs) in Obesity and Lipidic Metabolism
1.2.2. Proanthocyanidins and Gut Microbiota Modulation
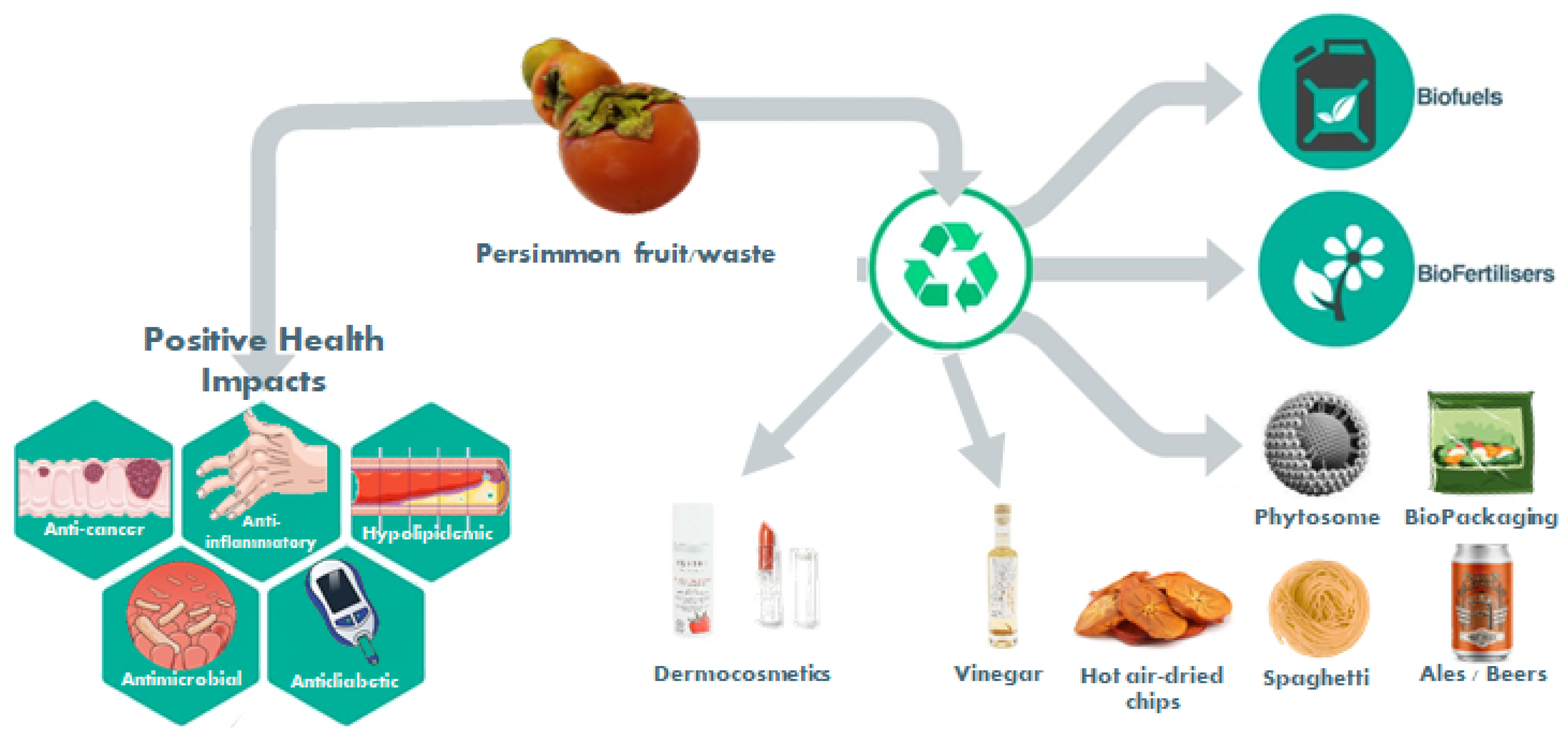
1.3. New Products and Byproducts Valorization
1.3.1. Reformulation of Traditional Foods (Spaghetti; Pork Liver Pâté; Rice Noodles; Cheese; Yogurt; Cupcakes; Persimmon Pulp; Hot-Air-Dried Chips; Ale Beers; Vinegar; Probiotic Food Products; Emulsifier)
1.3.2. Antimicrobial Activity and Food Packaging
1.3.3. Fabric Dyes
1.3.4. Plant Growth Regulation
1.3.5. Biofuel Production
1.3.6. Dermocosmetic Applications
1.3.7. Nanotechnology
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Izuchi, R.; Nakai, Y.; Takahashi, H.; Ushiama, S.; Okada, S.; Misaka, T.; Abe, K. Hepatic Gene Expression of the Insulin Signaling Pathway Is Altered by Administration of Persimmon Peel Extract: A DNA Microarray Study Using Type 2 Diabetic Goto-Kakizaki Rats. J. Agric. Food Chem. 2011, 59, 3320–3329. [Google Scholar] [CrossRef]
- Vieites, R.L. Persimmon Tree. Rev. Bras. De Frutic. 2012, 34, Ii. [Google Scholar]
- Del Bubba, M.; Giordani, E.; Pippucci, L.; Cincinelli, A.; Checchini, L.; Galvan, P. Changes in tannins, ascorbic acid and sugar content in astringent persimmons during on-tree growth and ripening and in response to different postharvest treatments. J. Food Compos. Anal. 2009, 22, 668–677. [Google Scholar] [CrossRef]
- FAO. Food and Agricultural Organization of the United Nations. 2017. Available online: http://www.fao.org/faostat/en/#data. (accessed on 25 January 2021).
- Bibi, N.; Khattak, A.B.; Mehmood, Z. Quality improvement and shelf life extension of persimmon fruit (Diospyros kaki). J. Food Eng. 2007, 79, 1359–1363. [Google Scholar] [CrossRef]
- OMAIAA. A Produção e Comercialização do Dióspiro em Portugal. Available online: http://www.observatorioagricola.pt/item.asp?id_item=117 (accessed on 25 January 2021).
- Toplu, C.; Kaplankiran, M.; Demirkeser, T.H.; Ozdemir, A.E.; Candir, E.E.; Yildiz, E. The performance of persimmon (Diospyros kaki Thumb.) Cultivars Under Mediterranean Coastal Conditions in Hatay, Turkey. J. Am. Pomol. Soc. 2009, 63, 33. [Google Scholar]
- Giordani, E. Varietal assortment of persimmon in the countries of the Mediterranean area and genetic improvement. In Proceedings of the First Mediterranean Symposium on Persimmon, Faenza, Italy, 23–24 November 2001; pp. 23–37. [Google Scholar]
- Gorinstein, S.; Zachwieja, Z.; Folta, M.; Barton, H.; Piotrowicz, J.; Zemser, M.; Weisz, M.; Trakhtenberg, S.; Martin-Belloso, O. Comparative contents of dietary fiber, total phenolics, and minerals in persimmons and apples. J. Agric. Food Chem. 2001, 49, 952–957. [Google Scholar] [CrossRef] [PubMed]
- de Ancos, B.; Gonzalez, E.; Cano, M.P. Effect of high-pressure treatment on the carotenoid composition and the radical scavenging activity of persimmon fruit purees. J. Agric. Food Chem. 2000, 48, 3542–3548. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Ozaki, M.; Akashi, T.; Yamashita, K.; Niwa, M.; Taniyama, K. Effects of (−)-epigallocatechin-3-O-gallate (green tea tannin) on the life span of stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. Suppl. 1995, 22, S302–S303. [Google Scholar] [CrossRef] [PubMed]
- Hibino, G.; Nadamoto, T.; Fujisawa, F.; Fushiki, T. Regulation of the peripheral body temperature by foods: A temperature decrease induced by the Japanese persimmon (kaki, Diospyros kaki). Biosci. Biotechnol. Biochem. 2003, 67, 23–28. [Google Scholar] [CrossRef]
- Gu, H.F.; Li, C.M.; Xu, Y.J.; Hu, W.F.; Chen, M.H.; Wan, Q.H. Structural features and antioxidant activity of tannin from persimmon pulp. Food Res. Int. 2008, 41, 208–217. [Google Scholar] [CrossRef]
- Briand, C. The common persimmon (Diospyros virginiana L.): The history of an underutilized fruit tree (16th–19th centuries). Huntia 2005, 12, 71–89. [Google Scholar]
- Chen, X.N.; Fan, J.F.; Yue, X.; Wu, X.R.; Li, L.T. Radical scavenging activity and phenolic compounds in persimmon (Diospyros kaki L. cv. Mopan). J. Food Sci. 2008, 73, C24–C28. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; Motohashi, N.; Satoh, K.; Sakagami, H.; Nakashima, H.; Tani, S.; Shirataki, Y.; Kurihara, T.; Spengler, G.; Wolfard, K.; et al. Biological activity of persimmon (Diospyros kaki) peel extracts. Phytother. Res. 2003, 17, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Direito, R.; Lima, A.; Rocha, J.; Ferreira, R.B.; Mota, J.; Rebelo, P.; Fernandes, A.; Pinto, R.; Alves, P.; Bronze, R.; et al. Dyospiros kaki phenolics inhibit colitis and colon cancer cell proliferation, but not gelatinase activities. J. Nutr. Biochem. 2017, 46, 100–108. [Google Scholar] [CrossRef]
- Matsumoto, K.; Watanabe, Y.; Ohya, M.A.; Yokoyama, S. Young persimmon fruits prevent the rise in plasma lipids in a diet-induced murine obesity model. Biol. Pharm. Bull. 2006, 29, 2532–2535. [Google Scholar] [CrossRef][Green Version]
- Gorinstein, S.; Kulasek, G.W.; Bartnikowska, E.; Leontowicz, M.; Zemser, M.; Morawiec, M.; Trakhtenberg, S. The influence of persimmon peel and persimmon pulp on the lipid metabolism and antioxidant activity of rats fed cholesterol. J. Nutr. Biochem. 1998, 9, 223–227. [Google Scholar] [CrossRef]
- Esteban-Muñoz, A.; Sánchez-Hernández, S.; Samaniego-Sánchez, C.; Giménez-Martínez, R.; Olalla-Herrera, M. Differences in the Phenolic Profile by UPLC Coupled to High Resolution Mass Spectrometry and Antioxidant Capacity of Two Diospyros kaki Varieties. Antioxidants 2021, 10, 31. [Google Scholar] [CrossRef]
- Lee, S.O.; Chung, S.K.; Lee, I.S. The antidiabetic effect of dietary persimmon (Diospyros kaki L. cv. Sangjudungsi) peel in streptozotocin-induced diabetic rats. J. Food Sci. 2006, 71, S293–S298. [Google Scholar] [CrossRef]
- Grygorieva, O.; Kucharska, A.Z.; Piórecki, N.; Klymenko, S.; Vergun, O.; Brindza, J. Antioxidant activities and phenolic compounds in fruits of various genotypes of American persimmon (Diospyros virginiana L.). Acta Sci. Pol. Technol. Aliment 2018, 17, 117–124. [Google Scholar] [PubMed]
- Hirvonen, K.; Bai, Y.; Headey, D.; Masters, W.A. Cost and affordability of the EAT-Lancet diet in 159 countries. Lancet 2019. [Google Scholar] [CrossRef]
- Woolston, C. Healthy people, healthy planet: The search for a sustainable global diet. Nature 2020, 588, S54–S56. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; Declerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Veberic, R.; Jurhar, J.; Mikulic-Petkovsek, M.; Stampar, F.; Schmitzer, V. Comparative study of primary and secondary metabolites in 11 cultivars of persimmon fruit (Diospyros kaki L.). Food Chem. 2010, 119, 477–483. [Google Scholar] [CrossRef]
- Inaba, A.; Sobajima, Y.; Ishida, M. Seasonal changes in the major components of kaki fruits. Kyoto Prefect Univ. Fac. Agr. Sci. Rep. 1971, 23, 24–28. [Google Scholar]
- Zhou, C.; Sheng, Y.; Zhao, D.; Wang, Z.; Tao, J. Variation of oleanolic and ursolic acid in the flesh of persimmon fruit among different cultivars. Molecules 2010, 15, 6580–6587. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.A.; Kadioglu, A. Fatty acid compositional changes in developing persimmon (Diospyros lotus L.) fruit. N. Z. J. Crop. Hortic. Sci. 1999, 27, 257–261. [Google Scholar] [CrossRef]
- da Conceição Santos, A.D.; Fonseca, F.A.; Dutra, L.M.; Santos, M.D.F.C.; Menezes, L.R.A.; Campos, F.R.; Nagata, N.; Ayub, R.; Barison, A. 1H HR-MAS NMR-based metabolomics study of different persimmon cultivars (Diospyros kaki) during fruit development. Food Chem. 2018, 239, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Kim, D.S.; Kim, S.W.; Lim, S.H.; Kim, D.K.; Shin, T.Y.; Kim, S.H. Inhibitory effects of Diospyros kaki in a model of allergic inflammation: Role of cAMP, calcium and nuclear factor-kappaB. Int. J. Mol. Med. 2013, 32, 945–951. [Google Scholar] [CrossRef]
- Ozen, A.; Colak, A.; Dincer, B.; Guner, S. A diphenolase from persimmon fruits (Diospyros kaki L., Ebenaceae). Food Chem. 2004, 85, 431–437. [Google Scholar] [CrossRef]
- Ercisli, S.; Akbulut, M.; Ozdemir, O.; Sengul, M.; Orhan, E. Phenolic and antioxidant diversity among persimmon (Diospyrus kaki L.) genotypes in Turkey. Int. J. Food Sci. Nutr. 2008, 59, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Barea-Álvarez, M.; Delgado-Andrade, C.; Haro, A.; Olalla, M.; Seiquer, I.; Rufián-Henares, J.Á. Subtropical fruits grown in Spain and elsewhere: A comparison of mineral profiles. J. Food Compos. Anal. 2016, 48, 34–40. [Google Scholar] [CrossRef]
- INSA; Instituto Nacional de Sáude Ricardo Jorge. Tabela da Composição de Alimentos (TCA); Instituto Nacional de Sáude Ricardo Jorge: Lisbon, Portugal; Available online: http://portfir.insa.pt/foodcomp/search (accessed on 18 February 2021).
- Jung, S.T.; Park, Y.S.; Zachwieja, Z.; Folta, M.; Barton, H.; Piotrowicz, J.; Katrich, E.; Trakhtenberg, S.; Gorinstein, S. Some essential phytochemicals and the antioxidant potential in fresh and dried persimmon. Int. J. Food Sci. Nutr. 2005, 56, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.C.; Jo, E.K.; Bae, M.S.; Lee, H.J.; Jeon, G.I.; Park, E.; Yuk, H.G.; Ahn, G.H.; Lee, S.C. Antioxidant and antigenotoxic activities of different parts of persimmon (Diospyros kaki cv. Fuyu) fruit. J. Med. Plants Res. 2010, 4, 155–160. [Google Scholar]
- Jimenez-Sanchez, C.; Lozano-Sanchez, J.; Marti, N.; Saura, D.; Valero, M.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Characterization of polyphenols, sugars, and other polar compounds in persimmon juices produced under different technologies and their assessment in terms of compositional variations. Food Chem. 2015, 182, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Piretti, M.V. Polyphenol constituents of the Diospyros kaki fruit. A review. Fitoterapia 1991, 62, 3–13. [Google Scholar]
- Maulidiani, M.; Mediani, A.; Abas, F.; Park, Y.S.; Park, Y.-K.; Kim, Y.M.; Gorinstein, S. 1 H NMR and antioxidant profiles of polar and non-polar extracts of persimmon ( Diospyros kaki L )-metabolomics study based on cultivars and origins. Talanta 2018, 184, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Senter, S.; Chapman, G.; Forbus, W.; Payne, J. Sugar and nonvolatile acid composition of persimmons during maturation. J. Food Sci. 1991, 56, 989–991. [Google Scholar] [CrossRef]
- Ryu, S.; Furihata, K.; Koda, M.; Wei, F.; Miyakawa, T.; Tanokura, M. NMR-based analysis of the chemical composition of Japanese persimmon aqueous extracts. Magn. Reson. Chem. 2016, 54, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Giordani, E.; Doumett, S.; Nin, S.; Del Bubba, M. Selected primary and secondary metabolites in fresh persimmon (Diospyros kaki Thunb.): A review of analytical methods and current knowledge of fruit composition and health benefits. Food Res. Int. 2011, 44, 1752–1767. [Google Scholar] [CrossRef]
- Mir-Marqués, A.; Domingo, A.; Cervera, M.L.; de la Guardia, M. Mineral profile of kaki fruits (Diospyros kaki L.). Food Chem. 2015, 172, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Bing, Y.; HuaLong, X.; Ping, L. Content and Chemical Composition of Carotenoids in Persimmon Fruit. Chin. Agric. Sci. Bull. 2006, 10, 065. [Google Scholar]
- Ebert, G.; Gross, J. Carotenoid changes in the peel of ripening persimmon (Diospyros kaki) cv Triumph. Phytochemistry 1985, 24, 29–32. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T.; Aziz, M.; Naz, A.; Ahmed, W.; Kumar, N.; Imran, M. Persimmon (Diospyros Kaki) Fruit: Hidden Phytochemicals and Health Claims. Excli J. 2015, 14, 542–561. [Google Scholar] [PubMed]
- Block, G.; Patterson, B.; Subar, A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr. Cancer 1992, 18, 1–29. [Google Scholar] [CrossRef] [PubMed]
- van Poppel, G. Carotenoids and cancer: An update with emphasis on human intervention studies. Eur. J. Cancer 1993, 29, 1335–1344. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer prevention: A review. J. Am. Diet. Assoc. 1996, 96, 1027–1039. [Google Scholar] [CrossRef]
- Yaqub, S.; Farooq, U.; Shafi, A.; Akram, K.; Murtaza, M.A.; Kausar, T.; Siddique, F. Chemistry and Functionality of Bioactive Compounds Present in Persimmon. J. Chem. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Hitaka, Y.; Nakano, A.; Tsukigawa, K.; Manabe, H.; Nakamura, H.; Nakano, D.; Kinjo, J.; Nohara, T.; Maeda, H. Characterization of carotenoid fatty acid esters from the peels of the persimmon Diospyros kaki. Chem. Pharm. Bull. 2013, 61, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Tanaka, Y.; Murakawa, M.; Kadoshima-Yamaoka, K.; Inoue, H.; Murafuji, H.; Nagahira, A.; Kanki, S.; Hayashi, Y.; Nagahira, K.; et al. Inhibition of phosphodiesterase 7A ameliorates Concanavalin A-induced hepatitis in mice. Int. Immunopharmacol. 2009, 9, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Direito, R.; Rocha, J.; Serra, A.T.; Fernandes, A.; Freitas, M.; Fernandes, E.; Pinto, R.; Bronze, R.; Sepodes, B.; Figueira, M.E. Anti-inflammatory Effects of Persimmon (Diospyros kaki L.) in Experimental Rodent Rheumatoid Arthritis. J. Diet Suppl. 2019, 17, 663–683. [Google Scholar] [CrossRef] [PubMed]
- Parliament, E. Regulation (EU) 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Communities. L 2011, 304, 18. [Google Scholar]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.F. Vitamins, 4th ed.; Fennema, O.R., Damodaran, S., Parkin, K.L., Eds.; Food Chemistry Series; CRC Press: Cambridge, UK, 2007; pp. 531–616. [Google Scholar]
- Setha, S.; Kondo, S.; Hirai, N.; Ohigashi, H. Xanthoxin, abscisic acid and its metabolite levels associated with apple fruit development. Plant Sci. 2004, 166, 493–499. [Google Scholar] [CrossRef]
- Maulidiani, M.; Abdul-Hamid, N.A.; Abas, F.; Park, Y.S.; Park, Y.-K.; Kim, Y.M.; Gorinstein, S. Detection of bioactive compounds in persimmon (Diospyros kaki) using UPLC-ESI-Orbitrap-MS/MS and fluorescence analyses. Microchem. J. 2019, 149, 103978. [Google Scholar] [CrossRef]
- Senica, M.; Veberic, R.; Grabnar, J.J.; Stampar, F.; Jakopic, J. Selected chemical compounds in firm and mellow persimmon fruit before and after the drying process. J. Sci. Food Agric. 2016, 96, 3140–3147. [Google Scholar] [CrossRef] [PubMed]
- Domínguez Díaz, L.; Dorta, E.; Maher, S.; Morales, P.; Fernández-Ruiz, V.; Cámara, M.; Sánchez-Mata, M.-C. Potential Nutrition and Health Claims in Deastringed Persimmon Fruits (Diospyros kaki L.), Variety ‘Rojo Brillante’, PDO ’Ribera del Xúquer’. Nutrients 2020, 12, 1397. [Google Scholar] [CrossRef] [PubMed]
- Câmara, M.B.D.; Ferreira, J.P.B.; Coelho, A.V.; Feliciano, R.; Silva, A.B.D.; Mecha, E.; Bronze, M.D.R.; Direito, R.; Rocha, J.P.F.; Sepodes, B.; et al. Caracterização Química e Avaliação da Atividade Biológica da Framboesa (Rubus Idaeus L.). Contribuição para o Desenvolvimento de uma Alegação de Saúde. Atena Ed. 2019, 9–21. [Google Scholar] [CrossRef]
- Yoon, J.H.; Baek, S.J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med. J. 2005, 46, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Zemser, M.; Weisz, M.; Halevy, S.; Deutsch, J.; Tilus, K.; Feintuch, D.; Guerra, N.; Fishman, M.; Bartnikowska, E. Fluorometric analysis of phenolics in persimmons. Biosci. Biotech. Biochem. 1994, 58, 1087–1092. [Google Scholar] [CrossRef]
- Wollgast, J.; Anklam, E. Polyphenols in chocolate: Is there a contribution to human health? Food Res. Intern. 2000, 33, 449–459. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Direito, R.; Rocha, J.; Sepodes, B.; Eduardo-Figueira, M. Phenolic Compounds Impact on Rheumatoid Arthritis, Inflammatory Bowel Disease and Microbiota Modulation. Pharmaceutics 2021, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Mallavadhani, U.V.; Panda, A.K.; Rao, Y.R. Pharmacology and chemotaxonomy of Diospyros. Phytochemistry 1998, 49, 901–951. [Google Scholar] [CrossRef]
- Sentandreu, E.; Cerdan-Calero, M.; Halket, J.M.; Navarro, J.L. Rapid screening of low molecular weight phenols from persimmon (Diospyros kaki) pulp using liquid chromatography-UV/Visible-electrospray mass spectrometry analysis. J. Sci. Food Agric. 2015, 95, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Someya, S.; Hu, F.; Tanokura, M. Comparative study of catechin compositions in five Japanese persimmons (Diospyros kaki). Food Chem. 2005, 93, 149–152. [Google Scholar] [CrossRef]
- Li, C.M.; Leverence, R.; Trombley, J.D.; Xu, S.F.; Yang, J.; Tian, Y.; Reed, J.D.; Hagerman, A.E. High Molecular Weight Persimmon (Diospyros kaki L.) Proanthocyanidin: A Highly Galloylated, A-Linked Tannin with an Unusual Flavonol Terminal Unit, Myricetin. J. Agric. Food Chem. 2010, 58, 9033–9042. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Jesion, I.; Gorinstein, S. Nutraceutical value of persimmon (Diospyros kaki Thunb.) and its influence on some indices of atherosclerosis in an experiment on rats fed cholesterol-containing diet. Adv. Hortic. Sci. 2008, 22, 250–254. [Google Scholar]
- Denev, P.; Yordanov, A. Total polyphenol, proanthocyanidin and flavonoid content, carbohydrate composition and antioxidant activity of persimmon (Diospyros kaki L.) fruit in relation to cultivar and maturity stage. Bulg. J. Agric. Sci. 2013, 19, 981–988. [Google Scholar]
- Direito, R.; Reis, C.; Roque, L.; Gonçalves, M.; Sanches-Silva, A.; Gaspar, M.M.; Pinto, R.; Rocha, J.; Sepodes, B.; Rosário Bronze, M.; et al. Phytosomes with Persimmon (Diospyros kaki L.) Extract: Preparation and Preliminary Demonstration of In Vivo Tolerability. Pharmaceutics 2019, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Pu, F.; Ren, X.-L.; Zhang, X.-P. Phenolic compounds and antioxidant activity in fruits of six Diospyros kaki genotypes. Eur. Food Res. Technol. 2013, 237, 923–932. [Google Scholar] [CrossRef]
- Gao, H.; Cheng, N.; Zhou, J.; Wang, B.; Deng, J.; Cao, W. Antioxidant activities and phenolic compounds of date plum persimmon (Diospyros lotus L.) fruits. J. Food Sci. Technol. 2014, 51, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, Y.B.; Seo, W.D.; Kang, S.T.; Lim, J.W.; Cho, K.M. Comparative studies of antioxidant activities and nutritional constituents of persimmon juice (Diospyros kaki L. cv. Gapjubaekmok). Prev. Nutr. Food Sci. 2012, 17, 141. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.-T.; Xu, X.-R.; Gan, R.-Y.; Zhang, Y.; Xia, E.-Q.; Li, H.-B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Gorinstein, S.; Martín-Belloso, O.; Park, Y.-S.; Haruenkit, R.; Lojek, A.; Ĉíž, M.; Caspi, A.; Libman, I.; Trakhtenberg, S. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001, 74, 309–315. [Google Scholar] [CrossRef]
- Achiwa, Y.; Hibasami, H.; Katsuzaki, H.; Imai, K.; Komiya, T. Inhibitory effects of persimmon (Diospyros kaki) extract and related polyphenol compounds on growth of human lymphoid leukemia cells. Biosci. Biotechnol. Biochem. 1997, 61, 1099–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wan, L.; Wu, C.; Fang, Y.; Han, G.; Li, H.; Zhang, Z.; Wang, H. Simultaneous Determination of 14 Phenolic Compounds in Grape Canes by HPLC-DAD-UV Using Wavelength Switching Detection. Molecules 2013, 18, 14241–14257. [Google Scholar] [CrossRef] [PubMed]
- Taira, S. Astringency in persimmon. In Fruit Analysis; Springer: Berlin/Heidelberg, Germany, 1995; pp. 97–110. [Google Scholar]
- Akagi, T.; Suzuki, Y.; Ikegami, A.; Kamitakahara, H.; Takano, T.; Nakatsubo, F.; Yonemori, K. Condensed Tannin Composition Analysis in Persimmon (Diospyros kaki Thunb.) Fruit by Acid Catalysis in the Presence of Excess Phloroglucinol. J. Jpn. Soc. Hortic. Sci. 2010, 79, 275–281. [Google Scholar] [CrossRef]
- Pei, X.; Zhang, Q.; Guo, D.; Luo, Z. Effectiveness of the RO2 marker for the identification of non-astringency trait in Chinese PCNA persimmon and its possible segregation ratio in hybrid F1 population. Sci. Hortic. 2013, 150, 227–231. [Google Scholar] [CrossRef]
- Gorinstein, S.; Leontowicz, H.; Leontowicz, M.; Jesion, I.; Namiesnik, J.; Drzewiecki, J.; Park, Y.S.; Ham, K.S.; Giordani, E.; Trakhtenberg, S. Influence of two cultivars of persimmon on atherosclerosis indices in rats fed cholesterol-containing diets: Investigation in vitro and in vivo. Nutrition 2011, 27, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Oliveras, M.J.; Quesada, J.; Rufián-Henares, J.A.; Pastoriza, S. Relationship between composition and bioactivity of persimmon and kiwifruit. Food Res. Int. 2018, 105, 461–472. [Google Scholar] [CrossRef]
- Matsuo, S. The chemical structure of kaki-tannin from immature fruit of the persimmon (Diospyros kaki L.). Agric. Biol. Chem. 1978, 42, 1637–1643. [Google Scholar] [CrossRef]
- Gorinstein, S.; Bartnikowska, E.; Kulasek, G.; Zemser, M.; Trakhtenberg, S. Dietary persimmon improves lipid metabolism in rats fed diets containing cholesterol. J. Nutr. 1998, 128, 2023–2027. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yokoyama, S.; Gato, N. Bile acid-binding activity of young persimmon (Diospyros kaki) fruit and its hypolipidemic effect in mice. Phytother. Res. 2010, 24, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Chahoud, G.; Aude, Y.W.; Mehta, J.L. Dietary recommendations in the prevention and treatment of coronary heart disease: Do we have the ideal diet yet? Am. J. Cardiol. 2004, 94, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Jung, S.T.; Kang, S.G.; Delgado-Licon, E.; Ayala, A.L.M.; Tapia, M.S.; Martin-Belloso, O.; Trakhtenbergh, S.; Gorinstein, S. Drying of persimmons (Diospyros kaki L.) and the following changes in the studied bioactive compounds and the total radical scavenging activities. Lwt-Food Sci. Technol. 2006, 39, 748–755. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kadowaki, A.; Ozaki, N.; Takenaka, M.; Ono, H.; Yokoyama, S.; Gato, N. Bile acid-binding ability of kaki-tannin from young fruits of persimmon (Diospyros kaki) in vitro and in vivo. Phytother. Res. 2011, 25, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, J.; Arner, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free. Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Seifried, H.E.; Anderson, D.E.; Fisher, E.I.; Milner, J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007, 18, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.V.; Steenken, S.; Hara, Y.; Simic, M.G. Reduction potentials of flavonoid and model phenoxyl radicals. Which ring in flavonoids is responsible for antioxidant activity? J. Chem. Soc. Perkin Trans. 2 1996, 11, 2497–2504. [Google Scholar] [CrossRef]
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A review on anti-cancer properties of Quercetin in breast cancer. Life Sci. 2020, 248, 117463. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef] [PubMed]
- Ou, Q.; Zheng, Z.; Zhao, Y.; Lin, W. Impact of quercetin on systemic levels of inflammation: A meta-analysis of randomised controlled human trials. Int. J. Food Sci. Nutr. 2020, 71, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Saccon, T.D.; Nagpal, R.; Yadav, H.; Cavalcante, M.B.; Nunes, A.D.d.C.; Schneider, A.; Gesing, A.; Hughes, B.; Yousefzadeh, M.; Tchkonia, T. Senolytic combination of Dasatinib and Quercetin alleviates intestinal senescence and inflammation and modulates the gut microbiome in aged mice. J. Gerontol. Ser. A 2021. [Google Scholar] [CrossRef] [PubMed]
- Shree, A.; Islam, J.; Sultana, S. Quercetin ameliorates reactive oxygen species generation, inflammation, mucus depletion, goblet disintegration, and tumor multiplicity in colon cancer: Probable role of adenomatous polyposis coli, β-catenin. Phytother. Res. 2021, 35, 2171–2184. [Google Scholar] [CrossRef]
- Daood, H.G.; Biacs, P.; Czinkotai, B.; Hoschke, A. Chromatographic Investigation of Carotenoids, Sugars and Organic-Acids from Diospyros-Kaki Fruits. Food Chem. 1992, 45, 151–155. [Google Scholar] [CrossRef]
- Uchida, S.; Ohta, H.; Niwa, M.; Mori, A.; Nonaka, G.; Nishioka, I.; Ozaki, M. Prolongation of life span of stroke-prone spontaneously hypertensive rats (SHRSP) ingesting persimmon tannin. Chem. Pharm. Bull. 1990, 38, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Remesy, C.; Jimenez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Markovic, Z.; Milenkovic, D.; Dorovic, J.; Dimitric Markovic, J.M.; Stepanic, V.; Lucic, B.; Amic, D. Free radical scavenging activity of morin 2’-O(−) phenoxide anion. Food Chem. 2012, 135, 2070–2077. [Google Scholar] [CrossRef]
- Yu, L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Qian, M. Free radical scavenging properties of wheat extracts. J. Agric. Food Chem. 2002, 50, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Mochizuki, A.; Amakura, Y. Identification of Phenolic Constituents and Inhibitory Activity of Persimmon Calyx and Shiteito against Tumor Cell Proliferation. Chem. Pharm. Bull. 2021, 69, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Guler, M.C.; Tanyeli, A.; Eraslan, E.; Bozhuyuk, M.R.; Akdemir, F.N.E.; Toktay, E.; Kurt, N.; Guven, E.C.; Ozkan, G. Persimmon (Diospyros Kaki Alleviates Ethanol-Induced Gastric Ulcer in Rats/Persimmon (Diospyros Kaki L.) Sicanlarda Etanol ile induklenen Mide Ulserini Hafifletir. South. Clin. Istanb. Eurasia (SCIE) 2021, 32, 1–8. [Google Scholar]
- Gorinstein, S.; Kulasek, G.W.; Bartnikowska, E.; Leontowicz, M.; Zemser, M.; Morawiec, M.; Trakhtenberg, S. The effects of diets, supplemented with either whole persimmon or phenol-free persimmon, on rats fed cholesterol. Food Chem. 2000, 70, 303–308. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yokoyama, S.; Gato, N. Hypolipidemic effect of young persimmon fruit in C57BL/6.KOR-ApoEshl mice. Biosci. Biotechnol. Biochem. 2008, 72, 2651–2659. [Google Scholar] [CrossRef]
- Song, M.; Yang, G.; Hoa, T.Q.; Hieu, H.D.; Amin, A.S.M.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. Anti-obesity effect of fermented persimmon extracts via activation of AMP-activated protein kinase. Biol. Pharm. Bull. 2020, 43, 440–449. [Google Scholar] [CrossRef]
- Zou, B.; Li, C.M.; Chen, J.Y.; Dong, X.G.; Zhang, Y.; Du, J. High molecular weight persimmon tannin is a potent hypolipidemic in high-cholesterol diet fed rats. Food Res. Int. 2012, 48, 970–977. [Google Scholar] [CrossRef]
- Alfarafisa, N.M.; Kitaguchi, K.; Yabe, T. Diospyros kaki extract protects myoblasts from oxidative stress-induced cytotoxicity via secretions derived from intestinal epithelium. Biosci. Biotechnol. Biochem. 2021, 85, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zou, B.; Yang, L.; Xu, S.F.; Yang, J.; Yao, P.; Li, C.M. High molecular weight persimmon tannin ameliorates cognition deficits and attenuates oxidative damage in senescent mice induced by D-galactose. Food Chem. Toxicol. 2011, 49, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, F.; Torabi, S.; Askari, V.R.; Asadpour, E.; Sadeghnia, H.R. Protective Effect of Diospyros kaki against Glucose-Oxygen-Serum Deprivation-Induced PC12 Cells Injury. Adv. Pharmacol. Sci. 2016, 2016, 5. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Said, A.; Tundis, R.; Hawas, U.W.; Rashed, K.; Menichini, F.; Frega, N.G. Antioxidant and antiproliferative activity of Diospyros lotus L. extract and isolated compounds. Plant Foods Hum. Nutr. 2009, 64, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Ohguchi, K.; Nakajima, C.; Oyama, M.; Iinuma, M.; Itoh, T.; Akao, Y.; Nozawa, Y.; Ito, M. Inhibitory effects of flavonoid glycosides isolated from the peel of Japanese persimmon (Diospyros kaki ‘Fuyu’) on melanin biosynthesis. Biol. Pharm. Bull. 2010, 33, 122–124. [Google Scholar] [CrossRef]
- Sun, W.; Chen, X.; Nan, X.; Zhang, J.; Dong, L.; Ji, W.; Sheng, G.; Zhou, Q. Inhibition of persimmon tannin extract on guinea pig skin pigmentation. J. Cosmet. Dermatol. 2021, 20, 2648–2656. [Google Scholar] [CrossRef]
- Jung, S.K.; Kim, K.; Tae, K.; Kong, G.; Kim, M.K. The effect of raw vegetable and fruit intake on thyroid cancer risk among women: A case–control study in South Korea. Br. J. Nutr. 2013, 109, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zou, B.; Li, C.M.; Yang, J.; Xu, S.F.; Hagerman, A.E. High molecular weight persimmon tannin is a potent antioxidant both ex vivo and in vivo. Food Res. Int. 2012, 45, 26–30. [Google Scholar] [CrossRef]
- Kitabatake, M.; Matsumura, Y.; Ouji-Sageshima, N.; Nishioka, T.; Hara, A.; Kayano, S.-I.; Ito, T. Persimmon-derived tannin ameliorates the pathogenesis of ulcerative colitis in a murine model through inhibition of the inflammatory response and alteration of microbiota. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Borges-Argaez, R.; Canche-Chay, C.I.; Pena-Rodriguez, L.M.; Said-Fernandez, S.; Molina-Salinas, G.M. Antimicrobial activity of Diospyros anisandra. Fitoterapia 2007, 78, 370–372. [Google Scholar] [CrossRef]
- Min, B.R.; Pinchak, W.E.; Anderson, R.C.; Callaway, T.R. Effect of tannins on the in vitro growth of Escherichia coli O157: H7 and in vivo growth of generic Escherichia coli excreted from steers. J. Food Prot. 2007, 70, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Štumpf, S.; Hostnik, G.; Primožič, M.; Leitgeb, M.; Salminen, J.-P.; Bren, U. The effect of growth medium strength on minimum inhibitory concentrations of tannins and tannin extracts against E. coli. Molecules 2020, 25, 2947. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Yoshikawa, H.; Katayama, R. Antioxidant activity in astringent and non-astringent persimmons. J. Hortic. Sci. Biotechnol. 2004, 79, 390–394. [Google Scholar] [CrossRef]
- Fukai, S.; Tanimoto, S.; Maeda, A.; Fukuda, H.; Okada, Y.; Nomura, M. Pharmacological activity of compounds extracted from persimmon peel (Diospyros kaki THUNB.). J. Oleo Sci. 2009, 58, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Lee, Y.; Kim, Y.T. Preventive effects of Citrus unshiu peel extracts on bone and lipid metabolism in OVX rats. Molecules 2014, 19, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-N.; Shin, M.-R.; Shin, S.H.; Lee, A.R.; Lee, J.Y.; Seo, B.-I.; Kim, M.Y.; Kim, T.H.; Noh, J.S.; Rhee, M.H. Study of antiobesity effect through inhibition of pancreatic lipase activity of Diospyros kaki fruit and Citrus unshiu peel. BioMed Res. Int. 2016, 2016, 1–7. [Google Scholar]
- Matsumura, Y.; Ito, T.; Yano, H.; Kita, E.; Mikasa, K.; Okada, M.; Furutani, A.; Murono, Y.; Shibata, M.; Nishii, Y.; et al. Antioxidant potential in non-extractable fractions of dried persimmon (Diospyros kaki Thunb.). Food Chem. 2016, 202, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, C.; Bucheli, P.; Wei, D. Citrus flavonoids in fruit and traditional Chinese medicinal food ingredients in China. Plant Foods Hum. Nutr. 2006, 61, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Yeo, E.; Song, E.; Chang, Y.-H.; Han, B.-K.; Choi, H.-J.; Hwang, J. Bioconversion of Citrus unshiu peel extracts with cytolase suppresses adipogenic activity in 3T3-L1 cells. Nutr. Res. Pract. 2015, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Gato, N.; Kadowaki, A.; Hashimoto, N.; Yokoyama, S.I.; Matsumoto, K. Persimmon Fruit Tannin-Rich Fiber Reduces Cholesterol Levels in Humans. Ann. Nutr. Metab. 2012, 62, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.-C.; Cho, W.-K.; Jeong, Y.H.; Im, G.Y.; Yang, M.C.; Hwang, Y.-H.; Ma, J.Y. Anti-inflammatory effect of Citrus Unshiu peel in LPS-stimulated RAW 264.7 macrophage cells. Am. J. Chin. Med. 2012, 40, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ra, J.; Song, J.-Y.; Gwak, C.; Kwon, H.-J.; Yim, S.-V.; Hong, S.-P.; Kim, J.; Lee, K.-H.; Cho, J.-J. Extracts from Citrus unshiu promote immune-mediated inhibition of tumor growth in a murine renal cell carcinoma model. J. Ethnopharmacol. 2011, 133, 973–979. [Google Scholar] [CrossRef]
- Pereira, K.; Salsamendi, J.; Casillas, J. The global nonalcoholic fatty liver disease epidemic: What a radiologist needs to know. J. Clin. Imaging Sci. 2015, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.-R.; Shin, S.H.; Roh, S.-S. Diospyros kaki and Citrus unshiu Mixture Improves Disorders of Lipid Metabolism in Nonalcoholic Fatty Liver Disease. Can. J. Gastroenterol. Hepatol. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Zhu, W.; Peng, J.; Deng, X.; Li, C. Persimmon tannin regulates the expression of genes critical for cholesterol absorption and cholesterol efflux by LXRα independent pathway. J. Funct. Foods 2016, 23, 283–293. [Google Scholar] [CrossRef]
- Hwang, K.-A.; Hwang, Y.-J.; Hwang, I.G.; Song, J.; Cho, S.M. Cholesterol-lowering effect of astringent persimmon fruits (Diospyros kaki Thunb.) extracts. Food Sci. Biotechnol. 2017, 26, 229–235. [Google Scholar] [CrossRef] [PubMed]
- de la Iglesia, R.; Milagro, F.I.; Campion, J.; Boque, N.; Martinez, J.A. Healthy properties of proanthocyanidins. Biofactors 2010, 36, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Macia, A.; Romero, M.P.; Valls, J.; Blade, C.; Arola, L.; Motilva, M.J. Bioavailability of procyanidin dimers and trimers and matrix food effects in in vitro and in vivo models. Br. J. Nutr. 2010, 103, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Blade, C.; Arola, L.; Salvado, M.J. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol. Nutr. Food Res. 2010, 54, 37–59. [Google Scholar] [CrossRef] [PubMed]
- van’t Slot, G.; Humpf, H.U. Degradation and Metabolism of Catechin, Epigallocatechin-3-gallate (EGCG), and Related Compounds by the Intestinal Microbiota in the Pig Cecum Model. J. Agric. Food Chem. 2009, 57, 8041–8048. [Google Scholar] [CrossRef]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Li, D.; Ho, C.-T.; Li, J.; Wan, X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281. [Google Scholar] [CrossRef]
- Espin, J.C.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Seeram, N.P.; Henning, S.M.; Zhang, Y.J.; Suchard, M.; Li, Z.P.; Heber, D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J. Nutr. 2006, 136, 2481–2485. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Bladé, C.; Aragonès, G.; Arola-Arnal, A.; Muguerza, B.; Bravo, F.I.; Salvadó, M.J.; Arola, L.; Suárez, M. Proanthocyanidins in health and disease. Biofactors 2016, 42, 5–12. [Google Scholar] [PubMed]
- Nash, V.; Ranadheera, C.S.; Georgousopoulou, E.N.; Mellor, D.D.; Panagiotakos, D.B.; McKune, A.J.; Kellett, J.; Naumovski, N. The effects of grape and red wine polyphenols on gut microbiota–A systematic review. Food Res. Int. 2018, 113, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014, 59, 41–52. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimia, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Carmody, R.N.; Kalariya, H.M.; Duran, R.M.; Moskal, K.; Poulev, A.; Kuhn, P.; Tveter, K.M.; Turnbaugh, P.J.; Raskin, I. Grape proanthocyanidin-induced intestinal bloom of Akkermansia muciniphila is dependent on its baseline abundance and precedes activation of host genes related to metabolic health. J. Nutr. Biochem. 2018, 56, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Png, C.W.; Lindén, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H. Mucolytic bacteria with increased prevalence in ibd mucosa augmentin vitroutilization of mucin by other bacteria. Off. J. Am. Coll. Gastroenterol. ACG 2010, 105, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Williams, E.E.; Lorca, G.L.; Norris, J.M.; Dunne, J.L. A Triple Threat? The Role of Diet, Nutrition, and the Microbiota in T1D Pathogenesis. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Hänninen, A.; Toivonen, R.; Pöysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; De Vos, W.M. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut 2018, 67, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Mageshwaran, T.; Ebenezar, A.R.; Madhanamadhubala, M.; Kavitha, S.; Mahalaxmi, S. Counteraction of reactive oxygen species and determination of antibacterial efficacy of proanthocyanidin and lycopene when mixed with calcium hydroxide and chlorhexidine mixture: An in vitro comparative study. J. Conserv. Dent. JCD 2012, 15, 337. [Google Scholar] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Macià, A.; Motilva, M.-J. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: A review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Andrés, C.; Seguí, L.; Barrera, C.; Jiménez-Hernández, N.; Artacho, A.; Betoret, N.; Gosalbes, M.J. Valorization of Persimmon and Blueberry Byproducts to Obtain Functional Powders: In Vitro Digestion and Fermentation by Gut Microbiota. J. Agric. Food Chem. 2020, 68, 8080–8090. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Cho, E.J.; Yokozawa, T. Effects of proanthocyanidin preparations on hyperlipidemia and other biomarkers in mouse model of type 2 diabetes. J. Agric. Food Chem. 2008, 56, 7781–7789. [Google Scholar] [CrossRef] [PubMed]
- Béné, C.; Fanzo, J.; Haddad, L.; Hawkes, C.; Caron, P.; Vermeulen, S.; Herrero, M.; Oosterveer, P. Five priorities to operationalize the EAT–Lancet Commission report. Nat. Food 2020, 1, 457–459. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Pérez Álvarez, J.A.; Fernández-López, J. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018, 256, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Lucas-González, R.; Pérez-Álvarez, J.Á.; Moscaritolo, S.; Fernández-López, J.; Sacchetti, G.; Viuda-Martos, M. Evaluation of polyphenol bioaccessibility and kinetic of starch digestion of spaghetti with persimmon (Dyospyros kaki) flours coproducts during in vitro gastrointestinal digestion. Food Chem. 2021, 338, 128142. [Google Scholar] [CrossRef]
- Russell, E.; Lynch, A.; Lynch, P.; Kerry, J. Quality and shelf life of duck liver pâté as influenced by dietary supplementation with α-tocopheryl acetate and various fat sources. J. Food Sci. 2003, 68, 799–802. [Google Scholar] [CrossRef]
- Lorente-Mento, J.M.; Lucas-González, R.; Sayas-Barbera, E.; Pérez-Álvarez, J.Á.; Fernández-López, J.; Viuda-Martos, M. Turrón Coproducts as Source of Bioactive Compounds: Assessment of Chemical, Physico-Chemical, Techno-Functional and Antioxidant Properties. Foods 2020, 9, 727. [Google Scholar] [CrossRef]
- Lucas-González, R.; Pellegrini, M.; Viuda-Martos, M.; Pérez-Álvarez, J.Á.; Fernández-López, J. Persimmon (Diospyros kaki Thunb.) coproducts as a new ingredient in pork liver pâté: Influence on quality properties. Int. J. Food Sci. Technol. 2019, 54, 1232–1239. [Google Scholar] [CrossRef]
- Lucas-González, R.; Pérez-Álvarez, J.Á.; Viuda-Martos, M.; Fernández-López, J. Persimmon Flour Co-Products as Novel Ingredients in the Reformulation of Pork Liver Pâté. Proceedings 2020, 70, 72. [Google Scholar] [CrossRef]
- Lucas-González, R.; Pérez-Álvarez, J.Á.; Viuda-Martos, M.; Fernández-López, J. Pork Liver Pâté Enriched with Persimmon Coproducts: Effect of In Vitro Gastrointestinal Digestion on Its Fatty Acid and Polyphenol Profile Stability. Nutrients 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Qi, S.; Lu, Z.; Li, L. Effects of immature persimmon (Diospyros kaki linn. F.) juice on the pasting, textural, sensory and color properties of rice noodles. J. Texture Stud. 2012, 43, 187–194. [Google Scholar] [CrossRef]
- Abbas, H.; Zaky, W.; Hassan, L.; Shahein, N.; Mohamed, A.; Samy, N.; Farahat, E. Impact of Kaki (Diospyros kaki) juice on the rheological, sensory and color properties of spreadable processed cheese analogue. J. Biol. Sci. 2019, 19, 231–236. [Google Scholar] [CrossRef]
- Arslan, S.; Bayrakci, S. Physicochemical, functional, and sensory properties of yogurts containing persimmon. Turk. J. Agric. For. 2016, 40, 68–74. [Google Scholar] [CrossRef]
- Abdallah, D.A.; El-Mageed, A.; Siliha, H.; Rabie, M. Physicochemical Characteristics of Persimmon Puree and its utilization in cupcake. Zagazig J. Agric. Res. 2017, 44, 2629–2640. [Google Scholar]
- Gautam, A.; Dhiman, A.K.; Attri, S.; Kathuria, D. Optimization of pulping method for extraction of pulp from ripe persimmon (Diospyros kaki L.) and its stability during storage. J. Appl. Nat. Sci. 2020, 12, 618–627. [Google Scholar] [CrossRef]
- Milczarek, R.R.; Vilches, A.M.; Olsen, C.W.; Breksa, A.P.; Mackey, B.E.; Brandl, M.T. Physical, microbial, and chemical quality of hot-air-dried persimmon (Diospyros kaki) chips during storage. J. Food Qual. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Milczarek, R.R.; Woods, R.D.; Lafond, S.I.; Smith, J.L.; Sedej, I.; Olsen, C.W.; Vilches, A.M.; Breksa, A.P.; Preece, J.E. Texture of Hot-Air-Dried Persimmon (Diospyros kaki) Chips: Instrumental, Sensory, and Consumer Input for Product Development. Foods 2020, 9, 1434. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Vegara, S.; Martí, N.; Valero, M.; Saura, D. Physicochemical characterization of special persimmon fruit beers using bohemian pilsner malt as a base. J. Inst. Brew. 2017, 123, 319–327. [Google Scholar] [CrossRef]
- Cho, J.-H.; Kim, I.-D.; Dhungana, S.K.; Do, H.-M.; Shin, D.-H. Persimmon fruit enhanced quality characteristics and antioxidant potential of beer. Food Sci. Biotechnol. 2018, 27, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Bayram, Y.; Ozkan, K.; Sagdic, O. Bioactivity, physicochemical and antimicrobial properties of vinegar made from persimmon (diospyros kaki) peels. Sigma J. Eng. Nat. Sci. /Mühendislik Fen Bilimleri Derg. 2020, 38, 1641–1652. [Google Scholar]
- Moon, Y.-J.; Cha, Y.-S. Effects of persimmon-vinegar on lipid metabolism and alcohol clearance in chronic alcohol-fed rats. J. Med. Food 2008, 11, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Hurh, B.S.; Shin, K.-S. Chemical characteristics and immuno-stimulatory activity of polysaccharides from fermented vinegars manufactured with different raw materials. J. Korean Soc. Food Sci. Nutr. 2015, 44, 191–199. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef]
- Fan, L.; Ding, S.; Ai, L.; Deng, K. Antitumor and immunomodulatory activity of water-soluble polysaccharide from Inonotus obliquus. Carbohydr. Polym. 2012, 90, 870–874. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kim, H.; Shin, K.S. In vitro and in vivo effects of polysaccharides isolated from Korean persimmon vinegar on intestinal immunity. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 867–876. [Google Scholar] [CrossRef]
- Park, D.-W.; Lee, H.S.; Shim, M.-S.; Yum, K.J.; Seo, J.T. Do kimchi and cheonggukjang probiotics as a functional food improve androgenetic alopecia? A clinical pilot study. World J. Mens Health 2020, 38, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Lye, H.-S.; Kuan, C.-Y.; Ewe, J.-A.; Fung, W.-Y.; Liong, M.-T. The improvement of hypertension by probiotics: Effects on cholesterol, diabetes, renin, and phytoestrogens. Int. J. Mol. Sci. 2009, 10, 3755–3775. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Hashiguchi, M.; Shiga, T.; Tamura, H.-O.; Mochizuki, M. Meta-analysis: Effects of probiotic supplementation on lipid profiles in normal to mildly hypercholesterolemic individuals. PLoS ONE 2015, 10, e0139795. [Google Scholar] [CrossRef]
- Tsai, T.-Y.; Chu, L.-H.; Lee, C.-L.; Pan, T.-M. Atherosclerosis-preventing activity of lactic acid bacteria-fermented milk− soymilk supplemented with Momordica charantia. J. Agric. food Chem. 2009, 57, 2065–2071. [Google Scholar] [CrossRef]
- Abularrage, C.J.; Sidawy, A.N.; Aidinian, G.; Singh, N.; Weiswasser, J.M.; Arora, S. Evaluation of the microcirculation in vascular disease. J. Vasc. Surg. 2005, 42, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; McClements, D.J. Progress in natural emulsifiers for utilization in food emulsions. Curr. Opin. Food Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Berton-Carabin, C.; Schroën, K. Towards new food emulsions: Designing the interface and beyond. Curr. Opin. Food Sci. 2019, 27, 74–81. [Google Scholar] [CrossRef]
- Liu, Z.; Pi, F.; Guo, X.; Guo, X.; Yu, S. Characterization of the structural and emulsifying properties of sugar beet pectins obtained by sequential extraction. Food Hydrocoll. 2019, 88, 31–42. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, Y.; Li, F.; Li, D.; Huang, Q. Pectin extracted from persimmon peel: A physicochemical characterization and emulsifying properties evaluation. Food Hydrocoll. 2020, 101, 105561. [Google Scholar] [CrossRef]
- Abadi, A.T.B. Helicobacter pylori treatment: New perspectives using current experience. J. Glob. Antimicrob. Resist. 2017, 8, 123–130. [Google Scholar] [CrossRef]
- Tepes, B.; O’Connor, A.; Gisbert, J.P.; O’Morain, C. Treatment of Helicobacter pylori infection 2012. Helicobacter 2012, 17, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Han, C.; Hu, T.; Zhang, J.; Wu, D.; Wang, F.; Liu, Y.; Ding, J.; Chen, K.; Yue, J.; et al. Peptide deformylase is a potential target for anti-Helicobacter pyloridrugs: Reverse docking, enzymatic assay, and X-ray crystallography validation. Protein Sci. 2006, 15, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Lu, H.; Dore, M.P. Relative potency of proton-pump inhibitors, Helicobacter pylori therapy cure rates, and meaning of double-dose PPI. Helicobacter 2019, 24, e12554. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Venerito, M.; Schulz, C. Helicobacter pylori infection: New facts in clinical management. Curr. Treat. options Gastroenterol. 2018, 16, 605–615. [Google Scholar] [CrossRef]
- Zhou, J.-T.; Li, C.-L.; Tan, L.-H.; Xu, Y.-F.; Liu, Y.-H.; Mo, Z.-Z.; Dou, Y.-X.; Su, R.; Su, Z.-R.; Huang, P.; et al. Inhibition of Helicobacter pylori and Its Associated Urease by Palmatine: Investigation on the Potential Mechanism. PLoS ONE 2017, 12, e0168944. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-H.; Hong, K.-S.; Hong, H.; Hahm, K.B. Detouring the Undesired Route of Helicobacter pylori-Induced Gastric Carcinogenesis. Cancers 2011, 3, 3018–3028. [Google Scholar] [CrossRef]
- Follmer, C. Ureases as a target for the treatment of gastric and urinary infections. J. Clin. Pathol. 2010, 63, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Chellia, R.; Hu, X.; Kathiresan, K.; Oh, D.-H.; Wang, M.-H. Eradication of Helicobacter pylori through the inhibition of urease and peptide deformylase: Computational and biological studies. Microb. Pathog. 2019, 128, 236–244. [Google Scholar] [CrossRef]
- Brumfitt, W.; Hamilton-Miller, J.; Franklin, I. Antibiotic activity of natural products: 1. Propolis. Microbios 1990, 62, 19–22. [Google Scholar]
- Hickey, W.J.; Shetty, A.R.; Massey, R.J.; Toso, D.B.; Austin, J. Three-dimensional bright-field scanning transmission electron microscopy elucidate novel nanostructure in microbial biofilms. J. Microsc. 2017, 265, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Creno, R.J.; Wenk, R.E.; Bohlig, P. Automated Micromeasurement of Urea Using Urease and the Berthelot Reaction. Am. J. Clin. Pathol. 1970, 54, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, K.; Wang, J.; Zhang, J.; Qi, Y.; Wei, X.; Fan, M. Young astringent persimmon tannin inhibits methicillin-resistant Staphylococcus aureus isolated from pork. LWT 2019, 100, 48–55. [Google Scholar] [CrossRef]
- Pereira, V.; Lopes, C.; Castro, A.; Silva, J.; Gibbs, P.; Teixeira, P. Characterization for enterotoxin production, virulence factors, and antibiotic susceptibility of Staphylococcus aureus isolates from various foods in Portugal. Food Microbiol. 2009, 26, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Rasamiravaka, T.; Andriatsitohanana, T.T.; Rasamindrakotroka, A. Evaluation of methicillin-resistant Staphylococcus aureus nasal carriage in Malagasy pig and poultry non-industrial farmers. J. Infect. Dev. Ctries. 2017, 11, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kali, A. Antibiotics and bioactive natural products in treatment of methicillin resistant Staphylococcus aureus: A brief review. Pharmacogn. Rev. 2015, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Eliopoulos, G.M. Microbiology of drugs for treating multiply drug-resistant Gram-positive bacteria. J. Infect. 2009, 59, S17–S24. [Google Scholar] [CrossRef]
- Díaz-Gómez, R.; López-Solís, R.; Obreque-Slier, E.; Toledo-Araya, H. Comparative antibacterial effect of gallic acid and catechin against Helicobacter pylori. LWT-Food Sci. Technol. 2013, 54, 331–335. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Xu, Y.; Zhang, B.; Xia, X. Antimicrobial effect and mode of action of chlorogenic acid on Staphylococcus aureus. Eur. Food Res. Technol. 2014, 238, 589–596. [Google Scholar] [CrossRef]
- Sianglum, W.; Srimanote, P.; Taylor, P.W.; Rosado, H.; Voravuthikunchai, S.P. Transcriptome Analysis of Responses to Rhodomyrtone in Methicillin-Resistant Staphylococcus aureus. PLoS ONE 2012, 7, e45744. [Google Scholar] [CrossRef]
- Hussein, Z.; Caleb, O.J.; Opara, U.L. Perforation-mediated modified atmosphere packaging of fresh and minimally processed produce—A review. Food Packag. Shelf Life 2015, 6, 7–20. [Google Scholar] [CrossRef]
- Matheus, J.R.V.; De Assis, R.M.; Correia, T.R.; Da Costa Marques, M.R.; Leite, M.C.A.M.; Pelissari, F.M.; Miyahira, R.F.; Fai, A.E.C. Biodegradable and Edible Film Based on Persimmon (Diospyros kaki L.) Used as a Lid for Minimally Processed Vegetables Packaging. Food Bioprocess Technol. 2021, 14, 765–779. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total. Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.I.; Shin, Y. Application of persimmon (Diospyros kaki L.) peel extract in indigo dyeing as an eco-friendly alternative reductant. Fash. Text. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Huh, M.-W.; Bae, J.-S.; An, S.-Y. Dyeability and functionality of silk fabrics treated with persimmon juice. Fash. Text. Res. J. 2008, 10, 1036–1044. [Google Scholar]
- Huh, M.-W. Dyeability and functionality of cotton fabrics treated with persimmon juice. Text. Coloration Finish. 2011, 23, 241–249. [Google Scholar] [CrossRef]
- Kim, I.-D.; Dhungana, S.; Park, Y.-S.; Kim, D.; Shin, D.-H. Persimmon Fruit Powder May Substitute Indolbi, a Synthetic Growth Regulator, in Soybean Sprout Cultivation. Molecules 2017, 22, 1462. [Google Scholar] [CrossRef] [PubMed]
- Conesa, C.; Laguarda-Miró, N.; Fito, P.; Seguí, L. Evaluation of Persimmon (Diospyros kaki Thunb. cv. Rojo Brillante) industrial residue as a source for value added products. Waste Biomass Valorization 2019, 11, 3749–3760. [Google Scholar] [CrossRef]
- Ayşe, K.; Ertuğrul, K. Medical and Cosmetic Applications of Persimmon (Diospyros Kaki): Toxicity Assessment-A review. Int. J. Tradit. Complementary Med. Res. 2020, 1, 162–176. [Google Scholar]
- Déprez, S.; Brezillon, C.; Rabot, S.; Philippe, C.; Mila, I.; Lapierre, C.; Scalbert, A. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J. Nutr. 2000, 130, 2733–2738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, Y.; Li, X.; Wang, F. Proanthocyanidin Encapsulated in Ferritin Enhances Its Cellular Absorption and Antioxidant Activity. J. Agric. Food Chem. 2019, 67, 11498–11507. [Google Scholar] [CrossRef]
- Sun, X.; Wu, X.; Chen, X.; Guo, R.; Kou, Y.; Li, X.; Sheng, Y.; Wu, Y. Casein-maltodextrin Maillard conjugates encapsulation enhances the antioxidative potential of proanthocyanidins: An in vitro and in vivo evaluation. Food Chem. 2021, 346, 128952. [Google Scholar] [CrossRef] [PubMed]
- Moberg, E.; Allison, E.H.; Harl, H.K.; Arbow, T.; Almaraz, M.; Dixon, J.; Scarborough, C.; Skinner, T.; Rasmussen, L.V.; Salter, A. Combined innovations in public policy, the private sector and culture can drive sustainability transitions in food systems. Nat. Food 2021, 2, 282–290. [Google Scholar] [CrossRef]
- Eisenstein, M. Natural solutions for agricultural productivity. Nature 2020, 588, S58–S59. [Google Scholar] [CrossRef] [PubMed]
- Armitage, C. Sustainable nutrition. Nature 2020, 588, S53. [Google Scholar] [CrossRef] [PubMed]

| Parameters | 100 g of the Edible Part |
|---|---|
| Energy (Kcal) | 71.50 |
| Proteins (g) | 0.64 |
| Total lipids (g) | 0.25 |
| Carbohydrates (g) | 17.30 |
| Fibers (g) | 2.60 |
| Water (g) | 80.86 |
| Calcium (mg) | 8.00 |
| Iron (mg) | 0.20 |
| Magnesium (mg) | 9.25 |
| Zinc (mg) | 0.11 |
| Sodium (mg) | 2.50 |
| Potassium (mg) | 230.00 |
| Phosphorus (mg) | 19.50 |
| Selenium (µg) | 0.60 |
| Thiamine (mg) | 0.03 |
| Riboflavin (mg) | 0.03 |
| Niacin equivalents (mg) | 0.20 |
| Vitamin B6 (mg) | 0.10 |
| Vitamin C (mg) | 11.75 |
| Total Vitamin A (retinol equivalents) (µg) | 177.00 |
| Folate (µg) | 7.00 |
| Phenolic Compounds (PCs) | Quantification and Reference | |||
|---|---|---|---|---|
| Gallic acid (mg/100 g FW) | 0.953 ± 0.344 [20] | 2.794 ± 0.263 [75] | 2.789 ± 0.003 [76] | 2.43 ± 0.215 [26] |
| Caffeic acid (mg/100 g FW) | 0.078 ± 0.001 [76,77] | 0.1 ± 0.001 [75,76] | ||
| P-coumaric (mg/100 g FW) | 0.048 ± 0.004 [76] | 0.097 ± 0.004 [75] | 0.088 ± 0.046; 0.113 ± 0.055 [20] | |
| Ferulic acid (mg/100 g FW) | 0.1 ± 0.001 [75,76] | 0.008 ± 0.003 [20] | ||
| Chlorogenic acid (mg/100 g FW) | 0.171 ± 0.016 [75] | 0.274 ± 0.003 [76] | ||
| Protocatechuic acid (mg/100 g FW) | 0.013 ± 0.010; 0.004 ± 0.002 [20] | 0.005 ± 0.000 [75] | ||
| Ellagic acid (mg/100 g FW) | 0.327 ± 0.173 [20] | |||
| Quercetin (mg/100 g FW) | 0.224 ± 0.002;0.812 ± 0.006 [76] | |||
| Proanthocyanins (mg/100 g FW) | 540.2 ± 0.000 [74] | 744 ± 8.6 [75] | ||
| Identifications | ||||
| (Epi)catechin and (epi)gallocatechin | [13,59,70,71,72,82] | |||
| Quercetin 3--2′′-galloylglucoside), quercetin 3-O-glucoside and isomer and aglycone | [59] | |||
| Kaempferol-3-O-glucoside, kaempferol 3-(2′′-galloylglucoside) | [59] | |||
| 2-Methoxy-1, 4-benzoquinone | [59] | |||
| Target | Biological Effect (s) | References |
|---|---|---|
| Atherosclerosis | Male Wistar rats and male mice (C57BL/6.Cr) submitted to a high-cholesterol diet showed that fruit administration made it difficult to increase lipid levels in serum and made it difficult to decrease antioxidant activity in plasma. Rat diets enriched with either 7% of phenol-free dry persimmon or 7% whole dry persimmon enhanced lipid levels. This was considerable when entire dry persimmon was included. Persimmon’s antioxidant effect was primarily linked with its phenols and was attained through the addition of whole dry fruit to basal diet. The capacity of dried young persimmon fruit to bind bile acid adds to its hypolipidemic effect in mice, and tannins are one of the functional constituents in young persimmon fruit. | [73,91,111] |
| Lipidic metabolism | With male C57BL/6 mice, bile acid-binding ability of kaki-tannin was analyzed versus cholic acid, deoxycholic acid, glycocholic acid, and taurocholic acid in vitro. The impact on fecal bile acid excretion in mice was also analyzed. Kaki-tannin’s bile acid-binding ability was feebler than that of cholestyramine, all the bile acids analyzed were adsorbed by kaki-tannin and considerably fostered fecal bile acid excretion in mice when given at 1% (w/w) in the diet. Kaki-tannin was able to bind bile acids, within the range of concentrations of bile acids found in the human intestine. The cultivars of young persimmon (Fuyu and Hachiya) administered to rats induced a significant reduction in the levels of total cholesterol, low-density lipoprotein (LDL), and triglycerides in relation to the control and the group of the mature persimmon (also of the Fuyu and Hachiya type). It prevented high-fat diet induced liver steatosis. Expression of liver cholesterol 7 alpha-hydroxylase gene (CYP7A1) was increased by approximately three times. Highly polymerized tannins were the functional constituents related to the hypolipidemic effect demonstrated. Hypolipidemic impacts of young persimmon fruit on apolipoprotein E-deficient C57BL/6.KOR-ApoEshl mice fed a diet supplemented with dry young persimmon fruit. This treatment considerably decreased plasma chylomicron, very low-density lipoprotein (VLDL) and low-density lipoprotein (LDL) cholesterols, and triglyceride, and this reaction was complemented by an increase in the excretion of fecal bile acid. Within the liver, sterol regulatory element binding protein-2 gene expression was considerably greater in mice fed young persimmon fruit, while the mRNA and protein levels of the LDL receptor were unaltered. These findings suggest that increase in the speed of fecal bile acid excretion is a key mechanism of the hypolipidemic impact induced by young persimmon fruit in C57BL/6.KOR-ApoEshl mice. Fermented persimmon extract (FPE) is completely unknown. The impacts of FPE on mice metabolic parameters fed with a high-fat diet (HFD) was analyzed. Results showed that supplementation with FPE led to an approximate 15% body weight decrease, abdominal and liver fat decrease, and lowered serum levels of total cholesterol, triglycerides, and glucose. FPE was also found to hinder the differentiation of murine 3T3-L1 pre-adipocyte cells into mature adipocytes. It is suggested that gallic acid is a key bioactive element of FPE, and that AMP-activated protein kinase facilitates the positive impacts of FPE and gallic acid. Kaki-tannin adsorbed all the bile acids analyzed and meaningfully encouraged the excretion of fecal bile acid within the range of concentrations of bile acids found in the human intestine in male mice C57BL/6J when supplied at 1% (w/w) in the diet, proving useful in the prevention and amelioration of metabolic syndrome. | [18,93,112,113] |
| Male Sprague-Dawley rats were fed a 2% high-cholesterol diet and given distinct doses of high molecular weight persimmon tannin (HMWPT) or without HMWPT for 9 weeks. A treatment of 100 mg high molecular weight tannins/kg of body weight per day could significantly increase the activity of serum lecithin cholesterol acyl transferase (LCAT) and fecal excretion of bile acids. The deposition of hepatic lipid droplets and hepatic steatosis, prompted by the high cholesterol diet, were clearly hampered by high molecular weight tannins. HMWPT was accountable for the hypocholesterolemic impact of persimmon and it may well exert a hypolipidemic impact by stimulating serum LCAT activity, boosting fecal bile acid excretion and enhancing antioxidant profile. | [114] | |
| Glucose Metabolism/Type 2 Diabetes (T2D) | Expression of genes associated with fatty acid synthesis and glycolysis was increased, while gene expression associated with β-oxidation and gluconeogenesis was decreased in the liver of Goto-Kakizaki rats fed a diet supplemented with the extract of persimmon peel. Apoptosis-related gene expression decreased, while ribosome-related gene expression was increased in the group of rats that ingested persimmon peel extract. Results showed that the insulin signaling pathway was triggered in the persimmon peel group, with a subsequent increase in insulin sensitivity. Ingestion of this extract helped to maintain euglycemia and lipid homeostasis. Fat-soluble extract from persimmon peel and fed T2D Goto-Kakizaki (GK) rats an AIN-93G rodent diet enhanced with persimmon peel extract (PP diet) for 12 weeks. In contrast with the control AIN-93G diet, the PP diet considerably lowered the activity of plasma glutamic-pyruvatetransaminase, with buildup of β-cryptoxanthin in the liver. DNA microarray assessments showed that the persimmon peel diet modified hepatic gene expression profiles. Expression of insulin signaling pathway-related genes was considerably enhanced in differentially expressed gene sets. Western blotting analysis also demonstrated a rise in insulin receptor beta tyrosine phosphorylation in PP diet fed rats. Persimmon peel extract impacts gene expression related to the insulin signaling pathway, the PP diet increases insulin resistance in GK rats. Downregulation of Ptpσ through administration of persimmon peel extract promotes tyrosine phosphorylation of IRβ, leads to activation of the insulin signaling pathway, and upregulates genes related to both glucose homeostasis and lipid homeostasis. Results indicate that dietary intake of persimmon peel extract can assist in maintaining euglycemia. | [1] |
| Chronic inflammation of collagen-induced arthritis (CIA) | Persimmon extract’s anti-inflammatory activity in rats with collagen-induced arthritis (CIA) was demonstrated by the considerable decrease in both the edema volume and radiological variations credited to bone CIA. Administering persimmon extract (15 mg/kg p.o. per day) diminishes the extent of chronic inflammation and tissue damage typical of CIA in rats, effect related most likely to the potent antioxidant qualities of the extract. Taking into consideration the repressive effect of the fresh persimmon fruit extract (1.1–17.5 μg/mL) on human neutrophil oxidative burst, an IC50 of 7.5 ± 1.0 μg/mL was established. In both of these cases, the extract showed the ability to act as an intracellular antioxidant as well as the capability to interfere with the neutrophil function, hindering the release of deleterious reactive oxygen species that would magnify the inflammatory signals already activated. The valuable properties of persimmon extract in this model might be associated with a reduction in neutrophil activation and subsequent decrease in the release of proinflammatory neutrophil-derived products, which is also in harmony with the positive results seen in the paw edema models (induced by CIA and carrageenan), both of which represent inflammation models characteristically with high correlation to neutrophil activation. The extract proved capable of acting as an intracellular antioxidant as well as inhibiting the neutrophils’ function, constraining the release of deleterious ROS that amplified inflammatory signals already triggered. | [54] |
| Allergic inflammation | Mast cell-mediated allergic inflammation in vivo, systemic anaphylaxis model was induced in mice that were given an intraperitoneal injection [8 mg/kg of body weight (BW)] of the mast cell degranulator, compound 48/80 and passive cutaneous anaphylaxis (PCA) model in mice were induced and in vitro studies of histamine and β-hexosaminidase levels, cAMP, and intracellular calcium levels, real-time polymerase chain reaction (RT-PCR) to analyze the mRNA expression of TNF-α, IL-1β, and β-actin, nuclear and cytosolic p65 NF-κB, and IκBα were assayed using anti-NF-κB (p65) and anti-IκBα antibody. The persimmon extract repressed the release of histamine and β-hexosaminidase from mast cells by modulating intracellular calcium levels; diminished gene expression and the secretion of the pro-inflammatory cytokines, tumor necrosis factor (TNF)-α, and interleukin (IL)-1β was obtained by inhibiting nuclear factor-kB, effects comparable to those observed with disodium cromoglicate, suggesting the potential therapeutic use of a Diospyros kaki aqueous extract in allergic inflammatory disorders. AEDK constrains systemic allergic reaction and the delivery of histamine in serum, which is an index of mast cell degranulation. Additionally, administering AEDK to mice kept them from IgE-mediated PCA, one of the most significant in vivo models of acute local anaphylaxis. In systemic and local anaphylaxis, the repressive effects of AEDK were similar to those of DSCG, a clinically used medication for treating asthma and allergies. AEDK restrains the delivery of calcium from intracellular calcium stores, suggesting the regulatory role of cAMP in histamine release. Due to the structural similarity of EGCG and catechin, it was theorized that catechin may be one of the compounds accountable for the anti-allergic and anti-inflammatory effects of AEDK. | [31] |
| Sarcopenia (age-related syndrome characterized by progressive loss of mass and strength of skeletal muscles) | An extract of Diospyros kaki was tested using a Caco-2 cell coculture system. An in vitro model for studying the toxicity and metabolism of drugs including bioactive compounds of plants. Caco-2 cells subjected to 0.5 mg/ml D. Kaki extract diminished the oxidative stress-induced decline of mouse myoblast cell C2C12 viability, which indicates that the extract may be able to promote intestinal epithelial cells to produce secretions that decrease oxidative stress in myoblasts in vitro. This feature is linked to the ability of persimmon extracts to stimulate epithelial cells to produce secretions that possess the ability to inhibit intracellular ROS production. The concept of functional substances is highlighted here. The effect of the Japanese persimmon fractions tested on C2C12 growth activity was found to be different in a single culture and coculture system. This result showed that the metabolic product from Caco-2 cell culture treated with this fraction had a considerable impact on C2C12 cell viability. The effects of phytochemical compounds and cellular communications that may be involved in the metabolism of the test compounds is critical in assessing the bioavailability of tested compounds. DPPH method, ORAC assay, total phenolic content (TPC), and trans-endothelial electrical resistance (TEER) were measured for measuring electrical resistance of a cell, and ROS intracellular production quantification by the oxidation-sensitive fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) was performed. | [115] |
| Cognition deficits and oxidative damage in senescent mice | High molecular weight persimmon condensed tannin (HMWPT) meaningfully augmented the decreased activities of superoxide dismutase, catalase, raised the lowered total anti-oxidation capability, glutathione (GSH), and hydroxyproline contents, and reduced the raised monoamine oxidase, total cholinesterase activities, and malondialdehyde level in serum, liver, or brain of senescent mice, an aging mice model induced by D-galactose in a dose-dependent fashion. Additionally, HMWPT substantially diminished the D-galactose induced number decline, neuronal degeneration, and karyopycnosis in cells in the hippocampus and reduction in thickness of skin epidermis and dermis. This accounted for the amelioration of the spontaneous behavior and cognitive performance and skin aging inhibition. HMWPT may well decrease memory impairment and enhance behavior performance in the D-gal induced aging mice. | [116] |
| In vitro cerebral ischemia | Persimmon extract protected PC12 cells from oxidative stress generated by the deprivation of glucose, oxygen, and serum (Glucose-Oxygen-Serum Deprivation (GOSD)-Induced PC12 Cells Injury) via an antioxidant mechanism, with reduced intracellular ROS production. The intracellular ROS levels was accomplished with a fluorescent probe, H2DCF-DA, and the effects of PeHE and PuHE on ROS production following GOSD insult in PC12 cells revealed that pretreatment (2 h) with peel and pulp fruit extracts of D. kaki was able to encourage cell survival and reduce ROS growth upon GOSD stress in PC12 cells. A potential system behind the mitigation of ROS production following ischemic insult is due to antioxidant and free radical scavenger characteristics of persimmon. | [117] |
| Cancer | Nine human cancer cell lines (A375, A549, ACHN, C32, caco-2, COR-L23, Huh-12, LNCaP, and MCF-7) were tested with D. Lotus extract as well as eight compounds (quercetin, kaempferol, methylgallate, ellagic acid, gallic acid, myricetin, myricetin 3-O-α-ramnoside, and myricetin 3-O-β-glucuronide) isolated from the D. Lotus fruit. D. lotus extract tested in different in vitro systems (ABTS, DPPH, FRAP, and Fe2+ chelating activity assay) demonstrated considerable antioxidant activity. D. lotus extract exhibited high antioxidant activity and chelating properties, and these activities are related to the phenolic content. The extract exerted the greatest antiproliferative activity of the cells of the tumor line COR-L23, among the hydrolyzed tannins identified, ellagic acid showed strong antiproliferative activity against C32 and A375 cells. Gallic acid showed the highest cytotoxic activity against Caco-2 cells. | [118] |
| Melanoma | The prepared extract of the skin of the Japanese persimmon (Diospyros kaki Fuyu) inhibited the melanin biosynthesis in the B16 rat melanoma cells. From this extract, two active compounds were isolated, which were identified as flavonoid glycosides, isoquercitrin (quercetin-3-O-glucoside) and hyperine (quercetin-3-O-galactoside). These two glycosylated flavonoids showed a strong inhibitory effect on melanin production, this inhibitory effect was due to the suppression of tyrosinase protein expression and not on tyrosinase activity. | [119] |
| Inhibition of melanogenesis (skin whitening effects) | The guinea pig pigmentation model was established by ultraviolet B (UVB) irradiation (with a height of 10 cm from the skin and ultraviolet intensity of 1395 UW/cm2, each for 24 min per day for a total of 5 days). Half male and half female white guinea pigs were used. Masson–Fontana silver staining was employed to examine the impacts of persimmon tannin extract on melanin distribution in guinea pigs’ skin tissue and arbutin as a positive control. Tyrosinase activity was also assessed, and an enzyme-linked immunosorbent assay was employed to examine the contents of antioxidant enzymes, inflammatory factors, and signaling pathway inhibitors in the guinea pigs’ skin tissue. The persimmon (Diospyros Kaki Thunb.) tannin extract (the medium-dose group—20% persimmon tannin extract) could significantly reduce melanin density in white guinea pigs. The variations in experimental results were statistically significant (p < 0.01). The medium-dose group was more effective in inhibiting tyrosinase activity than arbutin. IL-6 and IL-8 expression were decreased by around 22.2% and 54%. Inhibition of inflammatory mediators can reduce melanogenesis in melanocytes. In contrast with the model group, catalase, glutathione peroxidase, superoxide dismutase, persimmon tannin extract (PTE) could significantly increase the content of CAT, GPX, and SOD in skin tissues after UVB irradiation. This reduces the level of active oxygen in melanocytes and prevents the activation of melanin synthesis. The Wnt/β-catenin signaling pathway can promote melanogenesis, while DKK1 might inhibit the binding of Wnt protein to its receptor. The effect of PTE in the medium-dose group was better than that in the arbutin group, and the DKK1 content was 8% higher than that in the arbutin group. Overexpression of DKK1 could significantly inhibit cell survival and melanogenesis. In conclusion, PTE could inhibit melanocyte growth and strongly inhibit melanogenesis, which could inhibit skin pigmentation caused by UVB irradiation. The optimal content of persimmon tannin for inhibiting pigmentation was 20%. The inhibitory tyrosinase activity was raised by 24.3%, 33.3%, 59.3%, 36.81%, and 17.16%, respectively. By now, the health-promoting potential of the tannins is widely recognized; however, the application of persimmon tannin in whitening and inhibiting pigmentation has rarely been examined and reported. | [120] |
| Human lymphoid leukemia Molt 4B cells | A persimmon extract and a few of its individual phenolic compounds (epicatechin gallate, epigallocatechin, epicatechin, and catechin) were studied on the growth of human lymphoid leukemia Molt 4B cells, and it was observed that the extract as well as epigallocatechin and epicatechin gallate reduced the growth of these cells in a dose dependent manner. After treating for 3 days, severe cell damage was observed such as DNA fragmentation. Apoptosis of these cells were induced by tested phenolic compounds. | [81] |
| Thyroid cancer | A case-controlled analysis of thyroid cancer in Korean women, emphasized an inverse correlation observed between eating persimmons and benign and malignant thyroid cancer risk. Elevated consumption of raw vegetables, tangerines, and persimmons might reduce the risk of developing thyroid cancer and assist in the prevention of early-stage thyroid cancer. | [121] |
| Colitis and colon cancer cell | A phenolic-rich extract was tested using an in vivo model (male CD-1 mice) of experimental colitis (TNBS-induced colitis) and an in vitro model of colon adenocarcinoma cells (HT-29). Results demonstrated beneficial effects of a phenolic extract of persimmon in the reduction in experimental colitis severity, reduction of diarrhea severity, hemorrhagic injury, the reduction of the mortality rate, and the successful impairment of cell proliferation and invasion in HT-29 cell model. A reduced expression of iNOS and COX-2 in the colonic tissue of colitis mice contributes to the impairment of the inflammatory process in the colon. There was no inhibition of the gelatinase MMP-9 and MMP-2 activities, something which could partly result from the colitis animal model, where the phenolic concentrations utilized were far more diminished than those necessary to reduce MMP-9. The NF-κB pathway is a potential aim for the beneficial effects of the persimmon phenolic extract, as evidenced in these experiments. Considering the part that inflammatory processes play in the progression of CRC and the significant link between inflammation and cancer, the results highlight the potential of persimmon phenolic compounds as a pharmacological tool in the treatment of IBD patients. Persimmon phenolic extract may well be acting pleiotropically in several mechanisms of action and/or acting on an upstream mediator of inflammatory processes that reduce the expression of COX-2 and iNOS. | [17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Direito, R.; Rocha, J.; Sepodes, B.; Eduardo-Figueira, M. From Diospyros kaki L. (Persimmon) Phytochemical Profile and Health Impact to New Product Perspectives and Waste Valorization. Nutrients 2021, 13, 3283. https://doi.org/10.3390/nu13093283
Direito R, Rocha J, Sepodes B, Eduardo-Figueira M. From Diospyros kaki L. (Persimmon) Phytochemical Profile and Health Impact to New Product Perspectives and Waste Valorization. Nutrients. 2021; 13(9):3283. https://doi.org/10.3390/nu13093283
Chicago/Turabian StyleDireito, Rosa, João Rocha, Bruno Sepodes, and Maria Eduardo-Figueira. 2021. "From Diospyros kaki L. (Persimmon) Phytochemical Profile and Health Impact to New Product Perspectives and Waste Valorization" Nutrients 13, no. 9: 3283. https://doi.org/10.3390/nu13093283
APA StyleDireito, R., Rocha, J., Sepodes, B., & Eduardo-Figueira, M. (2021). From Diospyros kaki L. (Persimmon) Phytochemical Profile and Health Impact to New Product Perspectives and Waste Valorization. Nutrients, 13(9), 3283. https://doi.org/10.3390/nu13093283







