L-Citrulline Supplementation and Exercise in the Management of Sarcopenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. CM Supplementation
2.3. Physical Tests
2.4. Blood Parameters
2.5. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lenk, K.; Schuler, G.; Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia Sarcopenia Muscle 2010, 1, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolland, Y.; Van Kan, G.A.; Gillette-Guyonnet, S.; Vellas, B. Cachexia versus sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Janssen, I.; Baumgartner, R.N.; Ross, R.; Rosenberg, I.H.; Roubenoff, R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am. J. Epidemiol. 2004, 159, 413–421. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 8, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Huygens, W.; Thomis, M.A.; Peeters, M.W.; Aerssens, J.; Janssen, R.; Vlietinck, R.F.; Beunen, G. Linkage of myostatin pathway genes with knee strength in humans. Physiol. Genom. 2004, 17, 264–270. [Google Scholar] [CrossRef]
- Calvani, R.; Joseph, A.M.; Adhihetty, P.J.; Miccheli, A.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; Marzetti, E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol. Chem. 2013, 394, 393–414. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K. Edward, F. Adolph distinguished lecture: Muscle as an endocrine organ: IL-6 and other myokines. J. Appl. Physiol. 2009, 107, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Porter, M.M.; Vandervoort, A.A.; Lexell, J. Aging of human muscle: Structure, function and adaptability. Scand. J. Med. Sci. Sports 1995, 5, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Aran, L.; Bulli, G.; Curcio, F.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Sarcopenia: Assessment of disease burden and strategies to improve outcomes. Clin. Interv. Aging 2018, 13, 913–927. [Google Scholar] [CrossRef] [Green Version]
- Palomero, J.; Jackson, M.J. Redox regulation in skeletal muscle during contractile activity and aging. J. Anim. Sci. 2010, 88, 1307–1313. [Google Scholar] [CrossRef]
- Weisiger, R.A.; Fridovich, I. Superoxide dismutase. Organelle specificity. J. Biol. Chem. 1973, 248, 3582–3589. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-Induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H.; Jones, D.P. Encyclopedia of Stress; Elsevier: San Diego, CA, USA, 2007. [Google Scholar]
- Wei, W.; Fareed, M.U.; Evenson, A.; Menconi, M.J.; Yang, H.; Petkova, V.; Hasselgren, P.O. Sepsis stimulates calpain activity in skeletal muscle by decreasing calpastatin activity but does not activate caspase-3. Am. J. Physiol. 2004, 288, 580–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, A.; López-Cepero, J.M.; Sánchez del Pino, M.J. Skeletal muscle and aging. Front. Biosci. 2001, 6, 26–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, M.B. Free radicals and muscle fatigue: Of ROS, canaries, and the IOC. Free Radic. Biol. Med. 2008, 44, 169–179. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [Green Version]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. Almost all about citrulline in mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef]
- Bescós, R.; Sureda, A.; Tur, J.A.; Pons, A. The effect of nitric-oxide-related supplements on human performance. Sports Med. 2012, 4, 99–117. [Google Scholar] [CrossRef]
- Cynober, L. Citrulline: Just a biomarker or a conditionally essential amino acid and a pharmaconutrient in critically ill patients? Crit. Care 2013, 17, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadia, C.; Osowska, S.; Cynober, L.; Forbes, A. Citrulline in health and disease. Review on human studies. Clin. Nutr. 2018, 37, 1823–1828. [Google Scholar] [CrossRef] [Green Version]
- Botchlett, R.; Lawler, J.M.; Wu, G. L-arginine and L-citrulline in sports nutrition and health. Nutrition and enhanced sports performance. In Muscle Building, Endurance, and Strength; Bagchi, D., Nair, S., Sen, C.K., Eds.; Elsevier: San Diego, CA, USA, 2019; pp. 645–652. [Google Scholar]
- Bahri, S.; Curis, E.; El Wafi, F.; Aussel, C.; Chaumeil, J.; Cynober, L.; Zerrouk, N. Mechanisms and kinetics of citrulline uptake in a model of human intestinal epithelial cells. Clin. Nutr. 2008, 27, 872. [Google Scholar] [CrossRef]
- Sureda, A.; Cordova, A.; Ferrer, M.D.; Tauler, P.; Pérez, G.; Tur, J.A.; Pons, A. Effects of L-citrulline oral supplementation on polymorphonuclear neutrophils oxidative burst and nitric oxide production after exercise. Free Radic. Res. 2009, 43, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Sakurada, M.; Watanabe, F.; Yamasaki, T.; Doi, H.; Ezaki, H.; Morishita, K. Effects of oral L-citrulline supplementation on lipoprotein oxidation and endothelial dysfunction in humans with vasospastic angina. Immunol. Endocrinol. Metab. Agents Med. Chem. 2013, 13, 214–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Poll, M.G.C.; Soeters, P.B.; Deutz, N.E.P.; Fearon, K.C.H.; Dejong, C.H.C. Renal metabolism of amino acids: Its role in interorgan amino acid exchange. Am. J. Clin. Nutr. 2004, 79, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. L-Citrulline supplementation: Impact on cardiometabolic health. Nutrients 2018, 10, 921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Bénazeth, S.; Cynober, L. Dose ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The citrudose pharmacokinetic study. Br. J. Nutr. 2008, 99, 855–862. [Google Scholar] [CrossRef]
- Huerta Ojeda, Á.; Domínguez de Hanna, A.; Barahona-Fuentes, G. The effect of supplementation with L-arginine and L-citrulline on physical performance: A systematic review. Nutr. Hosp. 2019, 36, 1389–1402. [Google Scholar] [CrossRef]
- Bailey, S.J.; Blackwell, J.R.; Williams, E.; Vanhatalo, A.; Wylie, L.J.; Winyard, P.G.; Jones, A.M. Two weeks of watermelon juice supplementation improves nitric oxide bioavailability but not endurance exercise performance in humans. Nitric. Oxide 2016, 59, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Gonzales, J.U.; Raymond, A.; Ashley, J.; Kim, Y. Does L-citrulline supplementation improve exercise blood flow in older adults? Exp. Physiol. 2017, 102, 1661–1671. [Google Scholar] [CrossRef] [Green Version]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef]
- Pascual-Fernández, J.; Fernández-Montero, A.; Córdova-Martínez, A.; Pastor, D.; Martínez-Rodríguez, A.; Roche, E. Sarcopenia: Molecular pathways and potential targets for intervention. Int. J. Mol. Sci. 2020, 21, 8844. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Seelaender, M.; Sotiropoulos, A.; Coletti, D.; Lancha, A.H., Jr. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition 2019, 60, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.J.; Allison, R.; Close, G.L. Vitamin D and the athlete: Current perspectives and new challenges. Sports Med. 2018, 48, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Guisado, J.; Jakeman, P.M. Citrulline malate enhances athletic anaerobic performance and relieves muscle soreness. J. Strength Cond. Res. 2010, 24, 1215–1222. [Google Scholar] [CrossRef]
- Wax, B.; Kavazis, A.N.; Weldon, K.; Sperlak, J. Effects of supplemental citrulline malate ingestion during repeated bouts of lower-body exercise in advanced weight lifters. J. Strength Cond. Res. 2014, 29, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Cutrufello, P.T.; Gadomski, S.J.; Zavorsky, G.S. The effect of l-citrulline and watermelon juice supplementation on anaerobic and aerobic exercise performance. J. Sports Sci. 2015, 33, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.M.; Gray, M.; Wethington, L.N.; Stone, M.S.; Stewart, R.W.; Moyen, N.E. Acute citrulline malate supplementation improves upper- and lower-body submaximal weightlifting exercise performance in resistance-trained females. Eur. J. Nutr. 2017, 56, 775–784. [Google Scholar] [CrossRef]
- Suzuki, I.; Sakuraba, K.; Horiike, T.; Kishi, T.; Yabe, J.; Suzuki, T.; Morita, M.; Nishimura, A.; Suzuki, Y. A combination of oral L-citrulline and L-arginine improved 10-min full-power cycling test performance in male collegiate soccer players: A randomized crossover trial. Eur. J. Appl. Physiol. 2019, 119, 1075–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sureda, A.; Pons, A. Arginine and citrulline supplementation in sports and exercise: Ergogenic nutrients? Med. Sport Sci. 2012, 59, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Meynial-Denis, D. Glutamine metabolism in advanced age. Nutr. Rev. 2016, 74, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, Z.A.; Kul, S.; Türkbeyler, İ.H.; Sayıner, Z.A.; Abiyev, A. Is increased neutrophil lymphocyte ratio remarking the inflammation in sarcopenia? Exp. Gerontol. 2018, 110, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Bendahan, D.; Mattei, J.P.; Ghattas, B.; Confort-Gouny, S.; Le Guern, M.E.; Cozzone, P.J. Citrulline/malate promotes aerobic energy production in human exercising muscle. Br. J. Sports Med. 2002, 36, 282–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannesini, B.; Le Fur, Y.; Cozzone, P.J.; Verleye, M.; Le Guern, M.E.; Bendahan, D. Citrulline malate supplementation increases muscle efficiency in rat skeletal muscle. Eur. J. Pharmacol. 2011, 667, 100–104. [Google Scholar] [CrossRef]
- Bailey, S.J.; Blackwell, J.R.; Lord, T.; Vanhatalo, A.; Winyard, P.G.; Jones, A.M. L-Citrulline supplementation improves O2 uptake kinetics and high-intensity exercise performance in humans. J. Appl. Physiol. 2015, 119, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Engelmann, M.; Landgraf, R.; Wotjak, C.T. The hypothalamic-neurohypophysial system regulates the hypothalamic–pituitary–adrenal axis under stress: An old concept revisited. Front. Neuroendocrinol. 2004, 25, 132–149. [Google Scholar] [CrossRef]
- Kraemer, W.J. Endocrine responses to resistance exercise. Med. Sci. Sports Exerc. 1988, 20, S152–S157. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, W.J.; Deschenes, M.R.; Fleck, S.J. Physiological adaptations to resistance exercise. Implications for athletic conditioning. Sports Med. 1988, 6, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Cordova-Martínez, A.; Seco-Calvo, J.; Tur-Marí, J.A.; Abecia-Inchaurregui, L.C.; Echevarría-Orella, E.; Pons-Biescas, A. Testosterone and cortisol changes in professional basketball players through a season competition. J. Strength Cond. Res. 2010, 24, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Vervoorn, C.; Quist, A.M.; Vermulst, L.J.; Erich, W.B.; de Vries, W.R.; Thijssen, J.H. The behaviour of the plasma free testosterone/cortisol ratio during a season of elite rowing training. Int. J. Sports Med. 1991, 12, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. Hormones and sarcopenia. Curr. Pharm. Des. 2017, 23, 4484–4492. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; Bremner, W.J.; McKinlay, J.B. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 2002, 87, 589–599. [Google Scholar] [CrossRef]
- Morley, J.E.; Perry, H.M., 3rd. Androgens and women at the menopause and beyond. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 409–416. [Google Scholar] [CrossRef]
- Visser, M.; Deeg, D.J.; Lips, P.; Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The longitudinal aging study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef]
- Tallis, J.; James, R.S.; Seebacher, F. The effects of obesity on skeletal muscle contractile function. J. Exp. Biol. 2018, 6, 221. [Google Scholar] [CrossRef] [Green Version]
- Chappell, A.J.; Allwood, D.M.; Johns, R.; Brown, S.; Sultana, K.; Anand, A.; Simper, T. Citrulline malate supplementation does not improve German Volume Training performance or reduce muscle soreness in moderately trained males and females. J. Int. Soc. Sports Nutr. 2018, 15, 42. [Google Scholar] [CrossRef]
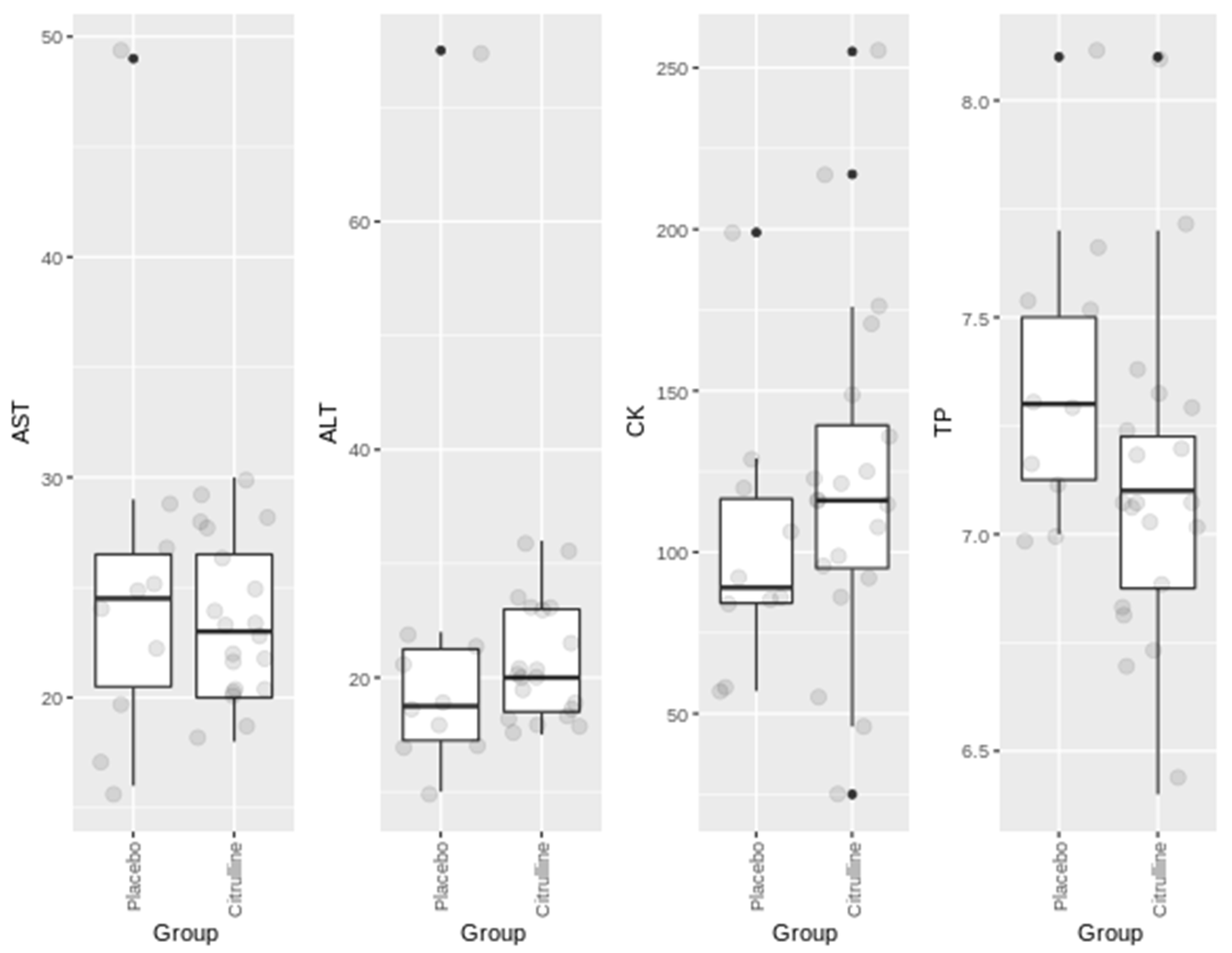
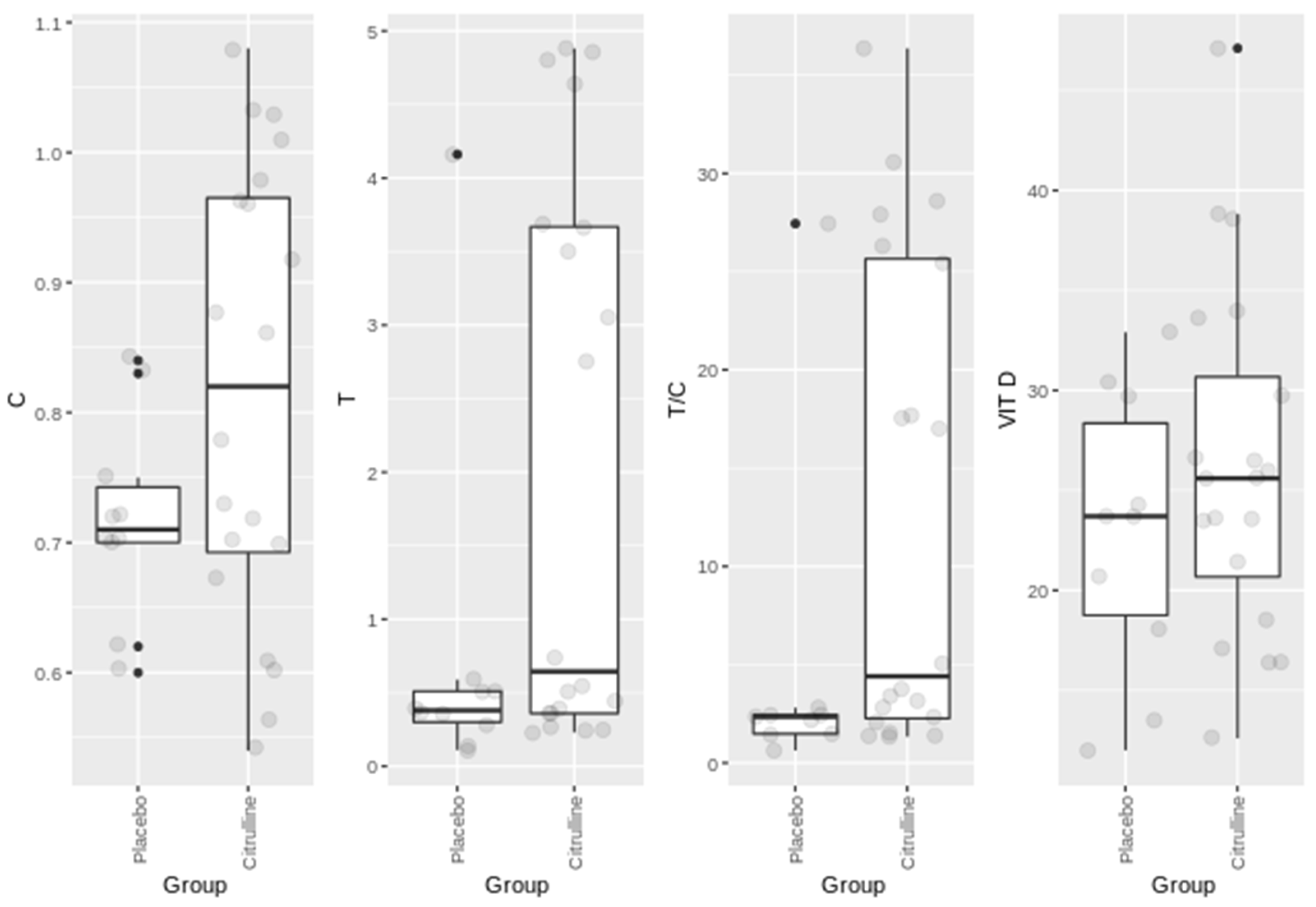
| Parameter | Men | Women |
|---|---|---|
| n | 18 | 26 |
| Height (cm) | 171.0 ± 4.3 | 158.1 ± 6.1 |
| Age (years) | 64.8 ± 3.6 | 65.4 ± 4.4 |
| Weight (kg) | 78.4 ± 8.9 | 63.9 ± 7.9 |
| BMI (kg/m2) | 26.6 ± 2.3 | 24.9 ± 2.9 |
| % Fat | 24.4 ± 2.7 | 34.9 ± 5.6 |
| Systolic BP | 133 ± 17 | 130 ± 21 |
| Diastolic BP | 79 ± 10 | 77 ± 11 |
| Session Protocol | Time (min) | Content | Level of Effort |
|---|---|---|---|
| Warm-up | 10 | General mobility, light movements | 4 |
| Balance | 5 | Standing and monopodial exercises | 3 |
| Aerobic endurance | 10 | Walking, slow running | 7 |
| Aerobic resistance | 20 | Overload exercises, with balls, dumbbells, elastic bands, steps | 8 |
| Tests | PL | CM | |
|---|---|---|---|
| t = 0 | * p | ||
| Endurance 6 min (m) | 931.1 ± 181.5 | 925.8 ± 191.0 | 0.913 |
| Strength-Dynamometer (Kg) | 37 (29–46) | 29.5 (26.5–37.5) | 0.540 |
| Speed (s) | 2.3 (2.1–2.8) | 2.1 (1.9–2.4) | 0.365 |
| Squat (s) | 9.9 (9.6–11.4) | 10.5 (10.0–11.8) | 0.092 |
| SPPB (score) | 12 (11–12) | 12 (11–12) | 0.563 |
| 6th week | ** p | ||
| Endurance 6 min (m) | 842.9 ± 208.9 | 969.8 ± 237.0 | 0.162 |
| Strength-Dynamometer (Kg) | 27.5 (25–29) | 31 (25.5–46.5) | 0.301 |
| Speed (s) | 2.4 (2.2–2.6) | 2.1 (1.9–2.3) | 0.038 |
| Squat (s) | 11.1 (4.5–12.8) | 10.0 (8.8–11.3) | 0.099 |
| SPPB (score) | 11 (11–12) | 12 (11.5–12) | 0.159 |
| Parameter | PL | CM | |
|---|---|---|---|
| t = 0 | * p | ||
| Hb (g/dL) | 14.2 (13.4–15.2) | 14.8 (14.35–15.45) | 0.931 |
| RBC (106 cells/μL) | 4.7 ± 0.3 | 4.8 ± 0.3 | 0.307 |
| Hematocrit (%) | 41.6 (40.2–44.5) | 42.7 (41.5–44.9) | 0.734 |
| WBC (103 cells/µL) | 6.4 (5.7–8.0) | 6.2 (5.2–6.8) | 0.582 |
| Lymphocytes (%) | 28.7 (17.2–38.1) | 34.3 (32.8–38.0) | 0.254 |
| Monocytes (%) | 6.4 (4.7–9.4) | 8.3 (7.3–9.1) | 0.768 |
| Neutrophils (%) | 63.1 (50.5–71.1) | 52.9 (49.1–55.0) | 0.228 |
| Serum iron (µg/dL) | 78 (64–106) | 89 (75–119.5) | 0.347 |
| Ferritin (ng/mL) | 62 (40–113) | 93 (81.5–193.5) | 0.524 |
| 6th week | ** p | ||
| Hb (g/dL) | 14.6 (14.2–15.0) | 15.1 (14.4–15.5) | 0.209 |
| RBC (106 cells/μL) | 4.8 ± 0.2 | 4.9 ± 0.3 | 0.463 |
| Hematocrit (%) | 43.3 (41.8–44.2) | 44.7 (41.6–45.5) | 0.172 |
| WBC (103 cells/µL) | 6.7 (5.5–7.2) | 6.8 (5.6–7.9) | 0.441 |
| Lymphocytes (%) | 34.4 (30.9–38.8) | 36.2 (29.5–40.2) | 0.441 |
| Monocytes (%) | 8.1 (7.7–9.6) | 8.6 (7.1–9.2) | 0.947 |
| Neutrophils (%) | 54.1 (50.1–57.9) | 53.3 (50.1–56.5) | 0.843 |
| Serum iron (µg/dL) | 96.5 (81–108) | 93.5 (83–100) | 0.774 |
| Ferritin (ng/mL) | 86.5 (47–120) | 91 (48.5–181.5) | 0.843 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero-García, A.; Pascual-Fernández, J.; Noriega-González, D.C.; Bello, H.J.; Pons-Biescas, A.; Roche, E.; Córdova-Martínez, A. L-Citrulline Supplementation and Exercise in the Management of Sarcopenia. Nutrients 2021, 13, 3133. https://doi.org/10.3390/nu13093133
Caballero-García A, Pascual-Fernández J, Noriega-González DC, Bello HJ, Pons-Biescas A, Roche E, Córdova-Martínez A. L-Citrulline Supplementation and Exercise in the Management of Sarcopenia. Nutrients. 2021; 13(9):3133. https://doi.org/10.3390/nu13093133
Chicago/Turabian StyleCaballero-García, Alberto, Jorge Pascual-Fernández, David César Noriega-González, Hugo J. Bello, Antoni Pons-Biescas, Enrique Roche, and Alfredo Córdova-Martínez. 2021. "L-Citrulline Supplementation and Exercise in the Management of Sarcopenia" Nutrients 13, no. 9: 3133. https://doi.org/10.3390/nu13093133
APA StyleCaballero-García, A., Pascual-Fernández, J., Noriega-González, D. C., Bello, H. J., Pons-Biescas, A., Roche, E., & Córdova-Martínez, A. (2021). L-Citrulline Supplementation and Exercise in the Management of Sarcopenia. Nutrients, 13(9), 3133. https://doi.org/10.3390/nu13093133










