Branched-Chain Amino Acids Can Predict Mortality in ICU Sepsis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Laboratory Analyses
2.3. Reagents
2.4. Lipoprotein Quantification Using NMR
2.5. Metabolic Quantification Using NMR
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics and Laboratory Results of the Study Population
3.2. Targeted Metabolomic Assessment of Lipoproteins in the Sepsis and Control Cohort
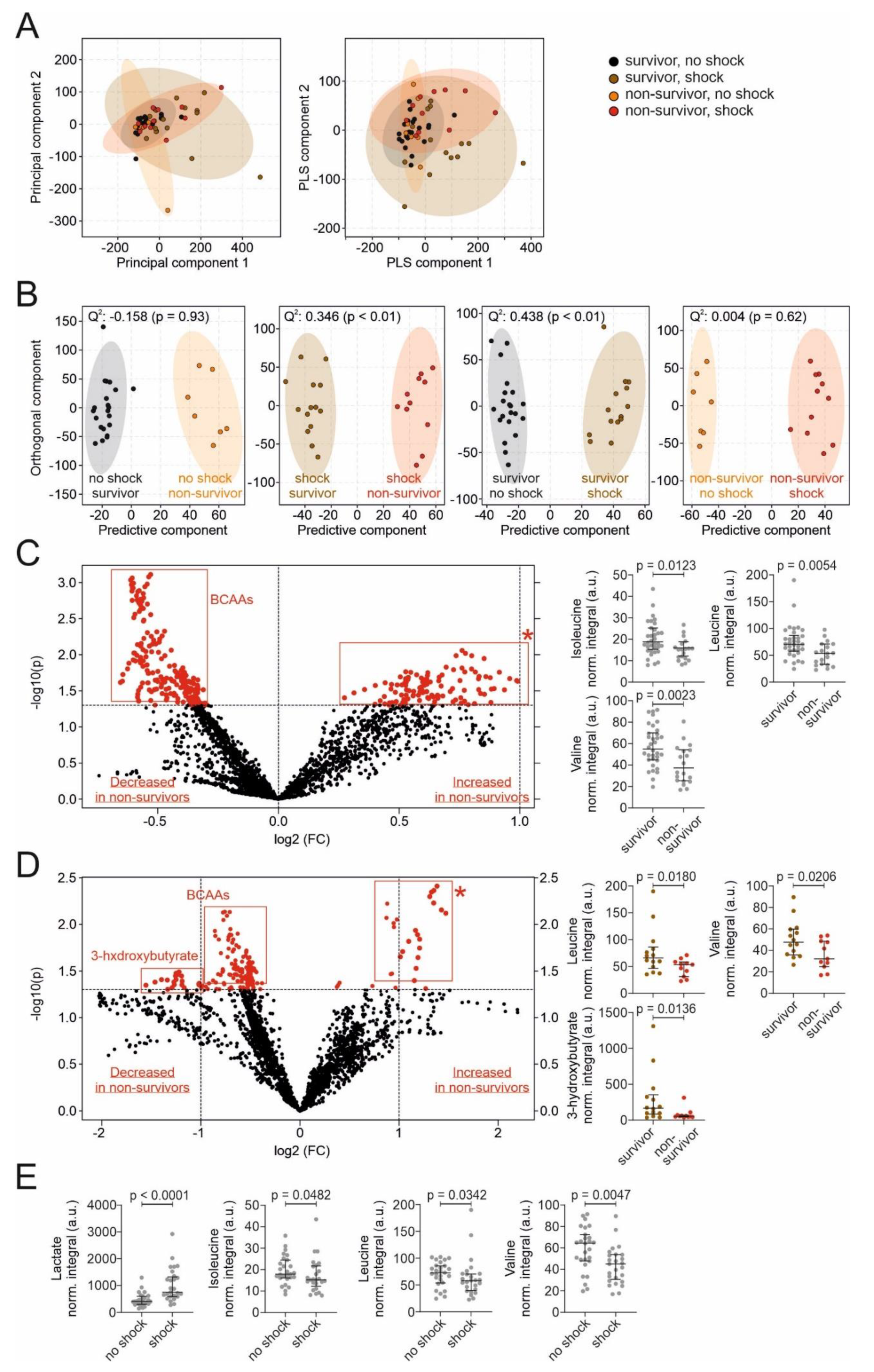
3.3. Untargeted Metabolomic Assessment of Metabolites in the Sepsis Cohort
3.4. Correlations between Metabolites and Other Parameters in the Sepsis Cohort
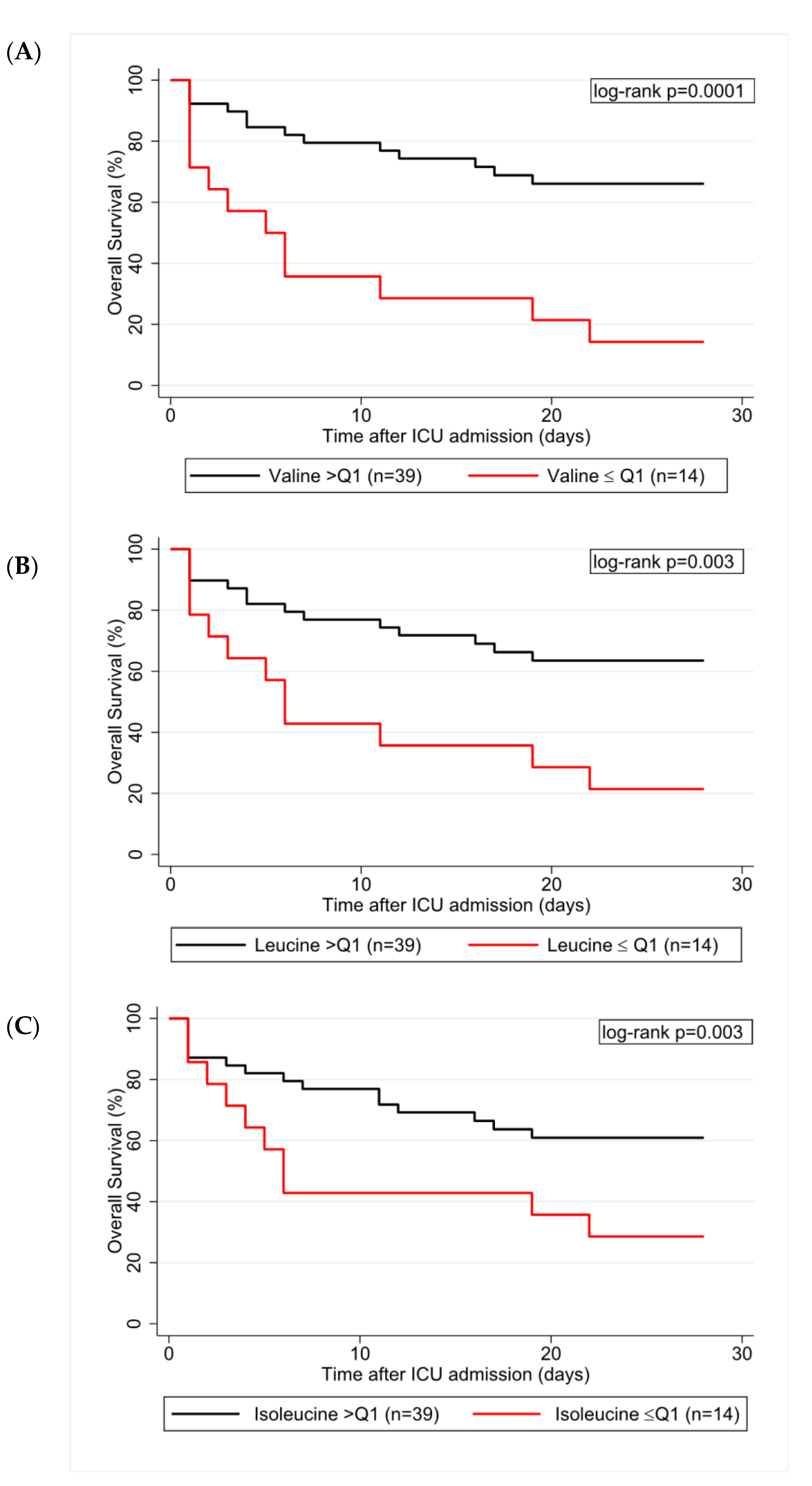
3.5. Univariable and Multivariable Regression Analyses
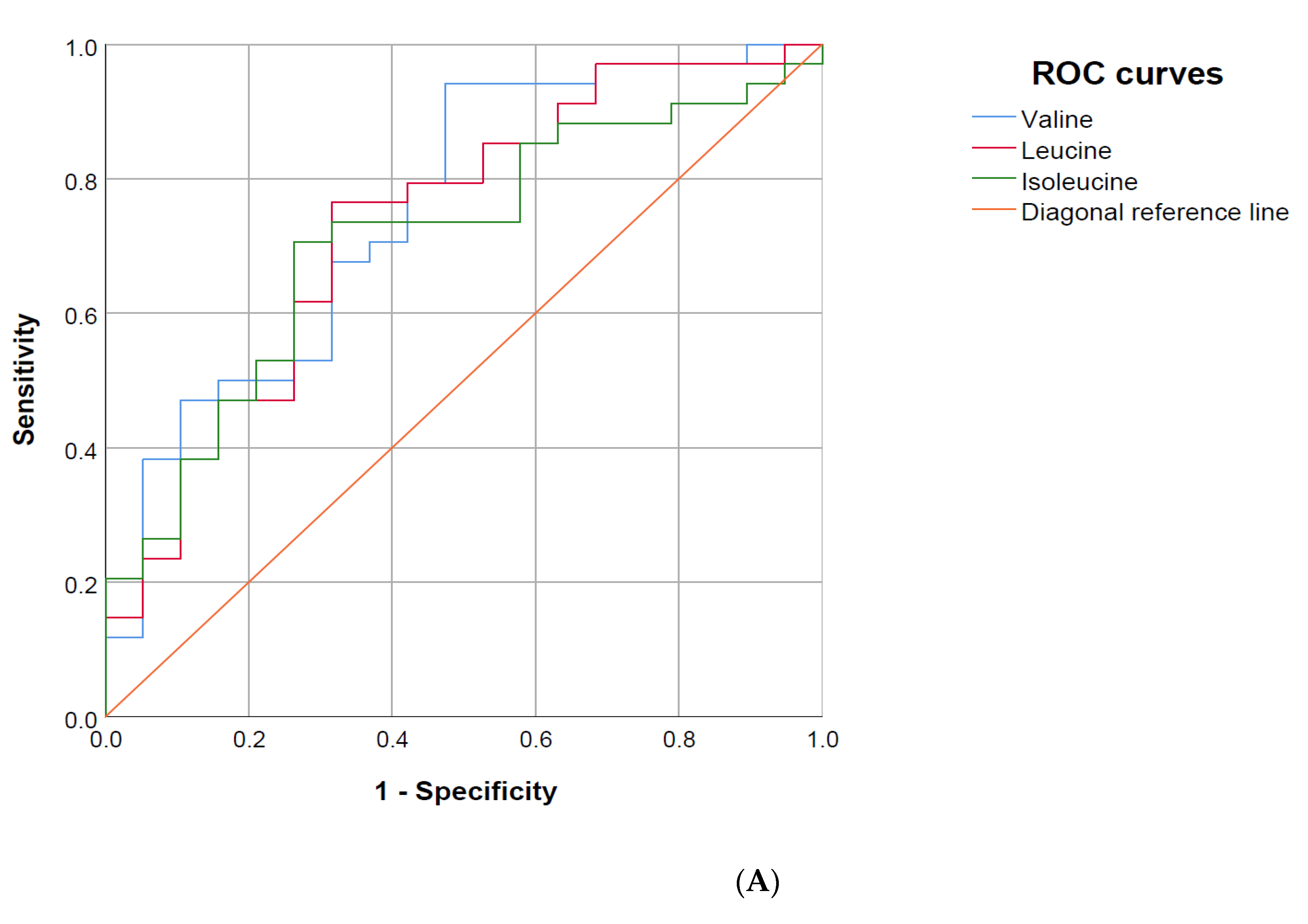
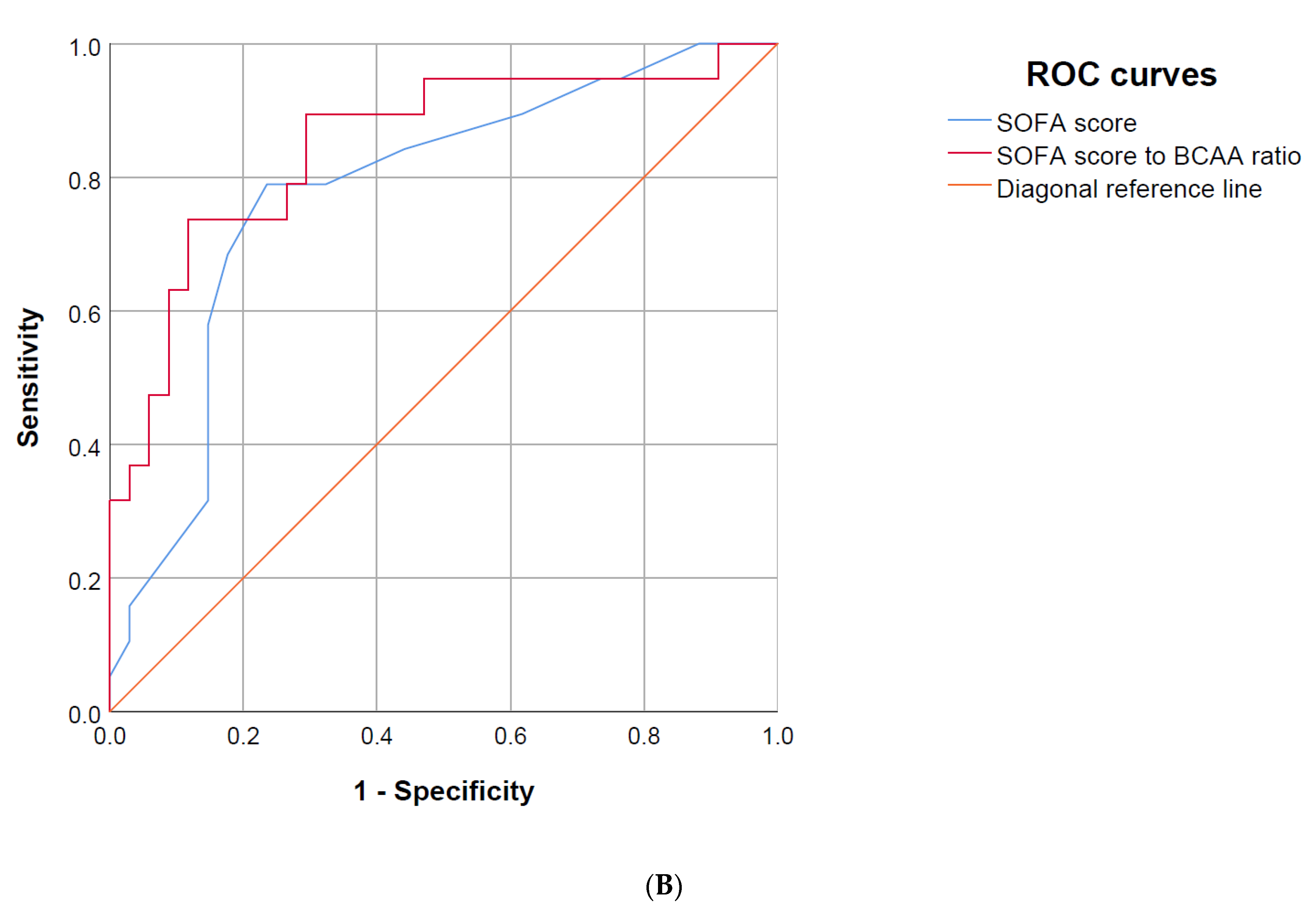
3.6. Area under the Receiver Operating Characteristics (AUROC) Curve
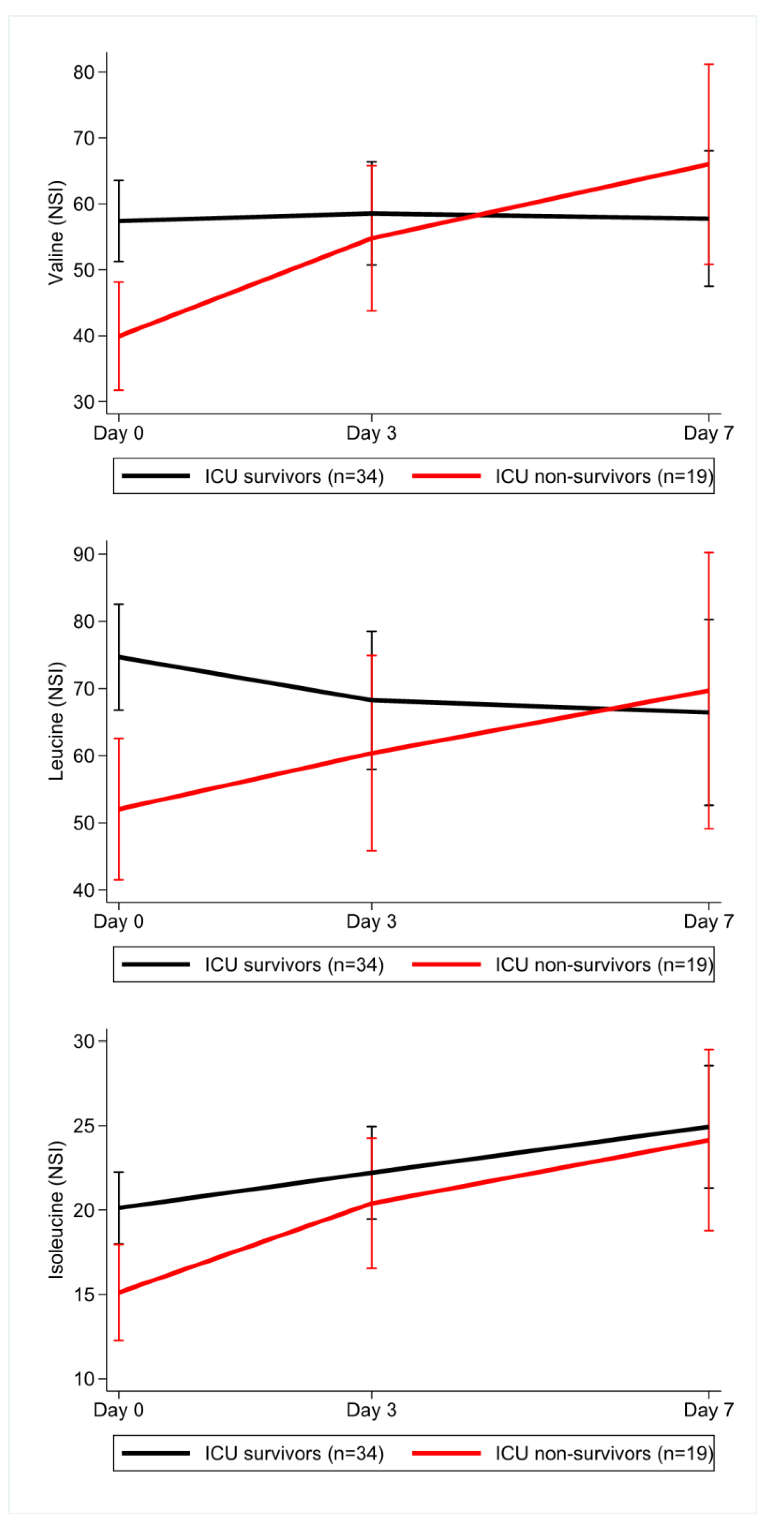
3.7. Longitudinal Data during the ICU Stay for Sepsis Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K.; International Forum of Acute Care, T. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef]
- Gobatto, A.L.; Besen, B.A.; Azevedo, L.C. How Can We Estimate Sepsis Incidence and Mortality? Shock 2017, 47, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Ryoo, S.M.; Han, K.S.; Ahn, S.; Shin, T.G.; Hwang, S.Y.; Chung, S.P.; Hwang, Y.J.; Park, Y.S.; Jo, Y.H.; Chang, H.L.; et al. The usefulness of C-reactive protein and procalcitonin to predict prognosis in septic shock patients: A multicenter prospective registry-based observational study. Sci. Rep. 2019, 9, 6579. [Google Scholar] [CrossRef]
- Reisinger, A.C.; Schuller, M.; Holzer, M.; Stadler, J.T.; Hackl, G.; Posch, F.; Marsche, G.; Sourij, H.; Ekart, R.; Eller, K.; et al. Arylesterase Activity of HDL Associated Paraoxonase as a Potential Prognostic Marker in Patients With Sepsis and Septic Shock-A Prospective Pilot Study. Front. Med. (Lausanne) 2020, 7, 579677. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.R.; Hummon, A.B. Mass spectrometry for the discovery of biomarkers of sepsis. Mol. Biosyst. 2017, 13, 648–664. [Google Scholar] [CrossRef] [PubMed]
- Everett, J.R. Pharmacometabonomics in humans: A new tool for personalized medicine. Pharmacogenomics 2015, 16, 737–754. [Google Scholar] [CrossRef]
- Lee, J.; Banerjee, D. Metabolomics and the Microbiome as Biomarkers in Sepsis. Crit. Care Clin. 2020, 36, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, A.; Ghini, V.; Meoni, G.; Licari, C.; Takis, P.G.; Tenori, L.; Turano, P.; Luchinat, C. High-Throughput Metabolomics by 1D NMR. Angew. Chem. Int. Ed. Engl. 2019, 58, 968–994. [Google Scholar] [CrossRef]
- Alkan, H.F.; Walter, K.E.; Luengo, A.; Madreiter-Sokolowski, C.T.; Stryeck, S.; Lau, A.N.; Al-Zoughbi, W.; Lewis, C.A.; Thomas, C.J.; Hoefler, G.; et al. Cytosolic Aspartate Availability Determines Cell Survival When Glutamine Is Limiting. Cell Metab. 2018, 28, 706–720 e706. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.; Hofer, D.C.; Trefely, S.; Pelzmann, H.J.; Madreiter-Sokolowski, C.; Duta-Mare, M.; Schlager, S.; Trausinger, G.; Stryeck, S.; Graier, W.F.; et al. N-acetylaspartate pathway is nutrient responsive and coordinates lipid and energy metabolism in brown adipocytes. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Maher, A.D.; Crockford, D.; Toft, H.; Malmodin, D.; Faber, J.H.; McCarthy, M.I.; Barrett, A.; Allen, M.; Walker, M.; Holmes, E.; et al. Optimization of human plasma 1H NMR spectroscopic data processing for high-throughput metabolic phenotyping studies and detection of insulin resistance related to type 2 diabetes. Anal. Chem. 2008, 80, 7354–7362. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Jones, G.; David, S.; Olariu, E.; Cadwell, K.K. Frequency and mortality of septic shock in Europe and North America: A systematic review and meta-analysis. Crit. Care 2019, 23, 196. [Google Scholar] [CrossRef]
- Sakr, Y.; Jaschinski, U.; Wittebole, X.; Szakmany, T.; Lipman, J.; Namendys-Silva, S.A.; Martin-Loeches, I.; Leone, M.; Lupu, M.N.; Vincent, J.L.; et al. Sepsis in Intensive Care Unit Patients: Worldwide Data From the Intensive Care over Nations Audit. Open Forum Infect. Dis. 2018, 5, ofy313. [Google Scholar] [CrossRef]
- Pierrakos, C.; Vincent, J.L. Sepsis biomarkers: A review. Crit. Care 2010, 14, R15. [Google Scholar] [CrossRef] [PubMed]
- Evans, T. Diagnosis and management of sepsis. Clin. Med. (Lond.) 2018, 18, 146–149. [Google Scholar] [CrossRef]
- Bonvini, A.; Coqueiro, A.Y.; Tirapegui, J.; Calder, P.C.; Rogero, M.M. Immunomodulatory role of branched-chain amino acids. Nutr. Rev. 2018, 76, 840–856. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Layman, D.K.; Walker, D.A. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J. Nutr. 2006, 136, 319S–323S. [Google Scholar] [CrossRef]
- Cummings, N.E.; Williams, E.M.; Kasza, I.; Konon, E.N.; Schaid, M.D.; Schmidt, B.A.; Poudel, C.; Sherman, D.S.; Yu, D.; Arriola Apelo, S.I.; et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol. 2018, 596, 623–645. [Google Scholar] [CrossRef]
- Fontana, L.; Cummings, N.E.; Arriola Apelo, S.I.; Neuman, J.C.; Kasza, I.; Schmidt, B.A.; Cava, E.; Spelta, F.; Tosti, V.; Syed, F.A.; et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep. 2016, 16, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Freund, H.R.; Ryan, J.A., Jr.; Fischer, J.E. Amino acid derangements in patients with sepsis: Treatment with branched chain amino acid rich infusions. Ann. Surg. 1978, 188, 423–430. [Google Scholar] [CrossRef]
- Englert, J.A.; Rogers, A.J. Metabolism, Metabolomics, and Nutritional Support of Patients with Sepsis. Clin. Chest Med. 2016, 37, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Platell, C.; Kong, S.E.; McCauley, R.; Hall, J.C. Branched-chain amino acids. J. Gastroenterol. Hepatol. 2000, 15, 706–717. [Google Scholar] [CrossRef]
- Holecek, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. (Lond.) 2018, 15, 33. [Google Scholar] [CrossRef]
- Sax, H.C.; Talamini, M.A.; Fischer, J.E. Clinical use of branched-chain amino acids in liver disease, sepsis, trauma, and burns. Arch. Surg. 1986, 121, 358–366. [Google Scholar] [CrossRef]
- Jimenez Jimenez, F.J.; Ortiz Leyba, C.; Morales Menedez, S.; Barros Perez, M.; Munoz Garcia, J. Prospective study on the efficacy of branched-chain amino acids in septic patients. JPEN J. Parenter. Enteral Nutr. 1991, 15, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Asai, Y.; Bajotto, G.; Yoshizato, H.; Hamada, K.; Higuchi, T.; Shimomura, Y. The effects of endotoxin on plasma free amino acid concentrations in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2008, 54, 460–466. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lang, C.H.; Lynch, C.J.; Vary, T.C. BCATm deficiency ameliorates endotoxin-induced decrease in muscle protein synthesis and improves survival in septic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R935–R944. [Google Scholar] [CrossRef]
- Druml, W.; Heinzel, G.; Kleinberger, G. Amino acid kinetics in patients with sepsis. Am. J. Clin. Nutr. 2001, 73, 908–913. [Google Scholar] [CrossRef]
- Su, L.; Li, H.; Xie, A.; Liu, D.; Rao, W.; Lan, L.; Li, X.; Li, F.; Xiao, K.; Wang, H.; et al. Dynamic changes in amino acid concentration profiles in patients with sepsis. PLoS ONE 2015, 10, e0121933. [Google Scholar] [CrossRef]
- Puskarich, M.A.; McHugh, C.; Flott, T.L.; Karnovsky, A.; Jones, A.E.; Stringer, K.A.; Investigators, R.T. Serum Levels of Branched Chain Amino Acids Predict Duration of Cardiovascular Organ Failure in Septic Shock. Shock 2021, 56, 65–72. [Google Scholar] [CrossRef]
- Sprung, C.L.; Cerra, F.B.; Freund, H.R.; Schein, R.M.; Konstantinides, F.N.; Marcial, E.H.; Pena, M. Amino acid alterations and encephalopathy in the sepsis syndrome. Crit. Care Med. 1991, 19, 753–757. [Google Scholar] [CrossRef]
- Liu, Z.; Triba, M.N.; Amathieu, R.; Lin, X.; Bouchemal, N.; Hantz, E.; Le Moyec, L.; Savarin, P. Nuclear magnetic resonance-based serum metabolomic analysis reveals different disease evolution profiles between septic shock survivors and non-survivors. Crit. Care 2019, 23, 169. [Google Scholar] [CrossRef]
- Mickiewicz, B.; Duggan, G.E.; Winston, B.W.; Doig, C.; Kubes, P.; Vogel, H.J.; Alberta Sepsis, N. Metabolic profiling of serum samples by 1H nuclear magnetic resonance spectroscopy as a potential diagnostic approach for septic shock. Crit. Care Med. 2014, 42, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Mickiewicz, B.; Tam, P.; Jenne, C.N.; Leger, C.; Wong, J.; Winston, B.W.; Doig, C.; Kubes, P.; Vogel, H.J.; Alberta Sepsis, N. Integration of metabolic and inflammatory mediator profiles as a potential prognostic approach for septic shock in the intensive care unit. Crit. Care 2015, 19, 11. [Google Scholar] [CrossRef]
- Blaise, B.J.; Gouel-Cheron, A.; Floccard, B.; Monneret, G.; Allaouchiche, B. Metabolic phenotyping of traumatized patients reveals a susceptibility to sepsis. Anal. Chem. 2013, 85, 10850–10855. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Huang, Y.; Zhu, Y.; Xia, L.; Wang, R.; Xiao, K.; Wang, H.; Yan, P.; Wen, B.; Cao, L.; et al. Discrimination of sepsis stage metabolic profiles with an LC/MS-MS-based metabolomics approach. BMJ Open Respir. Res. 2014, 1, e000056. [Google Scholar] [CrossRef]
- Fischer, J.E.; Rosen, H.M.; Ebeid, A.M.; James, J.H.; Keane, J.M.; Soeters, P.B. The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery 1976, 80, 77–91. [Google Scholar] [PubMed]
- Gluud, L.L.; Dam, G.; Les, I.; Marchesini, G.; Borre, M.; Aagaard, N.K.; Vilstrup, H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst. Rev. 2017, 5, CD001939. [Google Scholar] [CrossRef] [PubMed]
- Dam, G.; Aamann, L.; Vistrup, H.; Gluud, L.L. The role of Branched Chain Amino Acids in the treatment of hepatic Encephalopathy. J. Clin. Exp. Hepatol. 2018, 8, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schutz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. (Edinb. Scotl.) 2019, 38, 485–521. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. (Edinb. Scotl.) 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Basler, T.; Meier-Hellmann, A.; Bredle, D.; Reinhart, K. Amino acid imbalance early in septic encephalopathy. Intensive Care Med. 2002, 28, 293–298. [Google Scholar] [CrossRef]
- Garcia-de-Lorenzo, A.; Ortiz-Leyba, C.; Planas, M.; Montejo, J.C.; Nunez, R.; Ordonez, F.J.; Aragon, C.; Jimenez, F.J. Parenteral administration of different amounts of branch-chain amino acids in septic patients: Clinical and metabolic aspects. Crit. Care Med. 1997, 25, 418–424. [Google Scholar] [CrossRef]
- Mori, E.; Hasebe, M.; Kobayashi, K. Effect of total parenteral nutrition enriched in branched-chain amino acids on metabolite levels in septic rats. Metab. Clin. Exp. 1988, 37, 824–830. [Google Scholar] [CrossRef]
- Fujita, H.; Hirose, K.; Miyazaki, I. Effects of branched chain enriched amino acid solutions on septic rats. Clin. Nutr. (Edinb. Scotl.) 1986, 5, 171–177. [Google Scholar] [CrossRef]
- Vente, J.P.; Soeters, P.B.; von Meyenfeldt, M.F.; Rouflart, M.M.; van der Linden, C.J.; Gouma, D.J. Prospective randomized double-blind trial of branched chain amino acid enriched versus standard parenteral nutrition solutions in traumatized and septic patients. World J. Surg. 1991, 15, 128–132, discussion 133. [Google Scholar] [CrossRef]
- Nicastro, H.; da Luz, C.R.; Chaves, D.F.; Bechara, L.R.; Voltarelli, V.A.; Rogero, M.M.; Lancha, A.H., Jr. Does Branched-Chain Amino Acids Supplementation Modulate Skeletal Muscle Remodeling through Inflammation Modulation? Possible Mechanisms of Action. J. Nutr. Metab. 2012, 2012, 136937. [Google Scholar] [CrossRef]
- Shirabe, K.; Yoshimatsu, M.; Motomura, T.; Takeishi, K.; Toshima, T.; Muto, J.; Matono, R.; Taketomi, A.; Uchiyama, H.; Maehara, Y. Beneficial effects of supplementation with branched-chain amino acids on postoperative bacteremia in living donor liver transplant recipients. Liver Transpl. 2011, 17, 1073–1080. [Google Scholar] [CrossRef]
- Hey, P.; Gow, P.; Testro, A.G.; Apostolov, R.; Chapman, B.; Sinclair, M. Nutraceuticals for the treatment of sarcopenia in chronic liver disease. Clin. Nutr. ESPEN 2021, 41, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Conde, M.; Llop, E.; Gomez-Pimpollo, L.; Fernandez Carrillo, C.; Rodriguez, L.; Van Den Brule, E.; Perello, C.; Lopez-Gomez, M.; Abad, J.; Martinez-Porras, J.L.; et al. Adding Branched-Chain Amino Acids to an Enhanced Standard-of-Care Treatment Improves Muscle Mass of Cirrhotic Patients With Sarcopenia: A Placebo-Controlled Trial. Am. J. Gastroenterol. 2021. [Google Scholar] [CrossRef]
- Ko, C.H.; Wu, S.J.; Wang, S.T.; Chang, Y.F.; Chang, C.S.; Kuan, T.S.; Chuang, H.Y.; Chang, C.M.; Chou, W.; Wu, C.H. Effects of enriched branched-chain amino acid supplementation on sarcopenia. Aging (Albany NY) 2020, 12, 15091–15103. [Google Scholar] [CrossRef]
- Hanai, T.; Shiraki, M.; Nishimura, K.; Ohnishi, S.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M.; Moriwaki, H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 2015, 31, 193–199. [Google Scholar] [CrossRef]
- Vasques, J.; Guerreiro, C.S.; Sousa, J.; Pinto, M.; Cortez-Pinto, H. Nutritional support in cirrhotic patients with sarcopenia. Clin. Nutr. ESPEN 2019, 33, 12–17. [Google Scholar] [CrossRef]
- de Campos-Ferraz, P.L.; Andrade, I.; das Neves, W.; Hangai, I.; Alves, C.R.; Lancha, A.H., Jr. An overview of amines as nutritional supplements to counteract cancer cachexia. J. Cachexia Sarcopenia Muscle 2014, 5, 105–110. [Google Scholar] [CrossRef]
- Fedewa, M.V.; Spencer, S.O.; Williams, T.D.; Becker, Z.E.; Fuqua, C.A. Effect of branched-Chain Amino Acid Supplementation on Muscle Soreness following Exercise: A Meta-Analysis. Int. J. Vitam. Nutr. Res. 2019, 89, 348–356. [Google Scholar] [CrossRef]
- Park, S.; Chae, M.; Park, H.; Park, K. Higher Branched-Chain Amino Acid Intake Is Associated with Handgrip Strength among Korean Older Adults. Nutrients 2021, 13, 1522. [Google Scholar] [CrossRef] [PubMed]
- Waldron, M.; Whelan, K.; Jeffries, O.; Burt, D.; Howe, L.; Patterson, S.D. The effects of acute branched-chain amino acid supplementation on recovery from a single bout of hypertrophy exercise in resistance-trained athletes. Appl. Physiol. Nutr. Metab. 2017, 42, 630–636. [Google Scholar] [CrossRef] [PubMed]
- VanDusseldorp, T.A.; Escobar, K.A.; Johnson, K.E.; Stratton, M.T.; Moriarty, T.; Cole, N.; McCormick, J.J.; Kerksick, C.M.; Vaughan, R.A.; Dokladny, K.; et al. Effect of Branched-Chain Amino Acid Supplementation on Recovery Following Acute Eccentric Exercise. Nutrients 2018, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Cheng, B.; Xu, Z.; Ye, H.; Lu, W.; Luo, X.; Fu, S.; Fang, X. Impact of sarcopenic obesity on 30-day mortality in critically ill patients with intra-abdominal sepsis. J. Crit. Care 2018, 46, 50–54. [Google Scholar] [CrossRef]
- Loosen, S.H.; Schulze-Hagen, M.; Pungel, T.; Bundgens, L.; Wirtz, T.; Kather, J.N.; Vucur, M.; Paffenholz, P.; Demir, M.; Bruners, P.; et al. Skeletal Muscle Composition Predicts Outcome in Critically Ill Patients. Crit. Care Explor. 2020, 2, e0171. [Google Scholar] [CrossRef]
- Cox, M.C.; Booth, M.; Ghita, G.; Wang, Z.; Gardner, A.; Hawkins, R.B.; Darden, D.B.; Leeuwenburgh, C.; Moldawer, L.L.; Moore, F.A.; et al. The impact of sarcopenia and acute muscle mass loss on long-term outcomes in critically ill patients with intra-abdominal sepsis. J. Cachexia Sarcopenia Muscle 2021. [Google Scholar] [CrossRef] [PubMed]
- De Bandt, J.P.; Cynober, L. Therapeutic use of branched-chain amino acids in burn, trauma, and sepsis. J. Nutr. 2006, 136, 308S–313S. [Google Scholar] [CrossRef]
- Stringer, K.A.; Younger, J.G.; McHugh, C.; Yeomans, L.; Finkel, M.A.; Puskarich, M.A.; Jones, A.E.; Trexel, J.; Karnovsky, A. Whole Blood Reveals More Metabolic Detail of the Human Metabolome than Serum as Measured by 1H-NMR Spectroscopy: Implications for Sepsis Metabolomics. Shock 2015, 44, 200–208. [Google Scholar] [CrossRef]

| Variables | Sepsis Patients (N = 53) | Controls (N = 25) | p-Value |
|---|---|---|---|
| Demographics & Premedication | |||
| Age (years) | 66 (50–75) | 72 (65–79) | 0.012 |
| Female sex | 21 (40%) | 15 (60%) | 0.144 |
| Anti-diabetic medication | 12 (23%) | 8 (32%) | 0.413 |
| Pre-existing diabetes | 15 (28%) | 8 (32%) | 0.793 |
| Pre-existing liver disease | 3 (6%) | 2 (8%) | 0.653 |
| Disease severity and patient outcomes | |||
| SOFA score (points) | 9 (7–13) | 5 (3–9) | <0.0001 |
| Presence of shock | 26 (49%) # | 7 (28%) * | 0.079 |
| 28-day mortality | 25 (47%) | 4 (16%) | 0.011 |
| ICU mortality | 19 (36%) | 4 (16%) | 0.110 |
| Variables | Sepsis Patients (N = 52) | Controls (N = 25) | p-Value | Below Sidak-Threshold * |
|---|---|---|---|---|
| Main classes | ||||
| Triglycerides (mg/dL) | 185 (129–310) | 101 (87–157) | <0.001 | yes |
| Total cholesterol (mg/dL) | 117 (106–148) | 143 (119–194) | 0.011 | no |
| LDL cholesterol (mg/dL) | 57 (39–76) | 77 (53–106) | 0.012 | no |
| HDL cholesterol (mg/dL) | 20 (13–30) | 41 (32–51) | <0.001 | yes |
| Total ApoA1 (mg/dL) | 72 (52–98) | 120 (98–139) | <0.001 | yes |
| Total ApoA2 (mg/dL) | 19 (15–23) | 24 (20–27) | <0.001 | no |
| Total ApoB100 (mg/dL) | 82 (63–103) | 74 (59–87) | 0.171 | no |
| LDL to HDL ratio | 2.6 (1.7–3.7) | 1.9 (1.5–2.5) | 0.009 | no |
| ApoB100 to ApoA1 ratio | 1.3 (0.7–1.7) | 0.6 (0.5–0.8) | <0.001 | yes |
| Particles | ||||
| Total particle number (nmol/L) | 1494 (1149–1877) | 1338 (1063–1588) | 0.171 | no |
| VLDL particle number (nmol/L) | 324 (205–490) | 142 (118–233) | <0.001 | yes |
| IDL particle number (nmol/L) | 157 (79–300) | 87 (61–137) | 0.002 | no |
| LDL particle number (nmol/L) | 930 (737–1225) | 1028 (720–1254) | 0.640 | no |
| Triglycerides in subclasses | ||||
| VLDL (mg/dL) | 94 (63–199) | 50 (43–111) | 0.010 | no |
| IDL (mg/dL) | 13 (7–31) | 6 (3–15) | 0.007 | no |
| LDL (mg/dL) | 32 (19–58) | 22 (17–30) | 0.006 | no |
| HDL (mg/dL) | 15 (10–19) | 13 (10–16) | 0.124 | no |
| Cholesterol in subclasses | ||||
| VLDL (mg/dL) | 28 (20–41) | 17 (12–24)] | <0.001 | no |
| IDL (mg/dL) | 21 (11–37) | 11 (7–16) | 0.002 | no |
| LDL (mg/dL) | 57 (39–76) | 77 (53–106) | 0.012 | no |
| HDL (mg/dL) | 20 (13–30) | 41 (32–51) | <0.001 | yes |
| Free cholesterol in subclasses | ||||
| VLDL (mg/dL) | 13 (10–20) | 8 (6–13) | 0.002 | no |
| IDL (mg/dL) | 6 (3–11) | 3 (2–4) | <0.001 | no |
| LDL (mg/dL) | 24 (19–34) | 29 (21–39) | 0.107 | no |
| HDL (mg/dL) | 6 (1–11) | 14 (11–17) | <0.001 | yes |
| Phospholipids in subclasses | ||||
| VLDL (mg/dL) | 22 (15–41) | 15 (11–28) | 0.039 | no |
| IDL (mg/dL) | 5 (3–11) | 4 (2–6) | 0.133 | no |
| LDL (mg/dL) | 40 (28–55) | 51 (34–62) | 0.095 | no |
| HDL (mg/dL) | 36 (18–51) | 62 (49–73) | <0.001 | yes |
| Apolipoproteins in subclasses | ||||
| ApoA1 in HDL (mg/dL) | 67 (46–98) | 119 (98–138) | <0.001 | yes |
| ApoA2 in HDL (mg/dL) | 20 (17–25) | 25 (21–27) | 0.006 | no |
| ApoB in VLDL (mg/dL) | 18 (11–27) | 8 (7–13) | <0.001 | yes |
| ApoB in IDL (mg/dL) | 9 (4–17) | 5 (3–8) | 0.002 | no |
| ApoB in LDL (mg/dL) | 51 (41–67) | 57 (40–69) | 0.640 | no |
| Variable | Sepsis Cohort N = 53 | Shock N = 26 | No-Shock N = 27 | p-Value | ICU-Survivors N = 34 | Non-Survivors N = 19 | p-Value |
|---|---|---|---|---|---|---|---|
| Metabolomic results | |||||||
| Valine | 49.7 (33.3–65.6) | 43.3 (29.0–53.7) | 64.3 (47.7–72.3) | 0.005 | 55.0 (44.8–70.2) | 33.0 (24.9–53.9) | 0.002 |
| Leucine | 65.3 (44.1–81.3) | 57.0 (38.4–71.0) | 73.0 (54.3–86.3) | 0.034 | 70.8 (57.8–87.5) | 53.4 (32.0–71.0) | 0.005 |
| Isoleucine | 17.0 (13.7–22.4) | 15.2 (10.9–21.6) | 17.9 (16.1–24.4) | 0.048 | 18.1 (14.6–24.6) | 15.2 (11.1–17.9) | 0.012 |
| Acetate * | 35.6 (27.2–44.3) | 35.8 (30.4–48.4)] | 30.9 (24.5–44.1) | 0.292 | 34.8 (29.5–46.4) | 35.6 (24.1–48.6) | 1.000 |
| 3-Hydroxybutyrate | 93.4 (49.8–166.6) | 86.0 (47.5–208.9) | 93.4 (58.3–159.7) | 1.000 | 98.2 (57.8–205.3) | 64.2 (47.9–133.9) | 0.221 |
| Phenylalanine | 33.3 (23.7–47.4) | 35.6 (25.8–48.0) | 27.9 (21.9–47.3) | 0.188 | 30.0 (23.6–47.4) | 36.0 (24.2–48.2) | 0.458 |
| Tyrosine | 8.1 (6.3–11.3) | 8.2 (6.2–12.6) | 8.1 (6.3–11.3) | 0.715 | 8.7 (7.0–11.5) | 7.0 (5.6–9.3) | 0.156 |
| Lactate | 587 (383–914) | 815 (586–1394) | 409 (301–601) | < 0.0001 | 566 (392–995) | 587 (347–890) | 0.970 |
| Citrate | 22.8 (19.1–28.3) | 24.3 (18.6–29.7) | 22.5 (19.2–25.5) | 0.466 | 22.7 (19.1–28.7) | 23.5 (19.1–27.3) | 0.970 |
| Variables | Age | Valine | Leucine | Isoleucine | Acetate | 3-HB | Phenyl-alanine | Tyrosine | Citrate | Lactate | BMI | CRP | PCT | IL−6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOFA | −0.188 | −0.338 * | −0.220 | −0.284 * | −0.226 | −0.219 | 0.078 | −0.021 | 0.004 | 0.198 | 0.136 | 0.097 | 0.378 ** | 0.344 * |
| Age | −0.050 | −0.042 | 0.078 | 0.011 | 0.184 | 0.037 | −0.097 | 0.001 | −0.012 | 0.041 | 0.224 | −0.141 | −0.069 | |
| Valine | 0.860 ** | 0.833 ** | 0.321 * | 0.145 | −0.319 * | 0.566 ** | −0.004 | −0.009 | 0.084 | −0.271 * | −0.401 ** | −0.394 ** | ||
| Leucine | 0.798 ** | 0.310 * | 0.298 * | −0.304 * | 0.448 ** | 0.059 | −0.002 | −0.052 | −0.223 | −0.396 ** | −0.375 ** | |||
| Isoleucine | 0.215 | 0.303 * | −0.271 * | 0.564 ** | 0.108 | 0.021 | 0.043 | −0.208 | −0.408 ** | −0.270 | ||||
| Acetate | 0.194 | −0.109 | 0.287 * | −0.124 | 0.320 * | −0.010 | −0.428 ** | −0.219 | −0.292 * | |||||
| 3-HB | −0.053 | −0.017 | −0.082 | 0.045 | 0.039 | 0.006 | −0.276 * | −0.130 | ||||||
| Phenylalanine | 0.004 | 0.206 | 0.041 | 0.218 | 0.035 | 0.267 | 0.263 | |||||||
| Tyrosine | 0.006 | 0.436 ** | 0.160 | −0.476 ** | −0.125 | −0.024 | ||||||||
| Citrate | 0.090 | 0.048 | 0.055 | −0.112 | 0.096 | |||||||||
| Lactate | 0.149 | −0.114 | 0.120 | 0.268 | ||||||||||
| BMI | −0.040 | 0.036 | 0.015 | |||||||||||
| CRP | 0.255 | 0.375 ** | ||||||||||||
| PCT | 0.450 ** |
| Outcome Variable | 28-Day Mortality | ICU Mortality | ||||
|---|---|---|---|---|---|---|
| Variable | Odds Ratio | 95% Confidence Interval | p | Odds Ratio | 95% Confidence Interval | p |
| Demographics | ||||||
| Age (per 5 years increase) | 1.23 | 1.02–1.50 | 0.033 | 1.06 | 0.89–1.27 | 0.511 |
| Female sex | 2.71 | 0.87–8.42 | 0.085 | 1.65 | 0.53–5.17 | 0.390 |
| Anti-diabetic therapy | 0.75 | 0.20–2.75 | 0.665 | 0.87 | 0.22–3.37 | 0.836 |
| Type 2 diabetes | 0.45 | 0.13–1.57 | 0.210 | 0.56 | 0.15–2.08 | 0.384 |
| Liver disease | 2.35 | 0.20–27.6 | 0.497 | 3.88 | 0.33–45.93 | 0.282 |
| Laboratory parameters | ||||||
| White blood count (per 1 G/L increase) | 1.02 | 0.98–1.07 | 0.357 | 1.00 | 0.96–1.05 | 0.960 |
| Hemoglobin (per 1 g/dL increase) | 0.94 | 0.77–1.15 | 0.574 | 1.03 | 0.84–1.25 | 0.801 |
| Platelets (per 100 G/L increase) | 1.11 | 0.71–1.75 | 0.640 | 1.14 | 0.71–1.81 | 0.593 |
| C-reactive protein (per 100 mg/L increase) | 1.72 | 1.07–2.77 | 0.025 | 1.40 | 0.90–2.18 | 0.136 |
| Procalcitonin (per 10 ng/mL increase) | 1.03 | 0.97–1.09 | 0.339 | 1.04 | 0.98–1.11 | 0.170 |
| Serum creatinine (per 1 mg/dL increase) | 1.01 | 0.85–1.19 | 0.905 | 1.04 | 0.88–1.23 | 0.653 |
| Serum bilirubin (per 1 mg/dL increase) | 0.89 | 0.74–1.08 | 0.245 | 0.94 | 0.80–1.11 | 0.484 |
| Metabolites | ||||||
| Valine (per doubling) | 0.18 | 0.06–0.56 | 0.003 | 0.19 | 0.06–0.58 | 0.004 |
| Leucine (per doubling) | 0.19 | 0.06–0.59 | 0.004 | 0.22 | 0.07–0.66 | 0.007 |
| Isoleucine (per doubling) | 0.29 | 0.09–0.93 | 0.038 | 0.23 | 0.07–0.81 | 0.023 |
| Acetate (per doubling) | 1.26 | 0.57–2.80 | 0.572 | 1.24 | 0.54–2.85 | 0.609 |
| 3-Hydroxybutyrate (per doubling) | 0.91 | 0.61–1.38 | 0.668 | 0.79 | 0.50–1.26 | 0.326 |
| Phenylalanine (per doubling) | 1.77 | 0.75–4.19 | 0.194 | 1.23 | 0.53–2.88 | 0.631 |
| Tyrosine (per doubling) | 0.83 | 0.35–1.95 | 0.665 | 0.82 | 0.33–2.04 | 0.675 |
| Lactate (per doubling) | 1.15 | 0.65–2.04 | 0.632 | 1.00 | 0.55–1.81 | 0.996 |
| Citrate (per doubling) | 1.41 | 0.44–4.54 | 0.563 | 0.90 | 0.27–3.03 | 0.865 |
| Sepsis severity score | ||||||
| SOFA score (per 1 point increase) | 1.13 | 0.97–1.31 | 0.113 | 1.36 | 1.12–1.65 | 0.002 |
| Multivariable Model 1: 28-Day Mortality | Odds Ratio | 95% Confidence Interval | p |
| Age (per 5 year increase) | 1.25 | 1.00–1.56 | 0.048 |
| C-reactive protein (per 100 mg/L increase) | 1.37 | 0.80–2.35 | 0.257 |
| Valine (per doubling) | 0.19 | 0.05–0.66 | 0.009 |
| Multivariable Model 2: ICU Mortality | Odds Ratio | 95% Confidence Interval | p |
| SOFA score (per 1 point increase) | 1.29 | 1.06–1.57 | 0.012 |
| Valine (per doubling) | 0.26 | 0.08–0.85 | 0.026 |
| Outcome | ICU Mortality | 28-day Mortality | ||
|---|---|---|---|---|
| Variables | AUROC | 95% Confidence Interval | AUROC | 95% Confidence Interval |
| SOFA score | 0.78 | 0.65–0.91 | 0.62 | 0.46–0.78 |
| Valine | 0.75 | 0.62–0.89 | 0.75 | 0.62–0.89 |
| Leucine | 0.73 | 0.59–0.88 | 0.75 | 0.62–0.88 |
| Isoleucine | 0.71 | 0.57–0.85 | 0.69 | 0.54–0.83 |
| SOFA score/BCAA-ratio | 0.85 | 0.73–0.96 | 0.74 | 0.60–0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reisinger, A.C.; Posch, F.; Hackl, G.; Marsche, G.; Sourij, H.; Bourgeois, B.; Eller, K.; Madl, T.; Eller, P. Branched-Chain Amino Acids Can Predict Mortality in ICU Sepsis Patients. Nutrients 2021, 13, 3106. https://doi.org/10.3390/nu13093106
Reisinger AC, Posch F, Hackl G, Marsche G, Sourij H, Bourgeois B, Eller K, Madl T, Eller P. Branched-Chain Amino Acids Can Predict Mortality in ICU Sepsis Patients. Nutrients. 2021; 13(9):3106. https://doi.org/10.3390/nu13093106
Chicago/Turabian StyleReisinger, Alexander Christian, Florian Posch, Gerald Hackl, Gunther Marsche, Harald Sourij, Benjamin Bourgeois, Kathrin Eller, Tobias Madl, and Philipp Eller. 2021. "Branched-Chain Amino Acids Can Predict Mortality in ICU Sepsis Patients" Nutrients 13, no. 9: 3106. https://doi.org/10.3390/nu13093106
APA StyleReisinger, A. C., Posch, F., Hackl, G., Marsche, G., Sourij, H., Bourgeois, B., Eller, K., Madl, T., & Eller, P. (2021). Branched-Chain Amino Acids Can Predict Mortality in ICU Sepsis Patients. Nutrients, 13(9), 3106. https://doi.org/10.3390/nu13093106








