Enhancing Bioavailability of Nutraceutically Used Resveratrol and Other Stilbenoids
Abstract
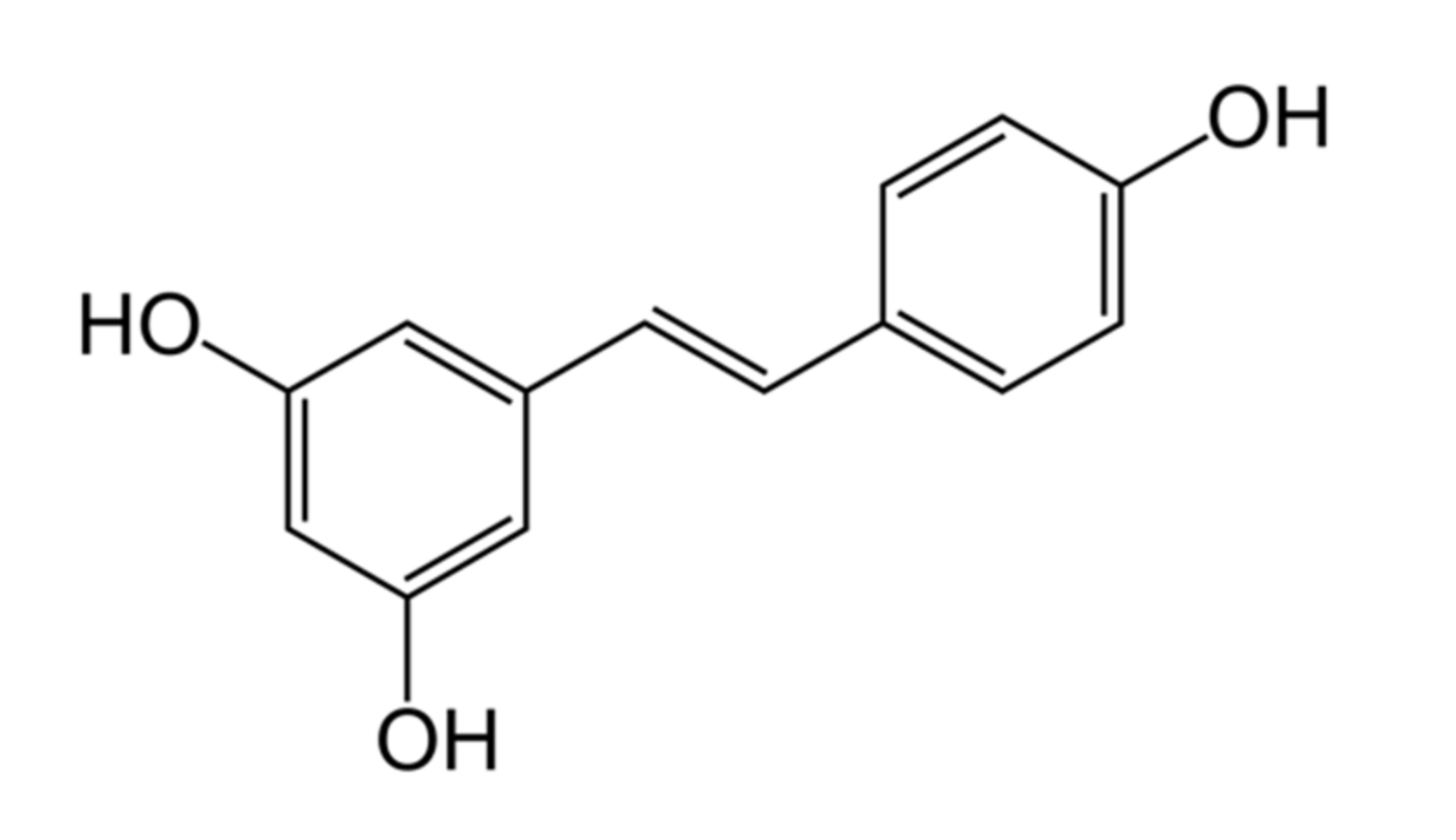
:1. Introduction
2. Chemical Properties
3. Physiological and Therapeutic Effects
4. Mechanism of Actions
5. Pharmacokinetic Characteristics of Stilbenoids
5.1. Pharmacokinetics in Animals
5.2. Pharmacokinetics in Humans
6. Enhancing the Bioavailability of Stilbenoids
6.1. Bioenhancers
6.2. Novel Methods
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renaud, S.; De Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Abou-Zeid, L.A.; El-Mowafy, A.M. Differential recognition of resveratrol isomers by the human estrogen receptor-α: Molecular dynamics evidence for stereoselective ligand binding. Chirality 2004, 16, 190–195. [Google Scholar] [CrossRef]
- Jarosova, V.; Vesely, O.; Doskocil, I.; Tomisova, K.; Marsik, P.; Jaimes, J.D.; Smejkal, K.; Kloucek, P.; Havlik, J. Metabolism of cis- and trans-Resveratrol and Dihydroresveratrol in an Intestinal Epithelial Model. Nutrients 2020, 12, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhanji, M.; Rao, C.N.; Sajish, M. Towards resolving the enigma of the dichotomy of resveratrol: Cis- and trans-resveratrol have opposite effects on TyrRS-regulated PARP1 activation. GeroScience 2020, 43, 1171–1200. [Google Scholar] [CrossRef] [PubMed]
- Rius, C.; Abu-Taha, M.; Hermenegildo, C.; Piqueras, L.; Cerda-Nicolas, J.-M.; Issekutz, A.C.; Estañ, L.; Cortijo, J.; Morcillo, E.J.; Orallo, F.; et al. Trans- but Not Cis-Resveratrol Impairs Angiotensin-II–Mediated Vascular Inflammation through Inhibition of NF-κB Activation and Peroxisome Proliferator-Activated Receptor-γ Upregulation. J. Immunol. 2010, 185, 3718–3727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freudenheim, J.L. A Review of Study Designs and Methods of Dietary Assessment in Nutritional Epidemiology of Chronic Disease. J. Nutr. 1993, 123, 401–405. [Google Scholar] [CrossRef]
- World Health Organization. Healthy Diet. No. WHO-EM/NUT/282/E; World Health Organization Regional Office for the Eastern Mediterranean: Albany, NY, USA, 2019. [Google Scholar]
- Chang, A.Y.; Skirbekk, V.F.; Tyrovolas, S.; Kassebaum, N.J.; Dieleman, J.L. Measuring population ageing: An analysis of the Global Burden of Disease Study 2017. Lancet Public Heal. 2019, 4, e159–e167. [Google Scholar] [CrossRef] [Green Version]
- Romagnolo, D.F.; Selmin, O.I. Mediterranean Diet and Prevention of Chronic Diseases. Nutr. Today 2017, 52, 208–222. [Google Scholar] [CrossRef] [Green Version]
- US Department of Agriculture; US Department of Health and Human Services. Dietary Guidelines for Americans; 2020-2025; US Department of Agriculture; US Department of Health and Human Services: Washington, DC, USA, 2020.
- Herforth, A.; Arimond, M.; Álvarez-Sánchez, C.; Coates, J.; Christianson, K.; Muehlhoff, E. A Global Review of Food-Based Dietary Guidelines. Adv. Nutr. 2019, 10, 590–605. [Google Scholar] [CrossRef] [Green Version]
- Dvorakova, M.; Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar] [CrossRef]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1155–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyenihi, O.R.; Oyenihi, A.B.; Adeyanju, A.A.; Oguntibeju, O.O. Antidiabetic Effects of Resveratrol: The Way Forward in Its Clinical Utility. J. Diabetes Res. 2016, 2016, 9737483. [Google Scholar] [CrossRef] [PubMed]
- Akinwumi, B.C.; Bordun, K.-A.M.; Anderson, H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2019, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, M.J. Of the Phenolic Substrate of White Hellebore (Veratrum Grandiflorum Loes. Fil.). J. Fac. Sci. Hokkaido Imper. Univ. 1940, 3, 1–16. [Google Scholar]
- Anisimova, N.Y.; Kiselevsky, M.V.; Sosnov, A.V.; Sadovnikov, S.V.; Stankov, I.N.; Gakh, A.A. Trans-, cis-, and dihydro-resveratrol: A comparative study. Chem. Central J. 2011, 5, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardile, V.; Chillemi, R.; Lombardo, L.; Sciuto, S.; Spatafora, C.; Tringali, C. Antiproliferative Activity of Methylated Analogues of E- and Z-Resveratrol. Zeitschrift für Naturforschun 2007, 62, 189–195. [Google Scholar] [CrossRef]
- Boocock, D.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I Dose Escalation Pharmacokinetic Study in Healthy Volunteers of Resveratrol, a Potential Cancer Chemopreventive Agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [Green Version]
- Smoliga, J.M.; Blanchard, O. Enhancing the Delivery of Resveratrol in Humans: If Low Bioavailability is the Problem, What is the Solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. HIGH ABSORPTION BUT VERY LOW BIOAVAILABILITY OF ORAL RESVERATROL IN HUMANS. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, D.M.; Yan, J.; Soleas, G.J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef]
- Willenberg, I.; Michael, M.; Wonik, J.; Bartel, L.C.; Empl, M.T.; Schebb, N.H. Investigation of the absorption of resveratrol oligomers in the Caco-2 cellular model of intestinal absorption. Food Chem. 2015, 167, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Kuhnle, G.; Spencer, J.P.; Chowrimootooc, G.; Schroeter, H.; Debnam, E.S.; Srai, S.S.; Rice-Evans, C.; Hahn, U. Resveratrol Is Absorbed in the Small Intestine as Resveratrol Glucuronide. Biochem. Biophys. Res. Commun. 2000, 272, 212–217. [Google Scholar] [CrossRef]
- Andlauer, W.; Kolb, J.; Siebert, K.; Fürst, P. Assessment of Resveratrol Bioavailability in the Perfused Small Intes-tine of the Rat. Drugs Exp. Clin. Res. 2000, 26, 47–55. [Google Scholar] [PubMed]
- Almeida, L.; Silva, M.V.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.-F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53, S7–S15. [Google Scholar] [CrossRef]
- La Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, B. Steady-State Pharmacokinetics and Tolerability of Trans-Resveratrol 2000 mg Twice Daily with Food, Quercetin and Alcohol (Ethanol) in Healthy Human Subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Bruguerolle, B. Chronopharmacokinetics. Clin. Pharmacokinet. 1998, 35, 83–94. [Google Scholar] [CrossRef]
- Silva, M.V.; Loureiro, A.; Falcao, A.; Nunes, T.; Rocha, J.-F.; Fernandes-Lopes, C.; Soares, E.; Wright, L.; Almeida, L.; Soares-Da-Silva, P. Effect of food on the pharmacokinetic profile of trans-resveratrol. Int. J. Clin. Pharmacol. Ther. 2008, 46, 564–570. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, C.; Qiu, S.; Yuan, X.; Li, L.; Zhu, X.; Wu, C.; Qiu, S.; Yuan, X.; Li, L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: Systematic review and meta-analysis. Nutr. Metab. 2017, 14, 60. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Resveratrol commonly displays hormesis: Occurrence and biomedical significance. Hum. Exp. Toxicol. 2010, 29, 980–1015. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [Green Version]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2018, 109, 2237–2251. [Google Scholar] [CrossRef]
- Catalogna, G.; Moraca, F.; D’Antona, L.; Dattilo, V.; Perrotti, G.; Lupia, A.; Costa, G.; Ortuso, F.; Iuliano, R.; Trapasso, F.; et al. Review about the multi-target profile of resveratrol and its implication in the SGK1 inhibition. Eur. J. Med. Chem. 2019, 183, 111675. [Google Scholar] [CrossRef]
- Varoni, E.M.; Faro, A.F.L.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Dyck, G.J.B.; Raj, P.; Zieroth, S.; Dyck, J.R.B.; Ezekowitz, J.A. The Effects of Resveratrol in Patients with Cardiovascular Disease and Heart Failure: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 904. [Google Scholar] [CrossRef] [Green Version]
- Asgary, S.; Karimi, R.; Momtaz, S.; Naseri, R.; Farzaei, M.H. Effect of resveratrol on metabolic syndrome components: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2019, 20, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites—Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabrizi, R.; Tamtaji, O.R.; Lankarani, K.B.; Akbari, M.; Dadgostar, E.; Dabbaghmanesh, M.H.; Kolahdooz, F.; Shamshirian, A.; Momen-Heravi, M.; Asemi, Z. The effects of resveratrol intake on weight loss: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2018, 60, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Ortega, I.; Duleba, A.J. Ovarian actions of resveratrol. Ann. N. Y. Acad. Sci. 2015, 1348, 86–96. [Google Scholar] [CrossRef]
- Kolesarova, A.; Capcarová, M.; Maruniakova, N.; Lukac, N.; Ciereszko, R.E.; Sirotkin, A.V. Resveratrol inhibits reproductive toxicity induced by deoxynivalenol. J. Environ. Sci. Heal. Part A 2012, 47, 1329–1334. [Google Scholar] [CrossRef]
- Brasnyó, P.; Molnar, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, Á.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, A.A.; Riehle, M.A. Resveratrol Fails to Extend Life Span in the MosquitoAnopheles stephensi. Rejuvenation Res. 2015, 18, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.; Pearson, K.J.; Price, N.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; López-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Christenson, J.; Whitby, S.J.; Mellor, D.; Thomas, J.; Mc Kune, A.; Roach, P.D.; Naumovski, N. The Effects of Resveratrol Supplementation in Overweight and Obese Humans: A Systematic Review of Randomized Trials. Metab. Syndr. Relat. Disord. 2016, 14, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Quintela, A.; Carpéné, C.; Fernández, M.; Aguirre, L.; Milton-Laskibar, I.; Contreras, J.; Portillo, M.P. Anti-obesity effects of resveratrol: Comparison between animal models and humans. J. Physiol. Biochem. 2016, 73, 417–429. [Google Scholar] [CrossRef]
- Li, S.; Chen, M.; Li, Y.; Tollefsbol, T.O. Prenatal epigenetics diets play protective roles against environmental pollution. Clin. Epigenetics 2019, 11, 82. [Google Scholar] [CrossRef]
- Bishayee, A.; Barnes, K.F.; Bhatia, D.; Darvesh, A.S.; Carroll, R.T. Resveratrol Suppresses Oxidative Stress and Inflammatory Response in Diethylnitrosamine-Initiated Rat Hepatocarcinogenesis. Cancer Prev. Res. 2010, 3, 753–763. [Google Scholar] [CrossRef] [Green Version]
- Riba, A.; Deres, L.; Sumegi, B.; Toth, K.; Szabados, E.; Halmosi, R. Cardioprotective Effect of Resveratrol in a Postinfarction Heart Failure Model. Oxidative Med. Cell. Longev. 2017, 2017, 6819281. [Google Scholar] [CrossRef]
- Gambini, J.; Ingles, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Bargues, C.M.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol:In VitroandIn VivoStudies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxidative Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [Green Version]
- Kataria, R.; Khatkar, A. Molecular docking, synthesis, kinetics study, structure–activity relationship and ADMET analysis of morin analogous as Helicobacter pylori urease inhibitors. BMC Chem. 2019, 13, 1–17. [Google Scholar] [CrossRef]
- Mirza, S.; Sharma, G.; Parshad, R.; Gupta, S.D.; Pandya, P.; Ralhan, R. Expression of DNA Methyltransferases in Breast Cancer Patients and to Analyze the Effect of Natural Compounds on DNA Methyltransferases and Associated Proteins. J. Breast Cancer 2013, 16, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Zhou, R.; Wang, B.; Mi, M.-T. Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2014, 99, 1510–1519. [Google Scholar] [CrossRef] [Green Version]
- Borra, M.T.; Smith, B.; Denu, J.M. Mechanism of Human SIRT1 Activation by Resveratrol. J. Biol. Chem. 2005, 280, 17187–17195. [Google Scholar] [CrossRef] [Green Version]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Vlavcheski, F.; Hartogh, D.J.D.; Giacca, A.; Tsiani, E. Amelioration of High-Insulin-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol Is Linked to Activation of AMPK and Restoration of GLUT4 Translocation. Nutrients 2020, 12, 914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penumathsa, S.V.; Thirunavukkarasu, M.; Zhan, L.; Maulik, G.; Menon, V.P.; Bagchi, D.; Maulik, N. Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/Akt/eNOS signalling pathway in diabetic myocardium. J. Cell. Mol. Med. 2008, 12, 2350–2361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, H.-C.; Hung, L.-M.; Chen, J.-K. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am. J. Physiol. Metab. 2006, 290, E1339–E1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szkudelski, T. The insulin-suppressive effect of resveratrol—An in vitro and in vivo phenomenon. Life Sci. 2008, 82, 430–435. [Google Scholar] [CrossRef]
- Bertelli, A.A.; Giovannini, L.; Stradi, R.; Tillement, J.P. Plasma, urine and tissue levels of trans- and cis-resveratrol (3,4’,5-trihydroxystilbene) after short-term or prolonged administration of red wine to rats. Int. J. Tissue React. 1996, 18, 67–71. [Google Scholar] [PubMed]
- Bertelli, A.A.; Giovannini, L.; Stradi, R.; Urien, S.; Tillement, J.P.; Bertelli, A. Kinetics of Trans- and Cis-Resveratrol (3,4’,5-Trihydroxystilbene) after Red Wine Oral Administration in Rats. Int. J. Clin. Pharmacol. Res. 1996, 16, 77–81. [Google Scholar] [CrossRef]
- Soleas, G.J.; Angelini, M.; Grass, L.; Diamandis, E.P.; Goldberg, D.M. Absorption of trans-resveratrol in rats. Methods Enzymol. 2001, 335, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Vitrac, X.; Desmoulière, A.; Brouillaud, B.; Krisa, S.; Deffieux, G.; Barthe, N.; Rosenbaum, J.; Mérillon, J.-M. Distribution of [14C]-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sci. 2003, 72, 2219–2233. [Google Scholar] [CrossRef]
- Yu, C.; Shin, Y.G.; Chow, A.; Li, Y.; Kosmeder, J.W.; Lee, Y.S.; Hirschelman, W.H.; Pezzuto, J.M.; Mehta, R.G.; van Breemen, R.B. Human, Rat, and Mouse Metabolism of Resveratrol. Pharm. Res. 2002, 19, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, E.; Soldo, T.; Erbersdobler, H.; Somoza, V. Bioactivity and metabolism oftrans-resveratrol orally administered to Wistar rats. Mol. Nutr. Food Res. 2005, 49, 482–494. [Google Scholar] [CrossRef]
- Azorín-Ortuño, M.; Yáñez-Gascón, M.J.; Vallejo, F.; Pallarés, F.J.; Larrosa, M.; Lucas, R.; Morales, J.C.; Tomas-Barberan, F.; Conesa, M.T.G.; Espín, J.C. Metabolites and tissue distribution of resveratrol in the pig. Mol. Nutr. Food Res. 2011, 55, 1154–1168. [Google Scholar] [CrossRef] [Green Version]
- Brill, S.S.; Furimsky, A.M.; Ho, M.N.; Furniss, M.J.; Li, Y.; Green, A.G.; Green, C.E.; Iyer, L.V.; Bradford, W.W.; Kapetanovic, I.M. Glucuronidation of trans-resveratrol by human liver and intestinal microsomes and UGT isoforms. J. Pharm. Pharmacol. 2006, 58, 469–479. [Google Scholar] [CrossRef]
- Planas, J.M.; Alfaras, I.; Colom, H.; Juan, M.E. The bioavailability and distribution of trans-resveratrol are constrained by ABC transporters. Arch. Biochem. Biophys. 2012, 527, 67–73. [Google Scholar] [CrossRef]
- Van De Wetering, K.; Burkon, A.; Feddema, W.; Bot, A.; De Jonge, H.; Somoza, V.; Borst, P. Intestinal Breast Cancer Resistance Protein (BCRP)/Bcrp1 and Multidrug Resistance Protein 3 (MRP3)/Mrp3 Are Involved in the Pharmacokinetics of Resveratrol. Mol. Pharmacol. 2008, 75, 876–885. [Google Scholar] [CrossRef] [Green Version]
- Kaldas, M.I.; Walle, U.K.; Walle, T. Resveratrol transport and metabolism by human intestinal Caco-2 cells. J. Pharm. Pharmacol. 2003, 55, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.-S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.A.P.; Kulling, E.S. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef]
- Jarosova, V.; Vesely, O.; Marsik, P.; Jaimes, J.D.; Smejkal, K.; Kloucek, P.; Havlik, J. Metabolism of Stilbenoids by Human Faecal Microbiota. Molecules 2019, 24, 1155. [Google Scholar] [CrossRef] [Green Version]
- Jaimes, J.D.; Jarosova, V.; Vesely, O.; Mekadim, C.; Mrazek, J.; Marsik, P.; Killer, J.; Smejkal, K.; Kloucek, P.; Havlik, J. Effect of Selected Stilbenoids on Human Fecal Microbiota. Molecules 2019, 24, 744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urpi-Sarda, M.; Zamora-Ros, R.; Lamuela-Raventos, R.M.; Cherubini, A.; Jauregui, O.; de la Torre, R.; Covas, M.I.; Estruch, R.; Jaeger, W.; Andres-Lacueva, C. HPLC–Tandem Mass Spectrometric Method to Characterize Resveratrol Metabolism in Humans. Clin. Chem. 2007, 53, 292–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delmas, D.; Aires, V.; Limagne, E.; Dutartre, P.; Mazué, F.; Ghiringhelli, F.; Latruffe, N. Transport, stability, and biological activity of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 48–59. [Google Scholar] [CrossRef]
- Fernández-Castillejo, S.; Macià, A.; Motilva, M.J.; Catalán, Ú.; Solà, R. Endothelial Cells Deconjugate Resveratrol Metabolites to Free Resveratrol: A Possible Role in Tissue Factor Modulation. Mol. Nutr. Food Res. 2018, 63, e1800715. [Google Scholar] [CrossRef]
- Vinson, J.A. Intracellular Polyphenols: How Little We Know. J. Agric. Food Chem. 2019, 67, 3865–3870. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Scott, E.; Brown, V.A.; Gescher, A.J.; Steward, W.P.; Brown, K. Clinical trials of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Koziolek, M.; Alcaro, S.; Augustijns, P.; Basit, A.W.; Grimm, M.; Hens, B.; Hoad, C.L.; Jedamzik, P.; Madla, C.M.; Maliepaard, M.; et al. The mechanisms of pharmacokinetic food-drug interactions—A perspective from the UNGAP group. Eur. J. Pharm. Sci. 2019, 134, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Tomas, M.; Ozdal, T.; Capanoglu, E. Effect of food matrix on the content and bioavailability of flavonoids. Trends Food Sci. Technol. 2020, in press. [Google Scholar] [CrossRef]
- Mhaske, D.B.; Sreedharan, S.; Mahadik, K.R. Role of Piperine as an Effective Bioenhancer in Drug Absorption. Phar-Maceutika Anal. Acta 2018, 9, 591. [Google Scholar]
- Wightman, E.L.; Reay, J.; Haskell, C.F.; Williamson, G.; Dew, T.; Kennedy, D. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: A randomised, double-blind, placebo-controlled, cross-over investigation. Br. J. Nutr. 2014, 112, 203–213. [Google Scholar] [CrossRef]
- Bailey, H.H.; Johnson, J.J.; Lozar, T.; Scarlett, C.O.; Wollmer, B.W.; Kim, K.; Havinghurst, T.; Ahmad, N. A randomized, double-blind, dose-ranging, pilot trial of piperine with resveratrol on the effects on serum levels of resveratrol. Eur. J. Cancer Prev. 2020, 30, 285–290. [Google Scholar] [CrossRef]
- Johnson, J.J.; Nihal, M.; Siddiqui, I.A.; Scarlett, C.O.; Bailey, H.H.; Mukhtar, H.; Ahmad, N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol. Nutr. Food Res. 2011, 55, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, P.; Bothiraja, C.; Pawar, A. Resveratrol-piperine loaded mixed micelles: Formulation, characterization, bioavailability, safety and in vitro anticancer activity. RSC Adv. 2016, 6, 112795–112805. [Google Scholar] [CrossRef]
- Junsaeng, D.; Anukunwithaya, T.; Songvut, P.; Sritularak, B.; Likhitwitayawuid, K.; Khemawoot, P. Comparative pharmacokinetics of oxyresveratrol alone and in combination with piperine as a bioenhancer in rats. BMC Complement. Altern. Med. 2019, 19, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, K.C.; Pantuso, T. Combination Effects of Quercetin, Resveratrol and Curcumin on In Vitro Intestinal Absorption. J. Restor. Med. 2014, 3, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-A.; Ha, S.K.; Cho, E.; Choi, I. Resveratrol as a Bioenhancer to Improve Anti-Inflammatory Activities of Apigenin. Nutrients 2015, 7, 9650–9661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detampel, P.; Beck, M.; Krähenbühl, S.; Huwyler, J. Drug interaction potential of resveratrol. Drug Metab. Rev. 2012, 44, 253–265. [Google Scholar] [CrossRef]
- Paine, M.F.; Hart, H.L.; Ludington, S.S.; Haining, R.L.; Rettie, A.E.; Zeldin, D. The human intestinal cytochrome P450 “pie”. Drug Metab. Dispos. 2006, 34, 880–886. [Google Scholar] [CrossRef]
- Syed, S.B.; Arya, H.; Fu, I.-H.; Yeh, T.-K.; Periyasamy, L.; Hsieh, H.-P.; Coumar, M.S.; Syed, S.B.; Arya, H.; Fu, I.-H.; et al. Targeting P-glycoprotein: Investigation of piperine analogs for overcoming drug resistance in cancer. Sci. Rep. 2017, 7, 7972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiebaut, F.; Tsuruo, T.; Hamada, H.; Gottesman, M.M.; Pastan, I.; Willingham, M.C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 7735–7738. [Google Scholar] [CrossRef] [Green Version]
- Quan, F.; Pan, C.; Ma, Q.; Zhang, S.; Yan, L. Reversal effect of resveratrol on multidrug resistance in KBv200 cell line. Biomed. Pharmacother. 2008, 62, 622–629. [Google Scholar] [CrossRef] [PubMed]
- El-Readi, M.Z.; Eid, S.; Abdelghany, A.A.; Al-Amoudi, H.S.; Efferth, T.; Wink, M. Resveratrol mediated cancer cell apoptosis, and modulation of multidrug resistance proteins and metabolic enzymes. Phytomedicine 2018, 55, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wu, X.-N.; Chen, J.; Wang, W.-X.; Lu, Z. Resveratrol reverses multidrug resistance in human breast cancer doxorubicin-resistant cells. Exp. Ther. Med. 2014, 7, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Mieszala, K.; Rudewicz, M.; Gomułkiewicz, A.; Ratajczak-Wielgomas, K.; Grzegrzółka, J.; Dziegiel, P.; Borska, S. Expression of genes and proteins of multidrug resistance in gastric cancer cells treated with resveratrol. Oncol. Lett. 2018, 15, 5825–5832. [Google Scholar] [CrossRef]
- Devi, P.; Sharma, P.; Rathore, C.; Negi, P. Novel Drug Delivery Systems of Resveratrol to Bioavailability and Therapeutic Effects. IntechOpen 2019. [Google Scholar] [CrossRef] [Green Version]
- Balata, G.F.; Eassa, E.; Shamrool, H.; Zidan, S.; Abourehab, M. Self-emulsifying drug delivery systems as a tool to improve solubility and bioavailability of resveratrol. Drug Des. Dev. Ther. 2016, 10, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.-B.; Ryu, J.-H.; Kim, J.-W.; Chang, I.-S.; Suh, K.-D. Stabilization of resveratrol immobilized in monodisperse cyano-functionalized porous polymeric microspheres. Polymer 2005, 46, 8956–8963. [Google Scholar] [CrossRef]
- Shi, G.; Rao, L.; Yu, H.; Xiang, H.; Yang, H.; Ji, R. Stabilization and encapsulation of photosensitive resveratrol within yeast cell. Int. J. Pharm. 2008, 349, 83–93. [Google Scholar] [CrossRef]
- Aloisio, C.; Bueno, M.S.; Ponte, M.P.; Paredes, A.; Palma, S.D.; Longhi, M. Development of solid self-emulsifying drug delivery systems (SEDDS) to improve the solubility of resveratrol. Ther. Deliv. 2019, 10, 626–641. [Google Scholar] [CrossRef]
- Seljak, K.B.; Berginc, K.; Trontelj, J.; Zvonar, A.; Kristl, A.; Gašperlin, M. A Self-Microemulsifying Drug Delivery System to Overcome Intestinal Resveratrol Toxicity and Presystemic Metabolism. J. Pharm. Sci. 2014, 103, 3491–3500. [Google Scholar] [CrossRef]
- Jaisamut, P.; Wanna, S.; Limsuwan, S.; Chusri, S.; Wiwattanawongsa, K.; Wiwattanapatapee, R. Enhanced Oral Bioavailability and Improved Biological Activities of a Quercetin/Resveratrol Combination Using a Liquid Self-Microemulsifying Drug Delivery System. Planta Medica 2020, 87, 336–346. [Google Scholar] [CrossRef]
- Luo, X.; Wang, D.; Wang, M.; Deng, S.; Huang, Y.; Xia, Z. Development of phospholipid complex loaded self-microemulsifying drug delivery system to improve the oral bioavailability of resveratrol. Nanomedicine 2021, 16, 721–739. [Google Scholar] [CrossRef]
- Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Cyclodextrin-Based Nanosponges for Delivery of Resveratrol: In Vitro Characterisation, Stability, Cytotoxicity and Permeation Study. AAPS PharmSciTech 2011, 12, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Sessa, M.; Balestrieri, M.L.; Ferrari, G.; Servillo, L.; Castaldo, D.; D’Onofrio, N.; Donsì, F.; Tsao, R. Bioavailability of encapsulated resveratrol into nanoemulsion-based delivery systems. Food Chem. 2014, 147, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Sessa, M.; Mediouni, H.; Mgaidi, A.; Ferrari, G. Encapsulation of bioactive compounds in nanoemulsion- based delivery systems. Procedia Food Sci. 2011, 1, 1666–1671. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Pai, R.S. Trans-resveratrol self-nano-emulsifying drug delivery system (SNEDDS) with enhanced bioavailability potential: Optimization, pharmacokinetics andin situsingle pass intestinal perfusion (SPIP) studies. Drug Deliv. 2013, 22, 522–530. [Google Scholar] [CrossRef] [Green Version]
- Yen, C.-C.; Chang, C.-W.; Hsu, M.-C.; Wu, Y.-T. Self-Nanoemulsifying Drug Delivery System for Resveratrol: Enhanced Oral Bioavailability and Reduced Physical Fatigue in Rats. Int. J. Mol. Sci. 2017, 18, 1853. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Zhu, W.; Liu, R.; Si, C. Lignin-Containing Self-Nanoemulsifying Drug Delivery System for Enhance Stability and Oral Absorption oftrans-Resveratrol. Part. Part. Syst. Charact. 2018, 35, 1700447. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Ahmad, J.; Alharbi, W.S.; Asfour, H.Z. Resveratrol loaded self-nanoemulsifying drug delivery system (SNEDDS) for pancreatic cancer: Formulation design, optimization and in vitro evaluation. J. Drug Deliv. Sci. Technol. 2021, 64, 102555. [Google Scholar] [CrossRef]
- Hu, C.; Wang, Q.; Ma, C.; Xia, Q. Non-aqueous self-double-emulsifying drug delivery system: A new approach to enhance resveratrol solubility for effective transdermal delivery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 360–369. [Google Scholar] [CrossRef]
- Ethemoglu, M.; Seker, F.; Akkaya, H.; Kilic, E.; Aslan, I.; Erdogan, C.S.; Yilmaz, B. Anticonvulsant activity of resveratrol-loaded liposomes in vivo. Neuroscience 2017, 357, 12–19. [Google Scholar] [CrossRef]
- Basavaraj, S.; Betageri, G.V. Improved oral delivery of resveratrol using proliposomal formulation: Investigation of various factors contributing to prolonged absorption of unmetabolized resveratrol. Expert Opin. Drug Deliv. 2014, 11, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Teskač, K.; Kristl, J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int. J. Pharm. 2010, 390, 61–69. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Xiong, Q.P.; Shi, Y.Y.; Zhang, D.Y. Study on Preparation and Characterization of Resveratrol Solid Li-pid Nanoparticles and Its Anticancer Effects in Vitro. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2010, 33, 1929–1932. [Google Scholar]
- Oganesyan, E.A.; Miroschichenko, I.I.; Vikhrieva, N.S.; Lyashenko, A.A.; Leshkov, S.Y. Use of Nanoparticles to In-crease the Systemic Bioavailability of trans-Resveratrol. Pharm. Chem. J. 2010, 44, 25–27. [Google Scholar] [CrossRef]
- Singh, G.; Pai, R.S. In-vitro/in-vivo characterization of trans-resveratrol-loaded nanoparticulate drug delivery system for oral administration. J. Pharm. Pharmacol. 2014, 66, 1062–1076. [Google Scholar] [CrossRef]
- Frozza, R.L.; Bernardi, A.; Paese, K.; Hoppe, J.B.; Da Silva, T.; Battastini, A.M.O.; Pohlmann, A.; Guterres, S.; Salbego, C. Characterization of trans-Resveratrol-Loaded Lipid-Core Nanocapsules and Tissue Distribution Studies in Rats. J. Biomed. Nanotechnol. 2010, 6, 694–703. [Google Scholar] [CrossRef]
- Lin, Y.F.; Lee, Y.H.; Hsu, Y.H.; Chen, Y.J.; Lin, Y.F.; Cheng, F.-Y.; Chiu, H.W. Resveratrol-Loaded Nanoparticles Conjugated with Kidney Injury Molecule-1 as a Drug Delivery System for Potential Use in Chronic Kidney Disease. Nanomedicine 2017, 12, 2741–2756. [Google Scholar] [CrossRef]
- Singh, A.; Ahmad, I.; Ahmad, S.; Iqbal, Z.; Ahmad, F. A novel monolithic controlled delivery system of resveratrol for enhanced hepatoprotection: Nanoformulation development, pharmacokinetics and pharmacodynamics. Drug Dev. Ind. Pharm. 2016, 42, 1524–1536. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Li, R.; Yang, X.; Zhong, Z.; Dai, Y.; Fan, Q.; Lin, Y.; Zhang, R.; Liang, T.; et al. Resveratrol-loaded PLGA nanoparticles functionalized with red blood cell membranes as a biomimetic delivery system for prolonged circulation time. J. Drug Deliv. Sci. Technol. 2019, 54, 101369. [Google Scholar] [CrossRef]
- Suktham, K.; Koobkokkruad, T.; Wutikhun, T.; Surassmo, S. Efficiency of resveratrol-loaded sericin nanoparticles: Promising bionanocarriers for drug delivery. Int. J. Pharm. 2018, 537, 48–56. [Google Scholar] [CrossRef]
- Reis, S.; Neves, A.R.; Lúcio, M.; Martins, S.; Lima, J. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Gao, Y.; Zhao, J.; Zhang, J.; Li, Q.; Zhao, Z.; Liu, J. Preparation and Optimization of Resveratrol Nanosuspensions by Antisolvent Precipitation Using Box-Behnken Design. AAPS PharmSciTech 2014, 16, 118–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostami, M.; Ghorbani, M.; Mohammadi, M.A.; Delavar, M.; Tabibiazar, M.; Ramezani, S. Development of resveratrol loaded chitosan-gellan nanofiber as a novel gastrointestinal delivery system. Int. J. Biol. Macromol. 2019, 135, 698–705. [Google Scholar] [CrossRef]
- Liu, C.; Tong, P.; Yang, R.; You, Y.; Liu, H.; Zhang, T. Solidified phospholipid-TPGS as an effective oral delivery system for improving the bioavailability of resveratrol. J. Drug Deliv. Sci. Technol. 2019, 52, 769–777. [Google Scholar] [CrossRef]
- Wang, P.-P.; Luo, Z.-G.; Tamer, T.M. Spiral–Dextrin Complex Crystals: Efficient Approach for Colon-Targeted Resveratrol Delivery. J. Agric. Food Chem. 2020, 69, 474–482. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Dvořák, J.; Rosiak, N.; Tykarska, E.; Szymańska, E.; Winnicka, K.; Ruchała, M.; Cielecka-Piontek, J. Buccal Resveratrol Delivery System as a Potential New Concept for the Periodontitis Treatment. Pharmaceutics 2021, 13, 417. [Google Scholar] [CrossRef]
- Chen, Z.; Farag, M.A.; Zhong, Z.; Zhang, C.; Yang, Y.; Wang, S.; Wang, Y. Multifaceted role of phyto-derived polyphenols in nanodrug delivery systems. Adv. Drug Deliv. Rev. 2021, 113870. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Castro, L.A.; Schiborr, C.; David, F.; Ehrt, H.; Voggel, J.; Sus, N.; Behnam, D.; Bosy-Westphal, A.; Frank, J. The Oral Bioavailability of Trans -Resveratrol from a Grapevine-Shoot Extract in Healthy Humans is Significantly Increased by Micellar Solubilization. Mol. Nutr. Food Res. 2018, 62, e1701057. [Google Scholar] [CrossRef] [PubMed]
- Briskey, D.; Rao, A. Trans-Resveratrol Oral Bioavailability in Humans Using LipiSperse™ Dispersion Technology. Pharmaceutics 2020, 12, 1190. [Google Scholar] [CrossRef] [PubMed]


| Compound | Dosage | Effect | Type | Animal/Cell | Results | Reference |
|---|---|---|---|---|---|---|
| Resveratrol | 5 g per person | Peak serum concentration 539 ng/mL RES Metabolites 3-8× higher compared to RES | Clinical studies | Low bioavailability | [20] | |
| Resveratrol + piperine | 250 mg RES vs. 250 mg RES + 20 mg PIP | No vs. Increase cerebral blood flow | No altering bioavailability | [85] | ||
| Resveratrol + piperine | 2.5 g RES + 0; 5 or 25 mg PIP | No relationship between dose and pharmacokinetic values | Dosage does not alter bioavailability | [86] | ||
| Resveratrol + quercetin + alcohol | 2 g 2×/person + 500 mg Q + 5 mL alcohol | Neither quercetin nor alcohol alter RES. Fat meal alter RES concentration | Fat meal decreases bioavailability | [28] | ||
| Resveratrol + piperine | 100 mg/kg RES vs. 100 mg/kg RES + 10 mg/kg PIP (oral) | After comparison maximum serum concentration increased to 1544% | Animal studies | C57BI/6 mice | Beneficial | [87] |
| Resveratrol + piperine | 30 mg/kg | RES with piperine loaded mixed micelles demonstrated 5.7-fold compared to RES | Wistar albino | Beneficial | [88] | |
| Oxyresveratrol + piperine | 100 mg/kg OXY vs. 100 mg/kg OXY + 10 mg/kg PIP (oral) | Piperine mix increased bioavailability of oxyresveratrol 2-fold | Wistar rat | Beneficial | [89] | |
| Resveratrol + curcumin + quercetin + piperine | 50 µM (RES, C, Q)200 nM (PIP) | RES received the greatest enhancement in permeability when combined with other agents: quercetin (310%), curcumin (300%), quercetin and curcumin (323%, 350% with piperine). Curcumin also demonstrated increased permeability when combined with quercetin alone (147%) and both quercetin and resveratrol (188%), addition of piperine resulted in a 229% increase in permeability. | In vitro/ex vivo studies | Caco-2 | Beneficial | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesely, O.; Baldovska, S.; Kolesarova, A. Enhancing Bioavailability of Nutraceutically Used Resveratrol and Other Stilbenoids. Nutrients 2021, 13, 3095. https://doi.org/10.3390/nu13093095
Vesely O, Baldovska S, Kolesarova A. Enhancing Bioavailability of Nutraceutically Used Resveratrol and Other Stilbenoids. Nutrients. 2021; 13(9):3095. https://doi.org/10.3390/nu13093095
Chicago/Turabian StyleVesely, Ondrej, Simona Baldovska, and Adriana Kolesarova. 2021. "Enhancing Bioavailability of Nutraceutically Used Resveratrol and Other Stilbenoids" Nutrients 13, no. 9: 3095. https://doi.org/10.3390/nu13093095
APA StyleVesely, O., Baldovska, S., & Kolesarova, A. (2021). Enhancing Bioavailability of Nutraceutically Used Resveratrol and Other Stilbenoids. Nutrients, 13(9), 3095. https://doi.org/10.3390/nu13093095






