Physical Growth of Patients with Hereditary Tyrosinaemia Type I: A Single-Centre Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Project Design
2.2. NTBC and succinylacetone concentrations
2.3. Dietary prescriptions
2.4. Anthropometry
2.5. Ethical statement
2.6. Statistics
3. Results
3.1. Patients
3.2. Dietary prescription
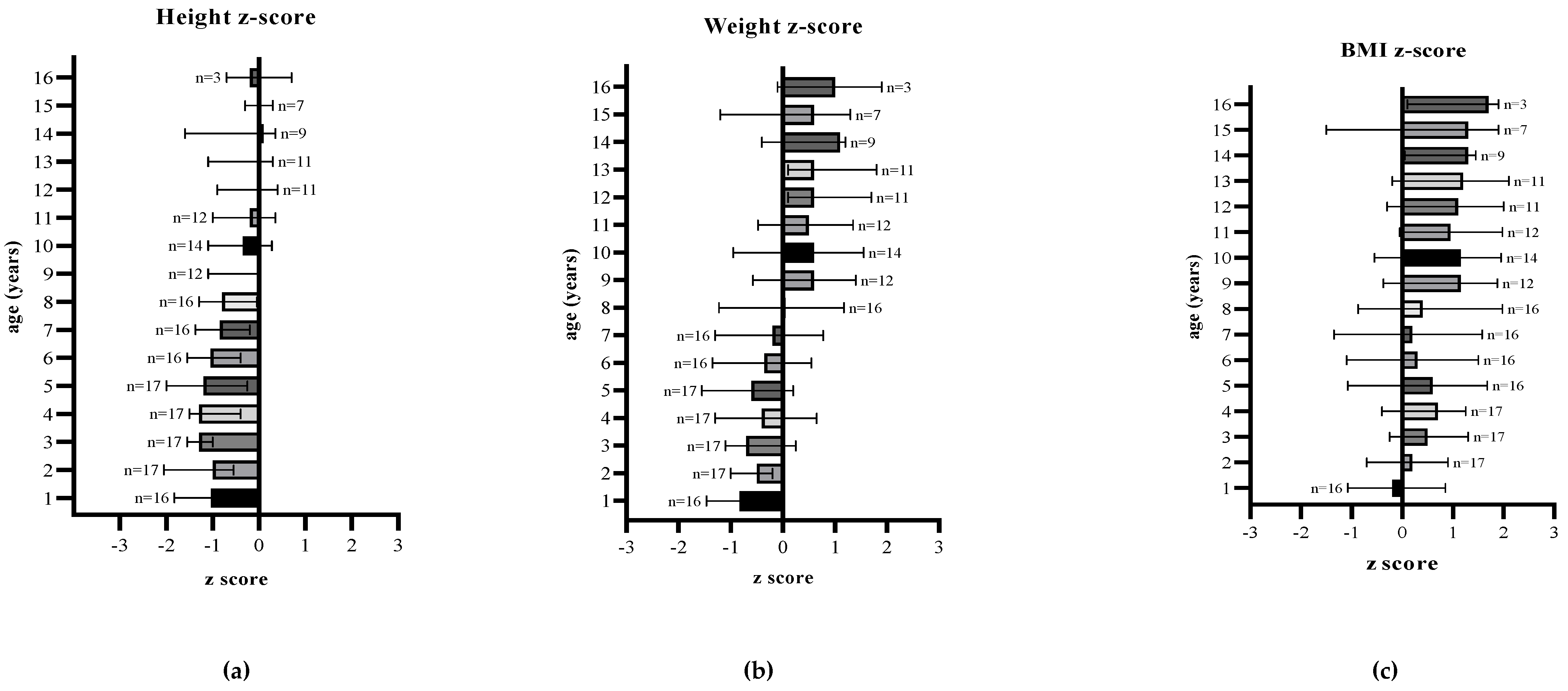
3.3. Anthropometric Parameters
3.4. Overweight and obesity by age
3.5. Serum NTBC and plasma succinylacetone concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dixon, M.; MacDonald, A.; White, F.; Stafford, J. Disorders of amino acid metabolism, organic acidaemias and urea cycle disorders. In Clinical Paediatric Dietetics, 4th ed.; John Wiley & Sons, Ltd.: Hoboken NJ, USA, 2015; pp. 381–525. [Google Scholar]
- Holme, E.; Lindstedt, S. Nontransplant treatment of tyrosinemia. Clin. Liver Dis. 2000, 4, 805–814. [Google Scholar] [CrossRef]
- McKiernan, P.J. Nitisinone in the treatment of hereditary tyrosinaemia type 1. Drugs 2006, 66, 743–750. [Google Scholar] [CrossRef]
- Lindstedt, S.; Holme, E.; Lock, E.; Hjalmarson, O.; Strandvik, B. Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 1992, 340, 813–817. [Google Scholar] [CrossRef]
- van Ginkel, W.G.; Jahja, R.; Huijbregts, S.C.; van Spronsen, F.J. Neurological and neuropsychological problems in tyrosinemia type I patients. In Hereditary Tyrosinemia; Springer: Cham, Switzerland, 2017; pp. 111–122. [Google Scholar]
- Mayorandan, S.; Meyer, U.; Gokcay, G.; Segarra, N.G.; De Baulny, H.O.; Van Spronsen, F.; Zeman, J.; De Laet, C.; Spiekerkoetter, U.; Thimm, E. Cross-sectional study of 168 patients with hepatorenal tyrosinaemia and implications for clinical practice. Orphanet J. Rare Dis. 2014, 9, 107. [Google Scholar] [CrossRef]
- Van Vliet, K.; van Ginkel, W.G.; Jahja, R.; Daly, A.; MacDonald, A.; De Laet, C.; Vara, R.; Rahman, Y.; Cassiman, D.; Eyskens, F. Emotional and behavioral problems, quality of life and metabolic control in NTBC-treated Tyrosinemia type 1 patients. Orphanet J. Rare Dis. 2019, 14, 1–9. [Google Scholar] [CrossRef]
- van Ginkel, W.G.; Jahja, R.; Huijbregts, S.C.; Daly, A.; MacDonald, A.; De Laet, C.; Cassiman, D.; Eyskens, F.; Körver-Keularts, I.M.; Goyens, P.J. Neurocognitive outcome in tyrosinemia type 1 patients compared to healthy controls. Orphanet J. Rare Dis. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Masurel-Paulet, A.; Poggi-Bach, J.; Rolland, M.-O.; Bernard, O.; Guffon, N.; Dobbelaere, D.; Sarles, J.; de Baulny, H.O.; Touati, G. NTBC treatment in tyrosinaemia type I: Long-term outcome in French patients. J. Inherit. Metab. Dis. 2008, 31, 81–87. [Google Scholar] [CrossRef]
- Thimm, E.; Richter-Werkle, R.; Kamp, G.; Molke, B.; Herebian, D.; Klee, D.; Mayatepek, E.; Spiekerkoetter, U. Neurocognitive outcome in patients with hypertyrosinemia type I after long-term treatment with NTBC. J. Inherit. Metab. Dis. Off. J. Soc. Study Inborn Errors Metab. 2012, 35, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Bendadi, F.; de Koning, T.J.; Visser, G.; Prinsen, H.C.; de Sain, M.G.; Verhoeven-Duif, N.; Sinnema, G.; van Spronsen, F.J.; van Hasselt, P.M. Impaired cognitive functioning in patients with tyrosinemia type I receiving nitisinone. J. Pediatrics 2014, 164, 398–401. [Google Scholar] [CrossRef] [PubMed]
- De Laet, C.; Terrones Munoz, V.; Jaeken, J.; François, B.; Carton, D.; Sokal, E.M.; Dan, B.; Goyens, P.J. Neuropsychological outcome of NTBC-treated patients with tyrosinaemia type 1. Dev. Med. Child Neurol. 2011, 53, 962–964. [Google Scholar] [CrossRef]
- Daly, A.; Gokmen Ozel, H.; MacDonald, A.; Preece, M.; Davies, P.; Chakrapani, A.; McKiernan, P. Diurnal variation of phenylalanine concentrations in tyrosinaemia type 1: Should we be concerned? J. Hum. Nutr. Diet. 2012, 25, 111–116. [Google Scholar] [CrossRef]
- Chinsky, J.M.; Singh, R.; Ficicioglu, C.; Van Karnebeek, C.D.; Grompe, M.; Mitchell, G.; Waisbren, S.E.; Gucsavas-Calikoglu, M.; Wasserstein, M.P.; Coakley, K. Diagnosis and treatment of tyrosinemia type I: A US and Canadian consensus group review and recommendations. Genet. Med. 2017, 19, 1380. [Google Scholar] [CrossRef]
- van Dam, E.; Daly, A.; Venema-Liefaard, G.; van Rijn, M.; Derks, T.G.; McKiernan, P.J.; Heiner-Fokkema, M.R.; MacDonald, A.; van Spronsen, F.J. What Is the Best Blood Sampling Time for Metabolic Control of Phenylalanine and Tyrosine Concentrations in Tyrosinemia Type 1 Patients? JIMD Rep. 2017, 36, 49–57. [Google Scholar] [PubMed]
- Wilson, C.; Van Wyk, K.; Leonard, J.; Clayton, P. Phenylalanine supplementation improves the phenylalanine profile in tyrosinaemia. J. Inherit. Metab. Dis. 2000, 23, 677–683. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, D.; van Dam, E.; van Rijn, M.; Derks, T.G.; Venema-Liefaard, G.; Hitzert, M.M.; Lunsing, R.J.; Heiner-Fokkema, M.R.; van Spronsen, F.J. Infants with Tyrosinemia Type 1: Should phenylalanine be supplemented? JIMD Rep. 2014, 18, 117–124. [Google Scholar]
- Harding, C.O.; Winn, S.R.; Gibson, K.M.; Arning, E.; Bottiglieri, T.; Grompe, M. Pharmacologic inhibition of L-tyrosine degradation ameliorates cerebral dopamine deficiency in murine phenylketonuria (PKU). J. Inherit. Metab. Dis. 2014, 37, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Daly, A.; Pinto, A.; Ashmore, C.; Evans, S.; Gupte, G.; Santra, S.; Preece, M.A.; Mckiernan, P.; Kitchen, S. Natural Protein Tolerance and Metabolic Control in Patients with Hereditary Tyrosinaemia Type 1. Nutrients 2020, 12, 1148. [Google Scholar] [CrossRef]
- Bärhold, F.; Meyer, U.; Neugebauer, A.-K.; Thimm, E.M.; Lier, D.; Rosenbaum-Fabian, S.; Och, U.; Fekete, A.; Möslinger, D.; Rohde, C. Hepatorenal Tyrosinaemia: Impact of a Simplified Diet on Metabolic Control and Clinical Outcome. Nutrients 2021, 13, 134. [Google Scholar] [CrossRef]
- Evans, M.; Truby, H.; Boneh, A. The relationship between dietary intake, growth, and body composition in inborn errors of intermediary protein metabolism. J. Pediatrics 2017, 188, 163–172. [Google Scholar] [CrossRef]
- MacDonald, A.; Rocha, J.; Van Rijn, M.; Feillet, F. Nutrition in phenylketonuria. Mol. Genet. Metab. 2011, 104, S10–S18. [Google Scholar] [CrossRef]
- McKiernan, P.; Preece, M.A.; Chakrapani, A. Outcome of children with hereditary tyrosinaemia following newborn screening. Arch. Dis. Child. 2015, 100, 738–741. [Google Scholar] [CrossRef] [PubMed]
- WHO Multicentre Growth Reference Study Group; De Onis, M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatrica 2006, 450, 76. [Google Scholar]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.J. Use of percentiles and z-scores in anthropometry. In Handbook of Anthropometry; Springer: Cham, Switzerland, 2012; pp. 29–48. [Google Scholar]
- Jack, R.M.; Scott, C.R. Validation of a therapeutic range for nitisinone in patients treated for tyrosinemia type 1 based on reduction of succinylacetone excretion. JIMD Rep. 2019, 46, 75–78. [Google Scholar]
- Conolly, A.; Craig, S. Health survey for England 2018—overweight and obesity in adults and children. In NHS Digital; NHS: Leeds, UK, 2019; p. 26. [Google Scholar]
- Veldhuis, J.D.; Roemmich, J.N.; Richmond, E.J.; Rogol, A.D.; Lovejoy, J.C.; Sheffield-Moore, M.; Mauras, N.; Bowers, C.Y. Endocrine control of body composition in infancy, childhood, and puberty. Endocr. Rev. 2005, 26, 114–146. [Google Scholar] [CrossRef]
- Wei, C.; Gregory, J.W. Physiology of normal growth. Paediatr. Child Health 2009, 19, 236–240. [Google Scholar] [CrossRef]
- Äärelä, L.; Hiltunen, P.; Soini, T.; Vuorela, N.; Huhtala, H.; Nevalainen, P.I.; Heikinheimo, M.; Kivelä, L.; Kurppa, K. Type 1 tyrosinemia in Finland: A nationwide study. Orphanet J. Rare Dis. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Cohn, R.; Yudkoff, M.; Yost, B.; Segal, S. Phenylalanine-tyrosine deficiency syndrome as a complication of the management of hereditary tyrosinemia. Am. J. Clin. Nutr. 1977, 30, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Aldámiz-Echevarría, L.; Llarena, M.; Andrade, F. Phenylketonuria Treatments Impact on Physical Growth: A Spanish Retrospective Longitudinal Study. J. Rare Disord. Diagn. Ther. 2015, 1, 2. [Google Scholar]
- MacDonald, A.; Singh, R.H.; Rocha, J.C.; van Spronsen, F.J. Optimising amino acid absorption: Essential to improve nitrogen balance and metabolic control in phenylketonuria. Nutr. Res. Rev. 2019, 32, 70–78. [Google Scholar] [CrossRef]
- Ilgaz, F.; Pinto, A.; Gokmen-Ozel, H.; Rocha, J.C.; van Dam, E.; Ahring, K.; Belanger-Quintana, A.; Dokoupil, K.; Karabulut, E.; MacDonald, A. Long-Term Growth in Phenylketonuria: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2070. [Google Scholar] [CrossRef]
- Couce, M.L.; Sánchez-Pintos, P.; Aldámiz-Echevarría, L.; Vitoria, I.; Navas, V.; Martín-Hernández, E.; García-Volpe, C.; Pintos, G.; Peña-Quintana, L.; Hernández, T. Evolution of tyrosinemia type 1 disease in patients treated with nitisinone in Spain. Medicine 2019, 98, e17303. [Google Scholar] [CrossRef]
- Aktuglu Zeybek, A.C.; Kiykim, E.; Soyucen, E.; Cansever, S.; Altay, S.; Zubarioglu, T.; Erkan, T.; Aydin, A. Hereditary tyrosinemia type 1 in Turkey: Twenty year single-center experience. Pediatrics Int. 2015, 57, 281–289. [Google Scholar] [CrossRef]
- Baumann, U.; Preece, M.; Green, A.; Kelly, D.; McKiernan, P. Hyperinsulinism in tyrosinaemia type I. J. Inherit. Metab. Dis. 2005, 28, 131–135. [Google Scholar] [CrossRef]
- Nasir, S.; Raza, M.; Siddiqui, S.I.; Saleem, A.; Abbas, A. Hereditary Tyrosinemia Compounded with Hyperinsulinemic Hypoglycemia: Challenging Diagnosis of a Rare Case. Cureus 2020, 12, e11541. [Google Scholar] [PubMed]
- Perry, T. Tyrosinemia associated with hypermethioninemia and islet cell hyperplasia. Can. Med Assoc. J. 1967, 97, 1067. [Google Scholar]
- Sotiridou, E.; Hoermann, H.; Aftab, S.; Dastamani, A.; Thimm, E.; Doodson, L.; Batzios, S.; Kummer, S.; Shah, P. Diazoxide-responsive hyperinsulinaemic hypoglycaemia in tyrosinaemia type 1. Endocrinol. Diabetes Metab. Case Rep. 2021, 2021. [Google Scholar] [CrossRef]
- Halvorsen, S.; Pande, H.; Loken, A.C.; Gjessing, L. Tyrosinosis. A study of 6 cases. Arch. Dis. Child. 1966, 41, 238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Larochelle, J.; Mortezai, A.; Belanger, M.; Tremblay, M.; Claveau, J.; Aubin, G. Experience with 37 infants with tyrosinemia. Can. Med Assoc. J. 1967, 97, 1051. [Google Scholar]
- Sass-Kortsak, A.; Ficici, S.; Paunier, L.; Kooh, S.; Fraser, D.; Jackson, S. Secondary metabolic derangements in patients with tyrosyluria. Can. Med Assoc. J. 1967, 97, 1079. [Google Scholar] [PubMed]
- Vora, S.; Chandran, S.; Rajadurai, V.S.; Hussain, K. Hyperinsulinemic hypoglycemia in infancy: Current concepts in diagnosis and management. Indian Pediatrics 2015, 52, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
| Definitions | BMI-for-Age z-Score | |
|---|---|---|
| <5 Years | 5–19 Years | |
| Overweight | ≥ +2 SD | ≥ +1 SD |
| Obesity | ≥ +3 SD | ≥ +2 SD |
| Age (years) | Number of patients | Height (cm) | Height-for-age z-score | Weight (kg) | Weight-for-age z-score | BMI (kg/m2) | BMI-for-age z-score | Protein (g/kg/day) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | NP | PE from PS | ||||||||
| Median (Q1, Q3) | ||||||||||
| 1 | 16 | 73.4 (71.6, 75.8) | −1.0 (−1.7, −0.3) | 8.9 (8.8, 10.2) | −0.8 (−1.4, −0.2) | 16.8 (16.2, 18.6) | −0.2 (−1.0, 0.8) | 3.4 (2.9, 3.6) | 0.5 (0.3, 0.7) | 2.8 (2.3, 3.1) |
| 2 | 17 | 84.0 (80.7, 87.0) | −1.0 (−2.0, −0.6) | 11.7 (11.2, 12.3) | −0.5 (−0.9, −0.2) | 16.6 (15.8, 17.5) | 0.2 (−0.6, 0.8) | 2.8 (2.7, 3.1) | 0.5 (0.3, 0.7) | 2.4 (2.3, 2.6) |
| 3 | 17 | 91.7 (90.3, 92.5) | −1.3 (−1.5, −1.0) | 13.7 (12.7, 15.0) | −0.7 (−1.1, 0.1) | 16.7 (15.8, 17.7) | 0.5 (−0.2, 1.2) | 2.7 (2.5, 2.9) | 0.4 (0.3, 0.5) | 2.2 (1.9, 2.6) |
| 4 | 17 | 99.3 (96.1, 100.3) | −1.3 (−1.5, −0.5) | 16.1 (14.4, 17.4) | −0.4 (−1.2, 0.4) | 16.6 (16.0, 17.5) | 0.7 (0.2, 1.2) | 2.5 (2.3, 3.0) | 0.4 (0.3, 0.5) | 2.1 (1.9, 2.7) |
| 5 | 17 | 104.9 (102.3, 109.3) | −1.2 (−2.0, −0.3) | 17.1 (15.8, 19.4) | −0.6 (−1.5, 0.1) | 16.3 (14.4, 17.5) | 0.6 (−1.0, 1.5) | 2.5 (2.4, 2.9) | 0.5 (0.3, 0.6) | 2.2 (1.9, 2.4) |
| 6 | 16 | 111.9 (109.5, 114.9) | −1.1 (−1.5, −0.4) | 20.1 (18.5, 22.3) | −0.3 (−1.1, 0.3) | 15.9 (15.0, 18.0) | 0.3 (−0.5, 1.5) | 2.3 (2.0, 2.7) | 0.4 (0.4, 0.5) | 2.0 (1.6, 2.2) |
| 7 | 16 | 118.8 (115.8, 121.5) | −0.9 (−1.3, −0.2) | 22.9 (19.9, 26.3) | −0.2 (−1.1, 0.7) | 15.8 (14.0, 18.4) | 0.2 (−1.3, 1.5) | 2.3 (2.0, 2.6) | 0.4 (0.3, 0.5) | 1.9 (1.6, 2.2) |
| 8 | 16 | 123.5 (121.4, 127.5) | −0.8 (−1.3, −0.2) | 25.8 (23.2, 31.4) | 0.0 (−0.9, 1.1) | 16.5 (14.9, 20.0) | 0.4 (−0.6, 1.9) | 2.2 (1.9, 2.2) | 0.4 (0.3, 0.5) | 1.8 (1.5, (1.9) |
| 9 | 12 | 133.0 (127.0, 134.0) | 0.0 (−1.1, 0.0) | 32.0 (27.1, 37.1) | 0.6 (−0.5, 1.4) | 17.2 (15.1, 20.1) | 1.1 (−0.1, 1.9) | 1.9 (1.7, 2.1) | 0.4 (0.3, 0.5) | 1.6 (1.4, 1.7) |
| 10 | 14 | 137.0 (133.8, 140.0) | −0.3 (−1.1, 0.2) | 36.6 (29.4, 42.0) | 0.6 (−0.6, 1.4) | 19.3 (15.7, 22.0) | 1.2 (−0.5, 1.9) | 1.8 (1.6, 2.2) | 0.5 (0.4, 0.6) | 1.5 (1.2,1.7) |
| 11 | 12 | 143.3 (138.9, 146.4) | −0.2 (−1.0, 0.2) | 39.3 (34.1, 46.7) | 0.5 (−0.2, 1.1) | 19.3 (17.8, 21.6) | 0.9 (0.3, 1.7) | 1.6 (1.5, 2.0) | 0.4 (0.3, 0.5) | 1.3 (1.1, 1.5) |
| 12 | 11 | 150.4 (147.1, 154.1) | 0.0 (−0.7, 0.4) | 47.1 (42.3, 51.0) | 0.6 (0.2, 1.3) | 20.5 (18.7, 22.8) | 1.1 (0.1, 1.8) | 1.5 (1.3, 1.9) | 0.4 (0.3, 0.5) | 1.0 (1.0, 1.4) |
| 13 | 11 | 154.6 (151.1, 159.7) | 0.0 (−0.9, 0.3) | 52.3 (49.1, 60.1) | 0.6 (0.3, 1.4) | 22.1 (19.3, 24.6) | 1.2 (0.4, 2.0) | 1.4 (1.2, 1.6) | 0.3 (0.3, 0.4) | 1.0 (0.9, 1.2) |
| 14 | 9 | 161.8 (151.6, 165.3) | 0.1 (−1.6, 0.3) | 62.2 (49.3, 63.1) | 1.1 (−0.3, 1.2) | 22.4 (21.6, 23.1) | 1.3 (0.7, 1.4) | 1.3 (1.2, 1.6) | 0.4 (0.3, 0.4) | 1.0 (0.9, 1.2) |
| 15 | 7 | 166.2 (162.9, 171.5) | 0.0 (−0.3, 0.3) | 61.9 (53.8, 66.7) | 0.6 (−0.5, 1.2) | 23.8 (18.7, 24.8) | 1.3 (−0.5, 1.7) | 1.5 (1.3, 1.7) | 0.4 (0.3, 0.5) | 1.0 (0.9, 1.2) |
| 16 | 3 | 172.1 (170.2, 175.6) | −0.2 (−0.4, 0.3) | 71.2 (65.6, 78.0) | 1.0 (0.5, 1.5) | 25.2 (22.7, 25.8) | 1.7 (0.9, 1.8) | 1.3 (1.2, 1.4) | 0.5 (0.3, 0.5) | 0.7 (0.6, 0.9) |
| Age (Years) | Number of Patients | Overweight n (%) | Obesity n (%) |
|---|---|---|---|
| 1 | 16 | 0 (0) | 0 (0) |
| 2 | 17 | 1 (6) | 1 (6) |
| 3 | 17 | 0 (0) | 1 (6) |
| 4 | 17 | 2 (12) | 1 (6) |
| 5 | 17 | 5 (29) | 2 (12) |
| 6 | 16 | 3 (19) | 2 (13) |
| 7 | 16 | 6 (38) | 1 (6) |
| 8 | 16 | 3 (19) | 4 (25) |
| 9 | 12 | 4 (33) | 2 (17) |
| 10 | 14 | 5 (36) | 3 (21) |
| 11 | 12 | 3 (25) | 3 (25) |
| 12 | 11 | 3 (27) | 3 (27) |
| 13 | 11 | 5 (46) | 3 (27) |
| 14 | 9 | 5 (56) | 1 (11) |
| 15 | 7 | 3 (43) | 1 (14) |
| 16 | 3 | 2 (67) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, O.; Daly, A.; Pinto, A.; Ashmore, C.; Evans, S.; Gupte, G.; Jackson, R.; Yabanci Ayhan, N.; MacDonald, A. Physical Growth of Patients with Hereditary Tyrosinaemia Type I: A Single-Centre Retrospective Study. Nutrients 2021, 13, 3070. https://doi.org/10.3390/nu13093070
Yilmaz O, Daly A, Pinto A, Ashmore C, Evans S, Gupte G, Jackson R, Yabanci Ayhan N, MacDonald A. Physical Growth of Patients with Hereditary Tyrosinaemia Type I: A Single-Centre Retrospective Study. Nutrients. 2021; 13(9):3070. https://doi.org/10.3390/nu13093070
Chicago/Turabian StyleYilmaz, Ozlem, Anne Daly, Alex Pinto, Catherine Ashmore, Sharon Evans, Girish Gupte, Richard Jackson, Nurcan Yabanci Ayhan, and Anita MacDonald. 2021. "Physical Growth of Patients with Hereditary Tyrosinaemia Type I: A Single-Centre Retrospective Study" Nutrients 13, no. 9: 3070. https://doi.org/10.3390/nu13093070
APA StyleYilmaz, O., Daly, A., Pinto, A., Ashmore, C., Evans, S., Gupte, G., Jackson, R., Yabanci Ayhan, N., & MacDonald, A. (2021). Physical Growth of Patients with Hereditary Tyrosinaemia Type I: A Single-Centre Retrospective Study. Nutrients, 13(9), 3070. https://doi.org/10.3390/nu13093070