Effect of Coffee Consumption on Non-Alcoholic Fatty Liver Disease Incidence, Prevalence and Risk of Significant Liver Fibrosis: Systematic Review with Meta-Analysis of Observational Studies
Abstract
:1. Introduction
2. Material & Methods
2.1. Study Search and Selection
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
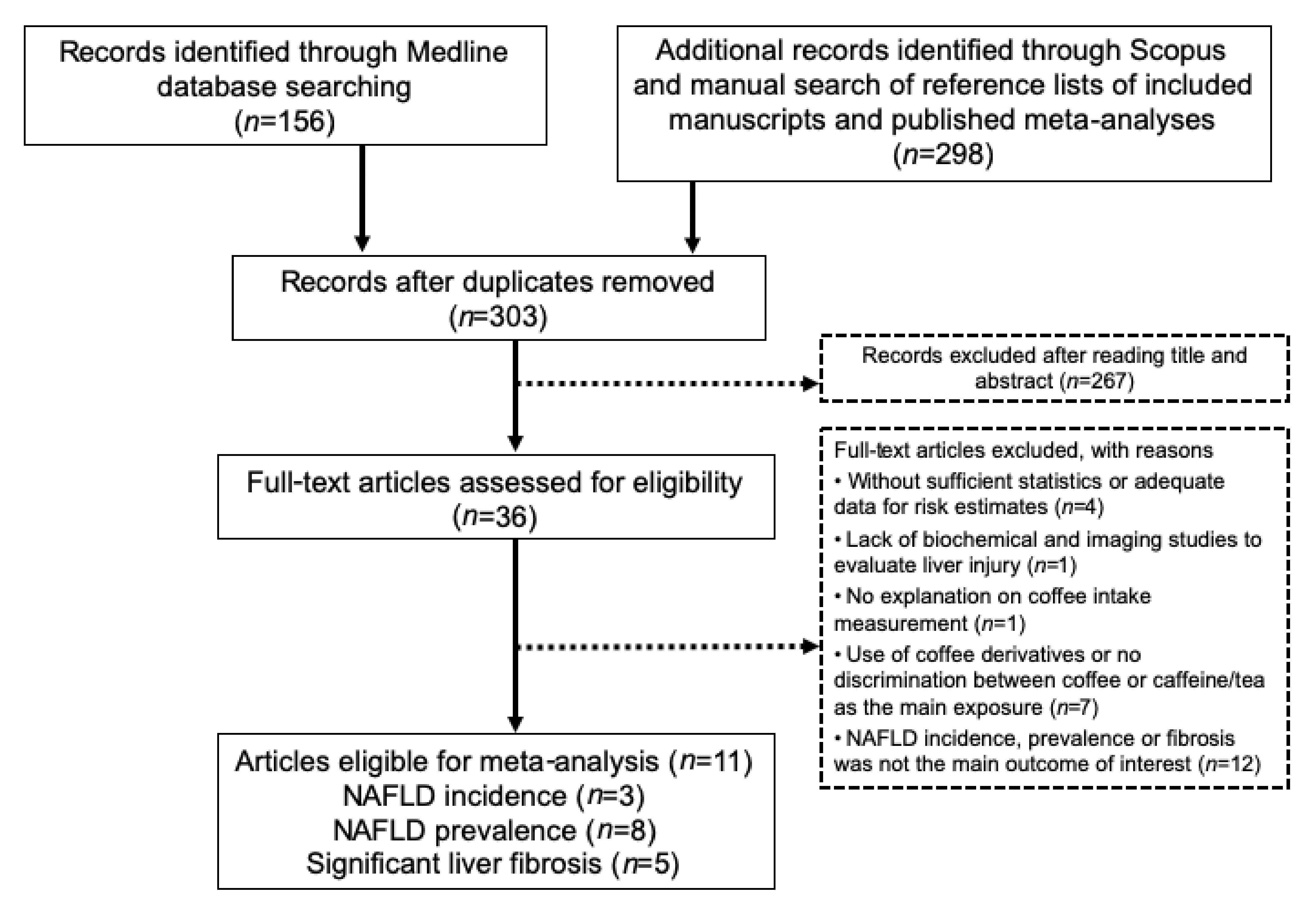
3.1. Publication Selection and Quality Assessment
3.2. Coffee Consumption and NAFLD
3.3. Meta-Analysis
3.3.1. Coffee Consumption and NAFLD Incidence
3.3.2. Coffee Consumption and NAFLD Prevalence
3.3.3. Coffee Consumption and Risk of Significant Liver Fibrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar]
- Eslam, M.; Sanyal, A.J.; George, J. International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar]
- Wong, R.J.; Aguilar, M.; Cheung, R.; Perumpail, R.B.; Harrison, S.A.; Younossi, Z.M.; Ahmed, A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015, 148, 547–555. [Google Scholar]
- Tesfay, M.; Goldkamp, W.J.; Neuschwander-Tetri, B.A. NASH: The Emerging Most Common Form of Chronic Liver Disease. Mol. Med. 2018, 115, 225–229. [Google Scholar]
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagström, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wong, V.W.-S.; Peleg, N.; et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1611–1625.e12. [Google Scholar]
- Armstrong, M.; Houlihan, D.D.; Bentham, L.; Shaw, J.C.; Cramb, R.; Olliff, S.; Gill, P.S.; Neuberger, J.M.; Lilford, R.J.; Newsome, P. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J. Hepatol. 2012, 56, 234–240. [Google Scholar]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2017, 67, 328–357. [Google Scholar]
- Molloy, J.W.; Calcagno, C.J.; Williams, C.D.; Jones, F.J.; Torres, D.M.; Harrison, S.A. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology 2011, 55, 429–436. [Google Scholar]
- Liu, R.; Guo, X.; Park, Y.; Huang, X.; Sinha, R.; Freedman, N.D.; Hollenbeck, A.R.; Blair, A.; Chen, H. Caffeine Intake, Smoking, and Risk of Parkinson Disease in Men and Women. Am. J. Epidemiol. 2012, 175, 1200–1207. [Google Scholar]
- Bambha, K.; Wilson, L.A.; Unalp, A.; Loomba, R.; Neuschwander-Tetri, B.A.; Brunt, E.M.; Bass, N.M.; The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN). Coffee consumption in NAFLD patients with lower insulin resistance is associated with lower risk of severe fibrosis. Liver Int. 2014, 34, 1250–1258. [Google Scholar]
- Shim, S.G.; Jun, D.W.; Kim, E.K.; Saeed, W.K.; Lee, K.N.; Lee, H.L.; Lee, O.Y.; Choi, H.S.; Yoon, B.C. Caffeine attenuates liver fibrosis via defective adhesion of hepatic stellate cells in cirrhotic model. J. Gastroenterol. Hepatol. 2013, 28, 1877–1884. [Google Scholar]
- Arauz, J.; Zarco, N.; Segovia, J.; Shibayama, M.; Tsutsumi, V.; Muriel, P. Caffeine prevents experimental liver fibrosis by blocking the expression of TGF-beta. Eur. J. Gastroenterol. Hepatol. 2014, 26, 164–173. [Google Scholar]
- Li, S.; Tan, H.Y.; Wang, N.; Cheung, F.; Hong, M.; Feng, Y. The Potential and Action Mechanism of Polyphenols in the Treatment of Liver Diseases. Oxidative Med. Cell. Longev. 2018, 2018, 8394818. [Google Scholar]
- Vitaglione, P.; Morisco, F.; Mazzone, G.; Amoruso, D.C.; Ribecco, M.T.S.; Romano, A.; Fogliano, V.; Caporaso, N.; D’Argenio, G. Coffee reduces liver damage in a rat model of steatohepatitis: The underlying mechanisms and the role of polyphenols and melanoidins. Hepatology 2010, 52, 1652–1661. [Google Scholar]
- Imatoh, T.; Kamimura, S.; Miyazaki, M. Coffee but not green tea consumption is associated with prevalence and severity of hepatic steatosis: The impact on leptin level. Eur. J. Clin. Nutr. 2015, 69, 1023–1027. [Google Scholar]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar]
- Herzog, R.; Álvarez-Pasquin, M.J.; Díaz, C.; Del Barrio, J.L.; Estrada, J.M.; Gil, Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? a systematic review. BMC Public Health 2013, 13, 154. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar]
- Chung, H.-K.; Nam, J.S.; Lee, M.-Y.; Kim, Y.-B.; Won, Y.-S.; Song, W.-J.; Kim, Y.-H.; Ahn, C.W.; Sung, K.-C. The increased amount of coffee consumption lowers the incidence of fatty liver disease in Korean men. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1653–1661. [Google Scholar]
- Funatsu, K.; Yamashita, T.; Nakamura, H. Coffee Consumption is Associated with a Lower Incidence of Fatty Liver in Middle-aged Men. J. Health Sci. 2011, 57, 406–413. [Google Scholar]
- Zelber-Sagi, S.; Salomone, F.; Webb, M.; Lotan, R.; Yeshua, H.; Halpern, Z.; Santo, E.; Oren, R.; Shibolet, O. Coffee consumption and nonalcoholic fatty liver onset: A prospective study in the general population. Transl. Res. 2015, 165, 428–436. [Google Scholar]
- Veronese, N.; Notarnicola, M.; Cisternino, A.M.; Reddavide, R.; Inguaggiato, R.; Guerra, V.; Rotolo, O.; Zinzi, I.; Leandro, G.; Correale, M.; et al. Coffee Intake and Liver Steatosis: A Population Study in a Mediterranean Area. Nutrients 2018, 10, 89. [Google Scholar]
- Alferink, L.J.; Fittipaldi, J.; Jong, J.C.K.-D.; Taimr, P.; Hansen, B.E.; Metselaar, H.J.; Schoufour, J.D.; Ikram, M.A.; Janssen, H.L.; Franco, O.; et al. Coffee and herbal tea consumption is associated with lower liver stiffness in the general population: The Rotterdam study. J. Hepatol. 2017, 67, 339–348. [Google Scholar]
- Graeter, T.; Niedermayer, P.C.; Mason, R.A.; Oeztuerk, S.; Haenle, M.M.; Koenig, W.; Boehm, B.O.; Kratzer, W.; EMIL-Study group. Coffee consumption and NAFLD: A community based study on 1223 subjects. BMC Res. Notes 2015, 8, 640. [Google Scholar]
- Katsagoni, C.N.; Georgoulis, M.; Papatheodoridis, G.; Fragopoulou, E.; Ioannidou, P.; Papageorgiou, M.; Alexopoulou, A.; Papadopoulos, N.; Deutsch, M.; Kontogianni, M. Associations Between Lifestyle Characteristics and the Presence of Nonalcoholic Fatty Liver Disease: A Case–Control Study. Metab. Syndr. Relat. Disord. 2017, 15, 72–79. [Google Scholar]
- Zhang, X.; Goh, G.B.; Chan, W.; Wong, G.L.; Fan, J.; Seto, W.; Huang, Y.; Lin, H.; Lee, I.; Lee, H.W.; et al. Unhealthy lifestyle habits and physical inactivity among Asian patients with non-alcoholic fatty liver disease. Liver Int. 2020, 40, 2719–2731. [Google Scholar]
- Anty, R.; Marjoux, S.; Iannelli, A.; Patouraux, S.; Schneck, A.-S.; Bonnafous, S.; Gire, C.; Amzolini, A.; Ben-Amor, I.; Saint-Paul, M.-C.; et al. Regular coffee but not espresso drinking is protective against fibrosis in a cohort mainly composed of morbidly obese European women with NAFLD undergoing bariatric surgery. J. Hepatol. 2012, 57, 1090–1096. [Google Scholar]
- Gutiérrez-Grobe, Y.; Chavez-Tapia, N.; Sánchez-Valle, V.; Gavilanes-Espinar, J.G.; Ponciano-Rodríguez, G.; Uribe, M.; Méndez-Sánchez, N. High coffee intake is associated with lower grade nonalcoholic fatty liver disease: The role of peripheral antioxidant activity. Ann. Hepatol. 2012, 11, 350–355. [Google Scholar]
- Chen, Y.-P.; Lu, F.-B.; Hu, Y.-B.; Xu, L.-M.; Zheng, M.-H.; Hu, E.-D. A systematic review and a dose–response meta-analysis of coffee dose and nonalcoholic fatty liver disease. Clin. Nutr. 2019, 38, 2552–2557. [Google Scholar]
- Mishra, P.; Younossi, Z.M. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD). Am. J. Gastroenterol. 2007, 102, 2716–2717. [Google Scholar]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar]
- Chartampilas, E. Imaging of nonalcoholic fatty liver disease and its clinical utility. Hormones 2018, 17, 69–81. [Google Scholar]
- Shen, H.; Rodriguez, A.C.; Shiani, A.; Lipka, S.; Shahzad, G.; Kumar, A.; Mustacchia, P. Association between caffeine consumption and nonalcoholic fatty liver disease: A systemic review and meta-analysis. Ther. Adv. Gastroenterol. 2016, 9, 113–120. [Google Scholar]
- Marventano, S.; Salomone, F.; Godos, J.; Pluchinotta, F.R.; Del Rio, D.; Mistretta, A.; Grosso, G. Coffee and tea consumption in relation with non-alcoholic fatty liver and metabolic syndrome: A systematic review and meta-analysis of observational studies. Clin. Nutr. 2016, 35, 1269–1281. [Google Scholar]
- Wijarnpreecha, K.; Thongprayoon, C.; Ungprasert, P. Coffee consumption and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2017, 29, e8–e12. [Google Scholar]
- Sewter, R.; Heaney, S.; Patterson, A. Coffee Consumption and the Progression of NAFLD: A Systematic Review. Nutrients 2021, 13, 2381. [Google Scholar]
- Kim, H.-J.; Cho, S.; Jacobs, D.R., Jr.; Park, K. Instant coffee consumption may be associated with higher risk of metabolic syndrome in Korean adults. Diabetes Res. Clin. Pract. 2014, 106, 145–153. [Google Scholar]
- Caldwell, S. NASH Therapy: Omega 3 supplementation, vitamin E, insulin sensitizers and statin drugs. Clin. Mol. Hepatol. 2017, 23, 103–108. [Google Scholar]
- Althubaiti, A. Information bias in health research: Definition, pitfalls, and adjustment methods. J. Multidiscip. Healthc. 2016, 9, 211–217. [Google Scholar]
- Stockwell, T.; Donath, S.; Cooper-Stanbury, M.; Chikritzhs, T.; Catalano, P.; Mateo, C. Under-reporting of alcohol consumption in household surveys: A comparison of quantity-frequency, graduated-frequency and recent recall. Addiction 2004, 99, 1024–1033. [Google Scholar]
- Alferink, L.J.M.; Jong, J.C.K.-D.; Murad, S.D. Potential Mechanisms Underlying the Role of Coffee in Liver Health. Semin. Liver Dis. 2018, 38, 193–214. [Google Scholar]
- Arauz, J.; Moreno, M.G.; Cortés-Reynosa, P.; Salazar, E.P.; Muriel, P. Coffee attenuates fibrosis by decreasing the expression of TGF-beta and CTGF in a murine model of liver damage. J. Appl. Toxicol. 2013, 33, 970–979. [Google Scholar]
- Gordillo-Bastidas, D.; Oceguera-Contreras, E.; Salazar-Montes, A.; González-Cuevas, J.; Hernández-Ortega, L.D.; Armendáriz-Borunda, J. Nrf2 and Snail-1 in the prevention of experimental liver fibrosis by caffeine. World J. Gastroenterol. 2013, 19, 9020–9033. [Google Scholar]
- Helal, M.G.; Ayoub, S.E.; Elkashefand, W.F.; Ibrahim, T.M. Caffeine affects HFD-induced hepatic steatosis by multifactorial intervention. Hum. Exp. Toxicol. 2018, 37, 983–990. [Google Scholar]
- Takahashi, S.; Egashira, K.; Saito, K.; Jia, H.; Abe, K.; Kato, H. Coffee intake down-regulates the hepatic gene expression of peroxisome proliferator-activated receptor gamma in C57BL/6J mice fed a high-fat diet. J. Funct. Foods 2014, 6, 157–167. [Google Scholar]
- Metro, D.; Cernaro, V.; Santoro, D.; Papa, M.; Buemi, M.; Benvenga, S.; Manasseri, L. Beneficial effects of oral pure caffeine on oxidative stress. J. Clin. Transl. Endocrinol. 2017, 10, 22–27. [Google Scholar]
- Seo, H.Y.; Kim, M.K.; Lee, S.H.; Hwang, J.S.; Park, K.G.; Jang, B.K. Kahweol Ameliorates the Liver Inflammation through the Inhibition of NF-kappaB and STAT3 Activation in Primary Kupffer Cells and Primary Hepatocytes. Nutrients 2018, 10, 863. [Google Scholar]
- Asbaghi, O.; Kashkooli, S.; Mardani, M.; Kelishadi, M.R.; Fry, H.; Kazemi, M.; Kaviani, M. Effect of green coffee bean extract supplementation on liver function and inflammatory biomarkers: A meta-analysis of randomized clinical trials. Complementary Ther. Clin. Pract. 2021, 43, 101349. [Google Scholar]

| Study | Study Design | Population | NAFLD Diagnosis | Steatosis Severity | Fibrosis Severity | Coffee Intake Measurement | Main Finding | RR/HR/OR Estimates for Extreme Categories of Coffee Intake (95% CI) | Adjustments for Confounding Variables | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Chung et al., 2020 [21] | Retrospective longitudinal cohort | 91,436 subjects participating in a comprehensive health-screening program were followed for a mean of 2.8 years, 13,362 (15% developed fatty liver) | Ultrasound | Steatosis incidence: increase in liver echogenicity compared with the renal cortex echogenicity | Self-administered food frequency questionnaire | No association between fatty liver incidence and the amount of coffee consumption at baseline | HR 1.09 (0.97–1.22) | Age, sex, education, exercise, smoking, alcohol intake, centre and year, BMI, total energy intake, triglyceride, LDL-C, HDL-C, glucose, alanine aminotransferase, aspartate aminotransferase, change of alcohol, change of BMI, and change of exercise | 8 | |
| Zhang et al., 2020 [28] | Cross-sectional | 555 NAFLD patients from multi-centre hepatology clinics | Radiological features, liver histology, or elevated alanine aminotransferase or aspartate aminotransferase levels with a CAP value ≥ 248 dB/m | Elastography: severe hepatic steatosis: CAP threshold of 280 dB/m | Elastography: advanced fibrosis: LSM threshold of 10 kPa | Standardized, self-administered questionnaires | Inverse association between coffee consumption and advanced fibrosis No association between coffee consumption and severe hepatic steatosis | OR 0.49 (0.27–0.88), p = 0.02 OR 0.92 (0.59–1.44), p = 0.71 | Age, sex, smoking and alcohol status, coffee, tea, and soft drinks drinker, time spent on different physical activities, energy expenditure, physical activity level and meeting physical activity guidelines (%), obesity and type 2 diabetes, and participating centre | 8 |
| Veronese et al., 2018 [24] | Cross-sectional | 2819 randomly sampled participants from electoral rolls Absence of fatty liver in 1627 subjects and 916 with NAFLD (134 patients with Liver Steatosis Score of 6) | Ultrasound | Fatty liver score ranged from 0 to 6, with higher values indicating a greater severity | Self-reported validated semi-quantitative food frequency questionnaire | No association between coffee consumption and lower odds of liver steatosis | OR 0.97 (0.71–1.32), p = 0.84 | Age, sex, smoking status, presence of diabetes, gastric ulcer, cancer, acute myocardial infarction, waist circumference, systolic and diastolic blood pressure, daily energy, and alcohol intake | 8 | |
| Alferink et al., 2017 [25] | Cross-sectional | Population-based cohort of 2424 participants (35% steatosis) | Ultrasound | Presence or absence of a hyper-echogenic liver parenchyma | Transient elastography: liver stiffness measurements (LSM) ≥8.0 kPa | Validated 389-item food frequency questionnaire | Independent association between frequent coffee consumption and lower probability of significant liver fibrosis No significant association between coffee intake and steatosis | OR 0.39 (0.18–0.86), p = 0.005 OR 1.15 (0.75–1.77), p = 0.192 | Tea, energy intake, BMI, gender, age, steatosis, alanine aminotransferase, excessive alcohol intake, current or former smoking and HOMA-IR, soda consumption, cream and sugar use, dietary quality, and physical activity Tea, energy intake, BMI, gender, age, HOMA-IR, excessive alcohol intake, current or former smoking | 9 |
| Katsagoni et al., 2017 [27] | Case-control | 100 newly ultrasound-proven NAFLD patients (21 with NASH), 55 healthy controls matched for age, sex, and BMI | Elevated alanine aminotransferase and/or gamma- glutamyl transpeptidase levels, elastography, and evidence of hepatic steatosis on ultrasound (available for 85) and/or compatible liver histology (n = 32) | Evidence of hepatic steatosis at ultrasonography Biopsy: NASH Diagnosis: NAFLD Activity score (NAS) ≥ 5 | Semi-quantitative validated food frequency questionnaire | Inverse association between coffee intake and NAFLD presence (OR 0.68, 95% CI 0.49–0.94, p = 0.02) Lost its significance once adjusted for adiponectin and TNF-a | OR 0.72 (0.49–1.04), p = 0.07 | Age, sex, waist circumference, HOMA-IR, adiponectin, and TNF-a | 7 | |
| Zelber-Sagi et al., 2015 [23] | Cross-sectional and prospective cohort | Cross-sectional cohort 347 general population, 31% diagnosed with NAFLD Prospective cohort A subgroup of patients without fatty liver at baseline who were followed up for 7 years (n = 147) | Ultrasound and SteatoTest | Hepatorenal index (HRI) on US, NashTest (borderline NASH or definite NASH) SteatoTest (≥5%, ≥S1–S2) | FibroTest (significant fibrosis (≥F2)) | Interviewer-administrated questionnaire and detailed semiquantitative food-frequency questionnaire | Cross-sectional cohort: Inverse association between coffee consumption and significant liver fibrosis and no association with the development of steatosis Prospective cohort: No association between coffee consumption and NAFLD incidence | NAFLD prevalence 0.92 (0.57–1.50), p = 0.75 Hepatic Fibrosis:0.49 (0.25–0.97), p = 0.04 NAFLD Incidence: 0.72 (0.28–1.85), p = 0.501 | Current smoking, sugar intake, physical activity (minutes per week), serum cholesterol levels, and dietary fat and calories intake Current smoking, sugar intake, physical activity | 8 |
| Imatoh et al., 2015 [16] | Cross-sectional | 1024 Japanese male workers receiving annual health checkups Non-steatosis (n = 270) Steatosis (n = 754) | Ultrasound | No, mild, or moderate-to-severe hepatic steatosis | Self-reported questionnaire | Dose-dependent protective effect of coffee on the prevalence of hepatic steatosis | OR 0.59 (0.38–0.90), p = 0.03 | BMI, age, smoking status, alcohol drinking, and green tea consumption | 7 | |
| Graeter et al., 2015 [26] | Cross-sectional | Random population-based sample with 1452 subjects (381 diagnosed with hepatic steatosis) | Ultrasound | No steatosis and steatosis grade I, II and III | Standardized questionnaire | No association between hepatic steatosis and coffee consumption | OR 0.77 (0.44–1.34), p = 0.81 | Age, BMI, gender, metabolic syndrome, and physical activity | 8 | |
| Bambha et al., 2014 [11] | Cross-sectional | 782 biopsy-proven NAFLD patients; Advanced fibrosis (>stage 2) in 25% (n = 199) NASH (definite or probable): in 79% (n = 616) | Biopsy | Presence versus absence of NASH histology ((1) definite steatohepatitis; (2) definitely not steatohepatitis; and (3) borderline steatohepatitis) | None to moderate (≤Stage 2) or advanced (>Stage 2) | Self-reported validated dietary questionnaire | Significant association between coffee intake and decreased odds of advanced fibrosis in patients with lower HOMA-IR | OR 0.68 (0.52–0.89), p = 0.005 | Age, sex, race, waist circumference, aspartate transaminase, gamma-glutamyl transferase diabetes, smoking, alcohol, biopsy length, HOMA-IR, and interaction between coffee and HOMA-IR | 8 |
| Anty et al., 2012 [29] | Cross-sectional | 195 severely and morbidly obese patients, referred for bariatric surgery of which NASH was present in 19.5% | Biopsy | NAFLD activity score (NAS) simple steatosis (NAS ≤ 2), borderline (3≤ NAS ≤ 4), or definitive NASH (NAS ≥ 5) | Significant fibrosis (F ≥ 2) | Interviewer- administered questionnaire | Regular coffee consumption was an independent protective factor for significant fibrosis | OR: 0.752 (0.578–0.980), p = 0.04 | Aspartate aminotransferase, presence of NASH, presence of the metabolic syndrome, and level of HOMA-IR | 7 |
| Funatsu et al., 2011 [22] | Nested case-control | 1236 subjects followed for 5 years; of those, 164 males with fatty liver were matched (age, BMI, and exercise level) with 328 without fatty liver | Ultrasound | Steatosis incidence: a bright liver, an increase in the liver−kidney contrast, and/or a decrease in liver deep echo | Self-administered questionnaire | Daily coffee intake was inversely associated with fatty liver development | 0.74 (0.61–0.89), p = 0.001 | Age, BMI, exercise, daily alcohol intake, and changes in BMI, exercise level, and daily alcohol intake over time | 7 |
| Subgroup | Number of Studies | Pooled RR | Heterogeneity |
|---|---|---|---|
| NAFLD Prevalence | |||
| All studies | 8 | 0.88 (0.76–1.02), p = 0.09 | I2 = 0%, p = 0.43 |
| Study design Case-control Cross-sectional | 1 7 | 0.72 (0.49–1.06), p = 0.09 0.91(0.77–1.07), p = 0.25 | I2 = 0%, p = 0.44 |
| Questionnaire Self-reported Interviewed | 6 2 | 0.84 (0.71–0.99), p = 0.04 1.04 (0.76–1.43), p = 0.80 | I2 = 3%, p = 0.40 I2 = 0%, p = 0.50 |
| NOS score 7 8-9 | 2 6 | 0.66 (0.49–0.88), p = 0.005 0.97 (0.82–1.16), p = 0.76 | I2 = 3%, p = 0.50 I2 = 0%, p = 0.91 |
| Energy Intake Adjustment | |||
| Yes No | 2 6 | 1.03 (0.80–1.32), p = 0.82 0.81 (0.67–0.97), p = 0.02 | I2 = 0%, p = 0.53 I2 = 0%, p = 0.50 |
| Significant Liver Fibrosis | |||
| All studies | 5 | 0.65 (0.54–0.78), p < 0.00001 | I2 = 11%, p = 0.34 |
| Questionnaire Self-reported Interviewed | 2 3 | 0.64 (0.50–0.82), p = 0.0004 0.60 (0.40–0.89), p = 0.01 | I2 = 0%, p = 0.33 I2 = 42%, p = 0.18 |
| NOS score 7 8-9 | 1 4 | 0.75 (0.58–0.98), p = 0.03 0.60 (0.48–0.75), p < 0.00001 | I2 = 0%, p = 0.42 |
| Energy Intake Adjustment | |||
| Yes No | 2 3 | 0.44 (0.27–0.74), p = 0.002 0.69 (0.58–0.83), p < 0.0001 | I2 = 0%, p = 0.66 I2 = 0%, p = 0.43 |
| Population NAFLD patients General population | 2 3 | 0.64 (0.50–0.82), p = 0.0004 0.60 (0.40–0.89), p = 0.01 | I2 = 0%, p = 0.33 I2 = 42%, p = 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebadi, M.; Ip, S.; Bhanji, R.A.; Montano-Loza, A.J. Effect of Coffee Consumption on Non-Alcoholic Fatty Liver Disease Incidence, Prevalence and Risk of Significant Liver Fibrosis: Systematic Review with Meta-Analysis of Observational Studies. Nutrients 2021, 13, 3042. https://doi.org/10.3390/nu13093042
Ebadi M, Ip S, Bhanji RA, Montano-Loza AJ. Effect of Coffee Consumption on Non-Alcoholic Fatty Liver Disease Incidence, Prevalence and Risk of Significant Liver Fibrosis: Systematic Review with Meta-Analysis of Observational Studies. Nutrients. 2021; 13(9):3042. https://doi.org/10.3390/nu13093042
Chicago/Turabian StyleEbadi, Maryam, Stephen Ip, Rahima A. Bhanji, and Aldo J. Montano-Loza. 2021. "Effect of Coffee Consumption on Non-Alcoholic Fatty Liver Disease Incidence, Prevalence and Risk of Significant Liver Fibrosis: Systematic Review with Meta-Analysis of Observational Studies" Nutrients 13, no. 9: 3042. https://doi.org/10.3390/nu13093042
APA StyleEbadi, M., Ip, S., Bhanji, R. A., & Montano-Loza, A. J. (2021). Effect of Coffee Consumption on Non-Alcoholic Fatty Liver Disease Incidence, Prevalence and Risk of Significant Liver Fibrosis: Systematic Review with Meta-Analysis of Observational Studies. Nutrients, 13(9), 3042. https://doi.org/10.3390/nu13093042







