Potential Roles of Iridoid Glycosides and Their Underlying Mechanisms against Diverse Cancer Growth and Metastasis: Do They Have an Inhibitory Effect on Cancer Progression?
Abstract
1. Introduction
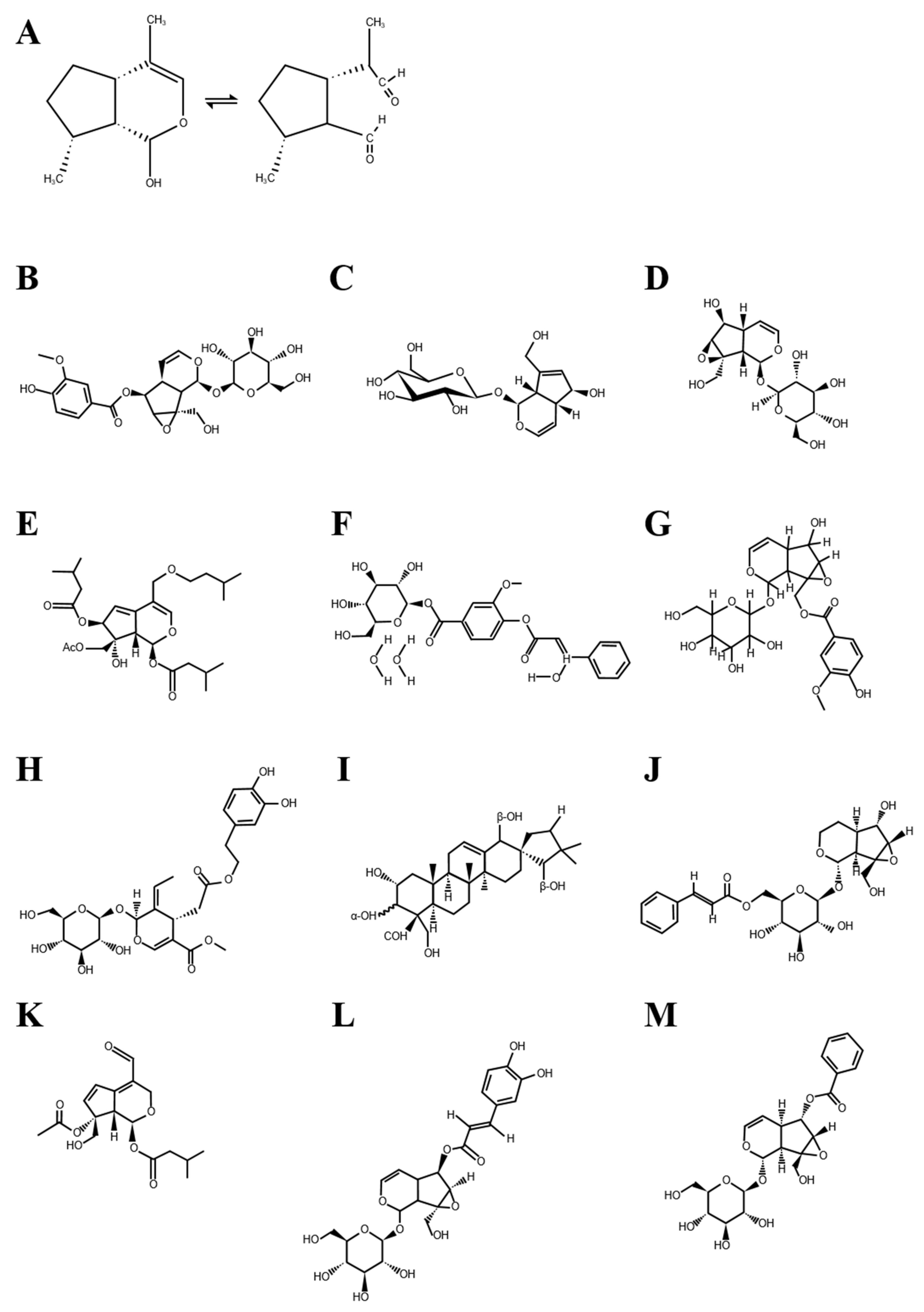
1.1. Chemical Nature of Iridoid Glycosides
1.2. Biological Activities of Iridoid Glycosides
2. Effects of Iridoid Glycosides on Cancer Development and Metastasis
2.1. Anti-Proliferative and Apoptotic Effects
2.2. Inhibitory Effects on Epithelial-Mesenchymal Transition
2.3. Inhibitory Effects on Cancer Migration and Invasion
2.4. Anti-Antiangiogenic Effects
3. Conclusions and Future Perspectives
- How do iridoid glycosides affect the tumor microenvironment?
- Can iridoid glycosides stimulate the immune system to suppress the development of cancer?
- Can iridoid glycosides inhibit lymphangiogenesis with respect to tumor metastasis?
- Can iridoid glycosides restrict growth and secondary metastasis through tumor dormancy?
- Can iridoid glycosides enhance the curative effect, acting as an adjuvant to existing anticancer drugs?
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Kim, C.W.; Hwang, K.A.; Choi, K.C. Anti-metastatic potential of resveratrol and its metabolites by the inhibition of epithelial-mesenchymal transition, migration, and invasion of malignant cancer cells. Phytomedicine 2016, 23, 1787–1796. [Google Scholar] [CrossRef]
- Hwang, K.A.; Kang, N.H.; Yi, B.R.; Lee, H.R.; Park, M.A.; Choi, K.C. Genistein, a soy phytoestrogen, prevents the growth of BG-1 ovarian cancer cells induced by 17beta-estradiol or bisphenol A via the inhibition of cell cycle progression. Int. J. Oncol. 2013, 42, 733–740. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Kivimaenpaa, M.; Julkunen-Tiitto, R. New Light for Phytochemicals. Trends Biotechnol. 2018, 36, 7–10. [Google Scholar] [CrossRef]
- Viljoen, A.; Mncwangi, N.; Vermaak, I. Anti-inflammatory iridoids of botanical origin. Curr. Med. Chem. 2012, 19, 2104–2127. [Google Scholar] [CrossRef] [PubMed]
- Konno, K.; Sabelis, J.M.; Takabayashi, J.; Otsu, S.; Sassa, J.T.; Oikawa, H. Comprehensive Natural Products II. In 4.08 Chemical Defence and Toxins of Plants, 385th ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 4, pp. 339–385. [Google Scholar]
- Kouda, R.; Yakushiji, F. Recent Advances in Iridoid Chemistry: Biosynthesis and Chemical Synthesis. Chem. Asian J. 2020, 15, 3771–3783. [Google Scholar] [CrossRef]
- Geu-Flores, F.; Sherden, N.H.; Courdavault, V.; Burlat, V.; Glenn, W.S.; Wu, C.; Nims, E.; Cui, Y.; O’Connor, S.E. An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis. Nature 2012, 492, 138–142. [Google Scholar] [CrossRef]
- Castejon, M.L.; Montoya, T.; Alarcon-de-la-Lastra, C.; Sanchez-Hidalgo, M. Potential Protective Role Exerted by Secoiridoids from Olea europaea L. in Cancer, Cardiovascular, Neurodegenerative, Aging-Related, and Immunoinflammatory Diseases. Antioxidants 2020, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research Advances in Their Phytochemistry, Biological Activities, and Pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Konno, K.; Sabelis, M.; Takabayashi, J.; Sassa, T.; Oikawa, H. Chemical Defence and Toxins of Plants; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Takayama, H.; Jia, Z.J.; Kremer, L.; Bauer, J.O.; Strohmann, C.; Ziegler, S.; Antonchick, A.P.; Waldmann, H. Discovery of inhibitors of the Wnt and Hedgehog signaling pathways through the catalytic enantioselective synthesis of an iridoid-inspired compound collection. Angew. Chem. Int. Ed. 2013, 52, 12404–12408. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef]
- Bhattamisra, S.K.; Yap, K.H.; Rao, V.; Choudhury, H. Multiple Biological Effects of an Iridoid Glucoside, Catalpol and Its Underlying Molecular Mechanisms. Biomolecules 2019, 10, 32. [Google Scholar] [CrossRef]
- Hussain, H.; Green, I.R.; Saleem, M.; Raza, M.L.; Nazir, M. Therapeutic potential of iridoid derivatives: Patent review. Inventions 2019, 4, 29. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, X.; Xia, Q.; Yin, T.; Bai, C.; Wang, Z.; Du, L.; Li, X.; Wang, W.; Sun, L.; et al. Comparative anti-arthritic investigation of iridoid glycosides and crocetin derivatives from Gardenia jasminoides Ellis in Freund’s complete adjuvant-induced arthritis in rats. Phytomedicine 2019, 53, 223–233. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.H.; He, Y.Q.; Zhang, Q.L.; Zhu, B.; Shen, Y.; Liu, M.Q.; Zhu, L.L.; Xin, H.L.; Qin, L.P.; et al. Iridoid glycosides from Morinda officinalis How. exert anti-inflammatory and anti-arthritic effects through inactivating MAPK and NF-kappaB signaling pathways. BMC Complement. Med. Ther. 2020, 20, 172. [Google Scholar] [CrossRef]
- Bachar, S.C.; Bachar, R.; Jannat, K.; Jahan, R.; Rahmatullah, M. Hepatoprotective natural products. Annu. Rep. Med. Chem. 2020, 55, 207–249. [Google Scholar]
- Eslami, S.Z.; Cortes-Hernandez, L.E.; Alix-Panabieres, C. The Metastatic Cascade as the Basis for Liquid Biopsy Development. Front. Oncol. 2020, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M.; Hausman, R.E. The development and causes of cancer. Cell Mol. Approach 2000, 2, 719–728. [Google Scholar]
- Martin, T.A.; Ye, L.; Sanders, A.J.; Lane, J.; Jiang, W.G. Cancer invasion and metastasis: Molecular and cellular perspective. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Sever, R.; Brugge, J.S. Signal transduction in cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a006098. [Google Scholar] [CrossRef] [PubMed]
- Loeb, L.A.; Loeb, K.R.; Anderson, J.P. Multiple mutations and cancer. Proc. Natl. Acad. Sci. USA 2003, 100, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, H.; Ushijima, T. Accumulation of genetic and epigenetic alterations in normal cells and cancer risk. NPJ Precis. Oncol. 2019, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Fei, H.; Wang, L.; Xu, H.; Zhang, H.; Zheng, L. PDCD5 regulates cell proliferation, cell cycle progression and apoptosis. Oncol. Lett. 2018, 15, 1177–1183. [Google Scholar] [CrossRef]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef]
- Matsui, W.H. Cancer stem cell signaling pathways. Medicine 2016, 95, S8–S19. [Google Scholar] [CrossRef]
- Liu, C.; Wu, F.; Liu, Y.; Meng, C. Catalpol suppresses proliferation and facilitates apoptosis of MCF-7 breast cancer cells through upregulating microRNA-146a and downregulating matrix metalloproteinase-16 expression. Mol. Med. Rep. 2015, 12, 7609–7614. [Google Scholar] [CrossRef][Green Version]
- Gao, N.; Tian, J.X.; Shang, Y.H.; Zhao, D.Y.; Wu, T. Catalpol suppresses proliferation and facilitates apoptosis of OVCAR-3 ovarian cancer cells through upregulating microRNA-200 and downregulating MMP-2 expression. Int. J. Mol. Sci. 2014, 15, 19394. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Cao, M.; Mu, X.; Yang, G.; Xue, W.; Huang, Y.; Chen, H. Catalpol Inhibited the Proliferation of T24 Human Bladder Cancer Cells by Inducing Apoptosis Through the Blockade of Akt-Mediated Anti-apoptotic Signaling. Cell Biochem. Biophys. 2015, 71, 1349–1356. [Google Scholar] [CrossRef]
- Qiao, P.F.; Yao, L.; Zeng, Z.L. Catalpolmediated microRNA34a suppresses autophagy and malignancy by regulating SIRT1 in colorectal cancer. Oncol. Rep. 2020, 43, 1053–1066. [Google Scholar] [CrossRef]
- Liu, L.; Gao, H.; Wang, H.; Zhang, Y.; Xu, W.; Lin, S.; Wang, H.; Wu, Q.; Guo, J. Catalpol promotes cellular apoptosis in human HCT116 colorectal cancer cells via microRNA-200 and the downregulation of PI3K-Akt signaling pathway. Oncol. Lett. 2017, 14, 3741–3747. [Google Scholar] [CrossRef]
- Xynos, N.; Abatis, D.; Argyropoulou, A.; Polychronopoulos, P.; Aligiannis, N.; Skaltsounis, A.L. Development of a Sustainable Procedure for the Recovery of Hydroxytyrosol from Table Olive Processing Wastewater Using Adsorption Resin Technology and Centrifugal Partition Chromatography. Planta Med. 2015, 81, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; De Bartolomeo, A.; Rosignoli, P.; Servili, M.; Selvaggini, R.; Montedoro, G.F.; Di Saverio, C.; Morozzi, G. Virgin olive oil phenols inhibit proliferation of human promyelocytic leukemia cells (HL60) by inducing apoptosis and differentiation. J. Nutr. 2006, 136, 614–619. [Google Scholar] [CrossRef]
- Mao, W.; Shi, H.; Chen, X.; Yin, Y.; Yang, T.; Ge, M.; Luo, M.; Chen, D.; Qian, X. Anti-proliferation and migration effects of oleuropein on human A549 lung carcinoma cells. Lat. Am. J. Pharm 2012, 31, 1217–1221. [Google Scholar]
- Yao, J.; Wu, J.; Yang, X.; Yang, J.; Zhang, Y.; Du, L. Oleuropein induced apoptosis in HeLa cells via a mitochondrial apoptotic cascade associated with activation of the c-Jun NH2-terminal kinase. J. Pharm. Sci. 2014, 125, 300–311. [Google Scholar] [CrossRef]
- Sarsour, E.H.; Goswami, M.; Kalen, A.L.; Lafin, J.T.; Goswami, P.C. Hydroxytyrosol inhibits chemokine C-C motif ligand 5 mediated aged quiescent fibroblast-induced stimulation of breast cancer cell proliferation. Age 2014, 36, 1213–1224. [Google Scholar] [CrossRef]
- Zhao, B.; Ma, Y.; Xu, Z.; Wang, J.; Wang, F.; Wang, D.; Pan, S.; Wu, Y.; Pan, H.; Xu, D.; et al. Hydroxytyrosol, a natural molecule from olive oil, suppresses the growth of human hepatocellular carcinoma cells via inactivating AKT and nuclear factor-kappa B pathways. Cancer Lett. 2014, 347, 79–87. [Google Scholar] [CrossRef]
- Terzuoli, E.; Donnini, S.; Giachetti, A.; Iniguez, M.A.; Fresno, M.; Melillo, G.; Ziche, M. Inhibition of hypoxia inducible factor-1alpha by dihydroxyphenylethanol, a product from olive oil, blocks microsomal prostaglandin-E synthase-1/vascular endothelial growth factor expression and reduces tumor angiogenesis. Clin. Cancer Res. 2010, 16, 4207–4216. [Google Scholar] [CrossRef]
- Nieminen, M.; Suomi, J.; Van Nouhuys, S.; Sauri, P.; Riekkola, M.L. Effect of iridoid glycoside content on oviposition host plant choice and parasitism in a specialist herbivore. J. Chem. Ecol. 2003, 29, 823–844. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C.C. Antileukemic activity of selected natural products in Taiwan. Am. J. Chin. Med. 2003, 31, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.B.; Kim, C.; Chung, W.S.; Cho, J.H.; Nam, D.; Kim, S.H.; Ahn, K.S. The hydrolysed products of iridoid glycosides can enhance imatinib mesylate-induced apoptosis in human myeloid leukaemia cells. Phytother. Res. 2015, 29, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Loscocco, F.; Visani, G.; Galimberti, S.; Curti, A.; Isidori, A. BCR-ABL Independent Mechanisms of Resistance in Chronic Myeloid Leukemia. Front. Oncol. 2019, 9, 939. [Google Scholar] [CrossRef]
- Hung, J.Y.; Yang, C.J.; Tsai, Y.M.; Huang, H.W.; Huang, M.S. Antiproliferative activity of aucubin is through cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Clin. Exp. Pharmacol. Physiol. 2008, 35, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Saracoglu, I.; Harput, U.S. In vitro cytotoxic activity and structure activity relationships of iridoid glucosides derived from Veronica species. Phytother. Res. 2012, 26, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Le, D.D.; Nguyen, D.H.; Zhao, B.T.; Kim, J.A.; Kim, S.K.; Min, B.S.; Choi, J.S.; Woo, M.H. 28-Noroleanane-derived spirocyclic triterpenoids and iridoid glucosides from the roots of Phlomoides umbrosa (Turcz.) Kamelin & Makhm with their cytotoxic effects. Phytochemistry 2018, 153, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhu, R.; Tian, S.; Wang, Y.; Lou, S.; Zhao, H. Jatamanvaltrate P induces cell cycle arrest, apoptosis and autophagy in human breast cancer cells in vitro and in vivo. Biomed. Pharmacother. 2017, 89, 1027–1036. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Heerboth, S.; Housman, G.; Leary, M.; Longacre, M.; Byler, S.; Lapinska, K.; Willbanks, A.; Sarkar, S. EMT and tumor metastasis. Clin. Transl. Med. 2015, 4, 6. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Li, W.; Shan, T.; Lin, W.R.; Ma, J.; Cui, X.; Yang, W.; Cao, G.; Li, Y.; et al. Conversion of epithelial-to-mesenchymal transition to mesenchymal-to-epithelial transition is mediated by oxygen concentration in pancreatic cancer cells. Oncol. Lett. 2018, 15, 7144–7152. [Google Scholar] [CrossRef]
- Roche, J. The Epithelial-to-Mesenchymal Transition in Cancer. Cancers 2018, 10, 52. [Google Scholar] [CrossRef]
- Choupani, J.; Alivand, M.R.; Derakhshan, S.M.; Zaeifizadeh, M.; Khaniani, M.S. Oleuropein inhibits migration ability through suppression of epithelial-mesenchymal transition and synergistically enhances doxorubicin-mediated apoptosis in MCF-7 cells. J. Cell Physiol. 2019, 234, 9093–9104. [Google Scholar] [CrossRef]
- Serrano-Gomez, S.J.; Maziveyi, M.; Alahari, S.K. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol. Cancer 2016, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Go, R.E.; Lee, H.M.; Hwang, K.A.; Lee, K.; Kim, B.; Lee, M.Y.; Choi, K.C. Cigarette smoke extracts induced the colon cancer migration via regulating epithelial mesenchymal transition and metastatic genes in human colon cancer cells. Environ. Toxicol. 2017, 32, 690–704. [Google Scholar] [CrossRef]
- Yao, D.; Dai, C.; Peng, S. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol. Cancer Res. 2011, 9, 1608–1620. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, Y.; Sheng, B.; Ding, Y.; Cheng, X. Catalpol inhibits TGF-beta1-induced epithelial-mesenchymal transition in human non-small-cell lung cancer cells through the inactivation of Smad2/3 and NF-kappaB signaling pathways. J. Cell Biochem. 2018, 120, 2251–2258. [Google Scholar] [CrossRef]
- Wang, L.; Xue, G.B. Catalpol suppresses osteosarcoma cell proliferation through blocking epithelial-mesenchymal transition (EMT) and inducing apoptosis. Biochem. Biophys. Res. Commun. 2018, 495, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Seoane, J.; Gomis, R.R. TGF-beta Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb. Perspect. Biol. 2017, 9, a022277. [Google Scholar] [CrossRef]
- Lee, H.K.; Shin, H.J.; Koo, J.; Kim, T.H.; Kim, C.W.; Go, R.E.; Seong, Y.H.; Park, J.E.; Choi, K.C. Blockade of transforming growth factor beta2 by anti-sense oligonucleotide improves immunotherapeutic potential of IL-2 against melanoma in a humanized mouse model. Cytotherapy 2021, 23, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, H.; Chen, S.; Wu, X.; Chen, X.; Wang, F. Catalpol inhibits the proliferation, migration and metastasis of HCC cells by regulating miR1405p expression. Mol. Med. Rep. 2021, 23, 29. [Google Scholar] [CrossRef]
- Yan, X.; Zhu, Z.; Xu, S.; Yang, L.N.; Liao, X.H.; Zheng, M.; Yang, D.; Wang, J.; Chen, D.; Wang, L.; et al. MicroRNA-140-5p inhibits hepatocellular carcinoma by directly targeting the unique isomerase Pin1 to block multiple cancer-driving pathways. Sci. Rep. 2017, 7, 45915. [Google Scholar] [CrossRef]
- Yang, P.; Xiong, J.; Zuo, L.; Liu, K.; Zhang, H. miR1405p regulates cell migration and invasion of nonsmall cell lung cancer cells through targeting VEGFA. Mol. Med. Rep. 2018, 18, 2866–2872. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef]
- Yilmaz, M.; Christofori, G. Mechanisms of motility in metastasizing cells. Mol. Cancer Res. 2010, 8, 629–642. [Google Scholar] [CrossRef]
- Krause, M.; Gautreau, A. Steering cell migration: Lamellipodium dynamics and the regulation of directional persistence. Nat. Rev. Mol. Cell Biol. 2014, 15, 577–590. [Google Scholar] [CrossRef]
- Wolgemuth, C.W. Lamellipodial contractions during crawling and spreading. Biophys. J. 2005, 89, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Blouw, B.; Patel, M.; Iizuka, S.; Abdullah, C.; You, W.K.; Huang, X.; Li, J.L.; Diaz, B.; Stallcup, W.B.; Courtneidge, S.A. The invadopodia scaffold protein Tks5 is required for the growth of human breast cancer cells in vitro and in vivo. PLoS ONE 2015, 10, e0121003. [Google Scholar] [CrossRef]
- Augoff, K.; Hryniewicz-Jankowska, A.; Tabola, R. Invadopodia: Clearing the way for cancer cell invasion. Ann. Transl. Med. 2020, 8, 902. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Liu, Q. Catalpol inhibits cell proliferation, invasion and migration through regulating miR-22-3p/MTA3 signalling in hepatocellular carcinoma. Exp. Mol. Pathol. 2019, 109, 51–60. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhan-Sheng, H. Catalpol inhibits migration and induces apoptosis in gastric cancer cells and in athymic nude mice. Biomed. Pharmacother. 2018, 103, 1708–1719. [Google Scholar] [CrossRef]
- Zhu, P.; Wu, Y.; Yang, A.; Fu, X.; Mao, M.; Liu, Z. Catalpol suppressed proliferation, growth and invasion of CT26 colon cancer by inhibiting inflammation and tumor angiogenesis. Biomed. Pharmacother. 2017, 95, 68–76. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M. Olive leaf extract and its main component oleuropein prevent chronic ultraviolet B radiation-induced skin damage and carcinogenesis in hairless mice. J. Nutr. 2009, 139, 2079–2086. [Google Scholar] [CrossRef]
- Hamdi, H.K.; Castellon, R. Oleuropein, a non-toxic olive iridoid, is an anti-tumor agent and cytoskeleton disruptor. Biochem. Biophys. Res. Commun. 2005, 334, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Rathee, D.; Thanki, M.; Bhuva, S.; Anandjiwala, S.; Agrawal, R. Iridoid glycosides-Kutkin, Picroside I, and Kutkoside from Picrorrhiza kurroa Benth inhibits the invasion and migration of MCF-7 breast cancer cells through the down regulation of matrix metalloproteinases: 1st Cancer Update. Arab. J. Chem. 2013, 6, 49–58. [Google Scholar] [CrossRef]
- Sun, Y.; Lan, M.; Chen, X.; Dai, Y.; Zhao, X.; Wang, L.; Zhao, T.; Li, Y.; Zhu, J.; Zhang, X.; et al. Anti-invasion and anti-metastasis effects of Valjatrate E via reduction of matrix metalloproteinases expression and suppression of MAPK/ERK signaling pathway. Biomed. Pharmacother. 2018, 104, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, Y.; Ikeda, T.; Saito-Takatsuji, H.; Yonekura, H. Emerging Role of AP-1 Transcription Factor JunB in Angiogenesis and Vascular Development. Int. J. Mol. Sci. 2021, 22, 2804. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Ibrahim, S.M. Molecular responses to hypoxia in tumor cells. Mol. Cancer 2003, 2, 23. [Google Scholar] [CrossRef]
- Siemann, D.W.; Horsman, M.R. Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol. Ther. 2015, 153, 107–124. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.O.; Park, S.J.; Yun, C.H.; Chung, A.S. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J. Biochem. Mol. Biol. 2003, 36, 128–137. [Google Scholar] [CrossRef]
- Quintero-Fabian, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argaez, V.; Lara-Riegos, J.; Ramirez-Camacho, M.A.; Alvarez-Sanchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Skobe, M.; Hawighorst, T.; Jackson, D.G.; Prevo, R.; Janes, L.; Velasco, P.; Riccardi, L.; Alitalo, K.; Claffey, K.; Detmar, M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 2001, 7, 192–198. [Google Scholar] [CrossRef]
- Hirakawa, S.; Brown, L.F.; Kodama, S.; Paavonen, K.; Alitalo, K.; Detmar, M. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 2007, 109, 1010–1017. [Google Scholar] [CrossRef]
- Moserle, L.; Casanovas, O. Anti-angiogenesis and metastasis: A tumour and stromal cell alliance. J. Intern. Med. 2013, 273, 128–137. [Google Scholar] [CrossRef]
- Han, Y.; Shen, M.; Tang, L.Y.; Tan, G.; Yang, Q.C.; Ye, L.; Ye, L.H.; Jiang, N.; Gao, G.P.; Shao, Y. Antiangiogenic effects of catalpol on rat corneal neovascularization. Mol. Med. Rep. 2018, 17, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Zhu, Z.; Xu, X.; Zhu, R.; Sheng, Y.; Zhao, H. Picroside II, an iridoid glycoside from Picrorhiza kurroa, suppresses tumor migration, invasion, and angiogenesis in vitro and in vivo. Biomed. Pharmacother. 2019, 120, 109494. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, A.M.; Camero, C.M.; D’Ambola, M.; D’Angelo, V.; Amira, S.; Bader, A.; Braca, A.; De Tommasi, N.; Germano, M.P. Antiangiogenic Iridoids from Stachys ocymastrum and Premna resinosa. Planta Med. 2019, 85, 1034–1039. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Shen, H.; Tang, F.R.; Arfuso, F.; Rajesh, M.; Wang, L.; Kumar, A.P.; Bian, J.; Goh, B.C.; Bishayee, A.; et al. Potential role of genipin in cancer therapy. Pharmacol. Res. 2018, 133, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Konoshima, T.; Takasaki, M.; Tokuda, H.; Nishino, H. Cancer chemopreventive activity of an iridoid glycoside, 8-acetylharpagide, from Ajuga decumbens. Cancer Lett. 2000, 157, 87–92. [Google Scholar] [CrossRef]
- Lin, C.M.; Jiang, Y.Q.; Chaudhary, A.G.; Rimoldi, J.M.; Kingston, D.G.; Hamel, E. A convenient tubulin-based quantitative assay for paclitaxel (Taxol) derivatives more effective in inducing assembly than the parent compound. Cancer Chemother. Pharmacol. 1996, 38, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Bocca, C.; Bozzo, F.; Bassignana, A.; Miglietta, A. Antiproliferative effect of a novel nitro-oxy derivative of celecoxib in human colon cancer cells: Role of COX-2 and nitric oxide. Anticancer Res. 2010, 30, 2659–2666. [Google Scholar]
- Pereyra, C.E.; Dantas, R.F.; Ferreira, S.B.; Gomes, L.P.; Silva, F.P., Jr. The diverse mechanisms and anticancer potential of naphthoquinones. Cancer Cell Int. 2019, 19, 207. [Google Scholar] [CrossRef] [PubMed]
- Fei, B.; Dai, W.; Zhao, S. Efficacy, Safety, and Cost of Therapy of the Traditional Chinese Medicine, Catalpol, in Patients Following Surgical Resection for Locally Advanced Colon Cancer. Med. Sci. Monit. 2018, 24, 3184–3192. [Google Scholar] [CrossRef]
| Stages | Iridoid Glycosides | Effective Dosages | Key Effects and Inhibitory Mechanisms | Types of Cancer | In Vitro /In Vivo | Ref. |
|---|---|---|---|---|---|---|
| Proliferation | Catalpol | 50 and 100 μg/mL |
| Ovarian cancer | In vitro | [30] |
| 50 and 100 μg/mL |
| Breast cancer | In vitro | [29] | ||
| 80 and 160 μM |
| Bladder cancer | In vitro | [31] | ||
| 50 and 100 μg/mL |
| Colorectal cancer | In vitro | [33] | ||
| 30, 40 and 50 μM |
| Colorectal cancer | In vitro | [32] | ||
| Oleuropein | 12.5 and 25 μM |
| Blood cancer | In vitro | [35] | |
| IC50 * = 59.96 μM |
| Lung cancer | In vitro | [36] | ||
| 150 and 200 μM |
| Cervical cancer | In vitro | [37] | ||
| Oleuropein (hydroxytyrosol **) | 100 and 200 μM |
| Breast cancer | In vitro | [38] | |
| 100, 200, 300 and 400 μM 10 and 20 mg/kg bw (i.p.) |
| Hepatocellular carcinoma | Both | [39] | ||
| 10 mg/kg bw (i.p.) |
| Colorectal cancer | In vivo | [40] | ||
| Aucubin | IC50 = 44.7 μM |
| Chronic myelogenous leukemia | In vitro | [42] | |
| 100, 150 and 200 μM |
| Chronic myelogenous leukemia | In vitro | [43] | ||
| 1, 5, 10 and 20 μM |
| Non-small cell lung cancer | In vitro | [45] | ||
| Amphicoside | IC50 = 340 μM (Epidermoid carcinoma) |
| Epidermoid carcinoma Rhabdomyosarcoma | In vitro | [46] | |
| Verminoside | IC50 = 128 μM (Epidermoid carcinoma) IC50 = 70 μM (Rhabdomyosarcoma) | |||||
| Veronicoside | IC50 = 153.3 μM (Epidermoid carcinoma) IC50 = 355 μM (Rhabdomyosarcoma) | |||||
| Phlomisu E | IC50 = 19.3 μM (Cervical cancer) IC50 = 8.4 μM (Leukemia) IC50 = 15.4 μM (Breast cancer) |
| Cervical cancer Leukemia Breast cancer | In vitro | [47] | |
| Jatamanvaltrate P | 10, 20, 50 μM 15 mg/kg bw (i.p.) |
| Breast cancer | Both | [48] | |
| EMT | Catalpol | 5 and 10 μM |
| Lung cancer | In vitro | [57] |
| 20, 40 and 80 μM |
| Osteosarcoma | In vitro | [58] | ||
| 50 μM |
| Hepatocellular carcinoma | In vitro | [62] | ||
| Oleuropein | 600 μg/mL |
| Breast cancer | In vitro | [53] | |
| Migration/ Invasion | Catalpol | 50 μM |
| Hepatocellular carcinoma | In vitro | [74] |
| 50 μM |
| Hepatocellular carcinoma | In vitro | [62] | ||
| 20, 40 and 80 μM |
| Gastric cancer | In vitro | [75] | ||
| 1.25, 2.5 and 5 μM |
| Colon cancer | In vitro | [76] | ||
| Oleuropein | 0.01 and 0.1% |
| Breast cancer | In vitro | [78] | |
| 25 mg/kg bw (p.o.) |
| Skin cancer | In vivo | [77] | ||
| Picroside I | 5 μM |
| Breast cancer | In vitro | [79] | |
| Kutkoside | 5 μM | |||||
| Kutkin | 5 μM | |||||
| Valjatrate E | 3, 6 and 12 μg/mL |
| Hepatocellular carcinoma | In vitro | [80] | |
| Angiogenesis | Catalpol | 1.25, 2.5 and 5 μM 7, 14, 28 mg/kg bw (p.o.) |
| Colon cancer | Both | [76] |
| Oleuropein | 25 mg/kg bw (p.o.) |
| Skin cancer | In vivo | [77] | |
| Oleuropein (hydroxytyrosol *) | 50 and 100 μM 10 mg/kg bw |
| Colon cancer | Both | [40] | |
| 10 and 20 mg/kg bw (i.p.) |
| Hepatocellular carcinoma | In vivo | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.-W.; Choi, K.-C. Potential Roles of Iridoid Glycosides and Their Underlying Mechanisms against Diverse Cancer Growth and Metastasis: Do They Have an Inhibitory Effect on Cancer Progression? Nutrients 2021, 13, 2974. https://doi.org/10.3390/nu13092974
Kim C-W, Choi K-C. Potential Roles of Iridoid Glycosides and Their Underlying Mechanisms against Diverse Cancer Growth and Metastasis: Do They Have an Inhibitory Effect on Cancer Progression? Nutrients. 2021; 13(9):2974. https://doi.org/10.3390/nu13092974
Chicago/Turabian StyleKim, Cho-Won, and Kyung-Chul Choi. 2021. "Potential Roles of Iridoid Glycosides and Their Underlying Mechanisms against Diverse Cancer Growth and Metastasis: Do They Have an Inhibitory Effect on Cancer Progression?" Nutrients 13, no. 9: 2974. https://doi.org/10.3390/nu13092974
APA StyleKim, C.-W., & Choi, K.-C. (2021). Potential Roles of Iridoid Glycosides and Their Underlying Mechanisms against Diverse Cancer Growth and Metastasis: Do They Have an Inhibitory Effect on Cancer Progression? Nutrients, 13(9), 2974. https://doi.org/10.3390/nu13092974







