Iron Deficiency in Vegetarian and Omnivorous Individuals: Analysis of 1340 Individuals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Issues
2.2. Sample
2.3. Sample Classification According to Eating Habits
2.4. Sample Classification Based on Nutritional Status
2.5. Analysis of Markers of Iron Metabolism and Metabolic Inflammation
2.6. Statistical Analysis
3. Results
3.1. Descriptive Characteristics of the Overall Sample (without Excluding Individuals with Inflammation or Insulin Resistance, Factors That Alter Ferritin Levels)
3.2. Vegetarian Habits Were Associated with a Lower Prevalence of Obesity and Lower Serum Ferritin Levels, Regardless of Inflammation
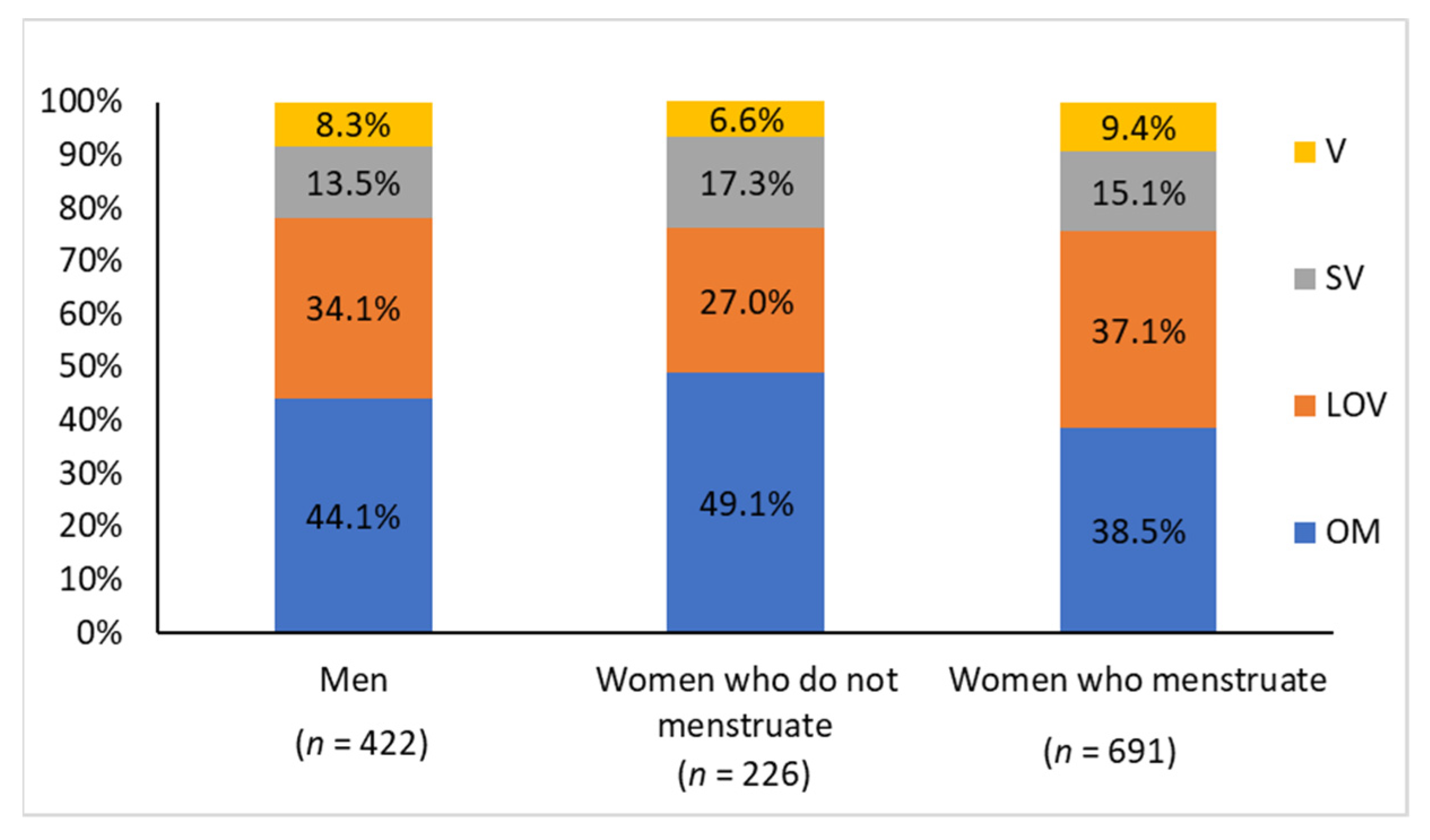
3.3. In the Overall Sample (without Excluding Individuals with Inflammation or Insulin Resistance), the Frequency of Iron Deficiency Was Higher among Vegetarians Than Omnivores, Regardless of Sex and Menstrual Blood Loss
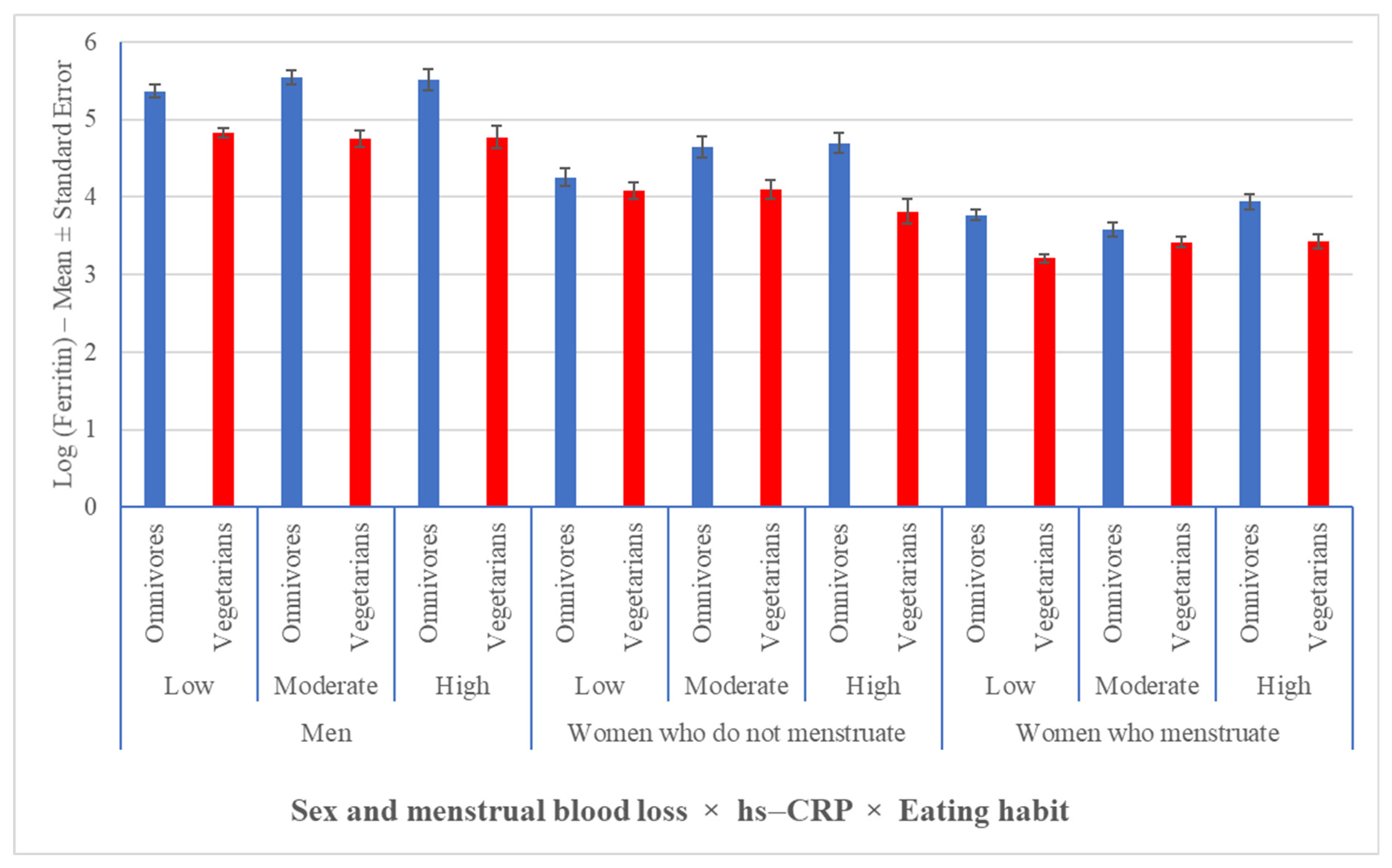
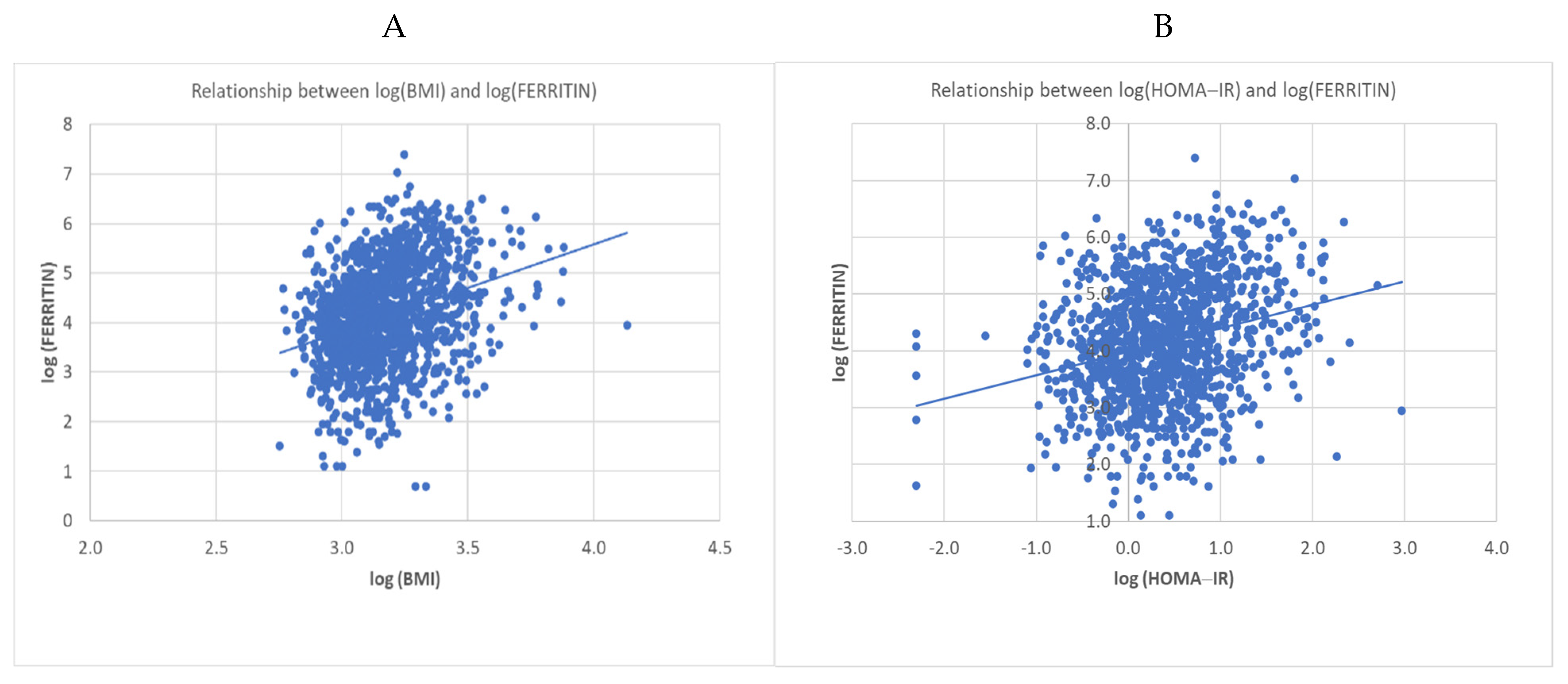
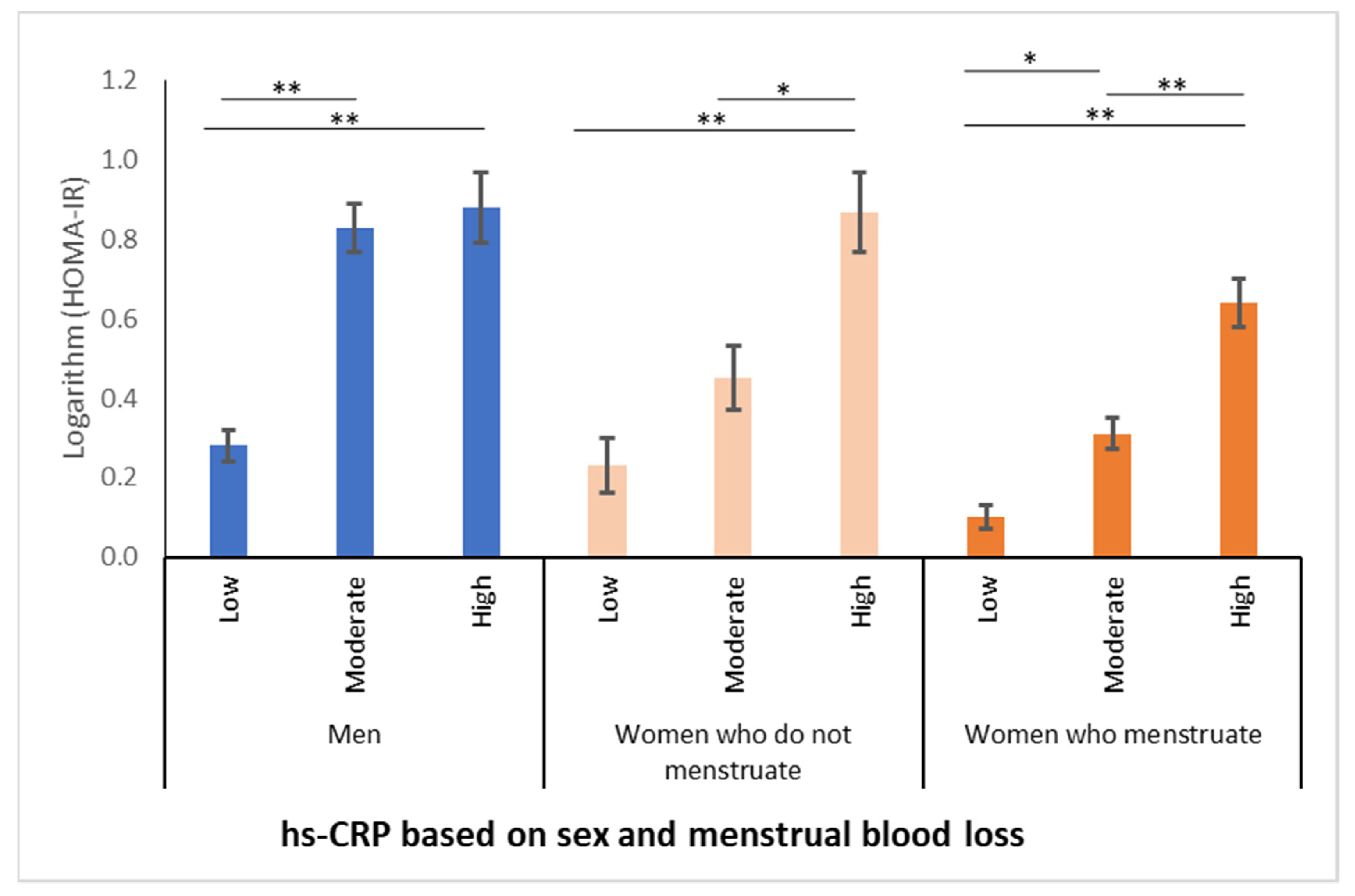
3.4. Serum Ferritin Concentrations Had a Positive Relationship with Increasing BMI and HOMA-IR Values Associated with Inflammation, Regardless of Eating Habit and Sex
3.5. Considering Only Individuals without Inflammation or Insulin Resistance, Enabling a True Diagnosis, Iron Deficiency Was the Same in Men and Women Who Do Not Menstruate, Regardless of Eating Habit, and More Prevalent Only in Vegetarian Women Who Menstruate
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial. Int. 2017, 21 (Suppl. 1), S6–S20. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Iron status of vegetarians. Am. J. Clin. Nutr. 1994, 59, 1233S–1237S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, L.J.; Armah, C.N.; Dainty, J.R.; Foxall, R.J.; Lewis, D.J.; Langford, N.J.; Fairweather-Tait, S.J. Impact of menstrual blood loss and diet on iron deficiency among women in the UK. Br. J. Nutr. 2005, 94, 557–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Jaster, B.; Seidl, K.; Green, A.A.; Talpers, S. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care 2006, 29, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Green, A.; Ferdowsian, H. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: A randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr. 2009, 89, 1588S–1596S. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Matoulek, M.; Malinska, H.; Oliyarnik, O.; Kazdova, L.; Neskudla, T.; Skoch, A.; Hajek, M.; Hill, M.; Kahle, M.; et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet. Med. 2011, 28, 549–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatti, A.S.; Mahida, V.I.; Gupte, S.C. Iron status of Hindu brahmin, Jain and Muslim communities in Surat, Gujarat. Indian J. Hematol. Blood Transfus. 2007, 23, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, N.S.; Chin, C.J.; Pan, W.H. A vegetarian diet rich in soybean products compromises iron status in young students. J. Nutr. 1995, 125, 212–219. [Google Scholar]
- Craig, W.J. Health effects of vegan diets. Am. J. Clin. Nutr. 2009, 89, 1627S–1633S. [Google Scholar] [CrossRef] [Green Version]
- Alida, M. Alida Maria Koorts and Margaretha Viljoen (October 5th 2011). In Acute Phase Proteins: Ferritin and Ferritin Isoforms; Acute Phase Proteins—Regulation and Functions of Acute Phase Proteins, Francisco Veas; IntechOpen: London, UK, 2011; Available online: https://www.intechopen.com/books/acute-phase-proteins-regulation-and-functions-of-acute-phase-proteins/acute-phase-proteins-ferritin-and-ferritin-isoforms (accessed on 22 June 2021). [CrossRef] [Green Version]
- Goodnough, L.T.; Nemeth, E.; Ganz, T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood 2010, 116, 4754–4761. [Google Scholar] [CrossRef]
- Camaschella, C. Iron deficiency: New insights into diagnosis and treatment. Hematol. Am. Soc. Hematol. Educ. Program 2015, 2015, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, I.M.; Bailey, K.; Flowers, C.H.; Garrigues, N.W.; Wesselius, L.J. Effects of TNF-alpha and IL-1beta on iron metabolism by A549 cells and influence on cytotoxicity. Am. J. Physiol. 1999, 277, L257–L263. [Google Scholar] [PubMed]
- Calder, P.C.; Ahluwalia, N.; Albers, R.; Bosco, N.; Bourdet-Sicard, R.; Haller, D.; Holgate, S.T.; Jönsson, L.S.; Latulippe, M.E.; Marcos, A.; et al. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br. J. Nutr. 2013, 109 (Suppl. 1), S1–S34. [Google Scholar] [CrossRef] [Green Version]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Real, J.M.; Lopez-Bermejo, A.; Ricart, W. Cross-talk between iron metabolism and diabetes. Diabetes 2002, 51, 2348–2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, E.A.; Appleby, P.N.; Davey, G.K.; Key, T.J. Diet and body mass index in 38000 EPIC-Oxford meat-eaters, fish-eaters, vegetarians and vegans. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 728–734. [Google Scholar] [CrossRef] [Green Version]
- WHO (World Health Organization). Obesity Preventing and Managing the Global Epidemic—Report of a WHO Consultation on Obesity; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Haider, L.M.; Schwingshackl, L.; Hoffmann, G.; Ekmekcioglu, C. The effect of vegetarian diets on iron status in adults: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2018, 58, 1359–1374. [Google Scholar] [CrossRef]
- Haddad, E.H.; Berk, L.S.; Kettering, J.D.; Hubbard, R.W.; Peters, W.R. Dietary intake and biochemical, hematologic, and immune status of vegans compared with nonvegetarians. Am. J. Clin. Nutr. 1999, 70, 586S–593S. [Google Scholar] [CrossRef] [Green Version]
- Craddock, J.C.; Neale, E.P.; Peoples, G.E.; Probst, Y.C. Vegetarian-Based Dietary Patterns and their Relation with Inflammatory and Immune Biomarkers: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Menzel, J.; Jabakhanji, A.; Biemann, R.; Mai, K.; Abraham, K.; Weikert, C. Systematic review and meta-analysis of the associations of vegan and vegetarian diets with inflammatory biomarkers. Sci. Rep. 2020, 10, 21736. [Google Scholar] [CrossRef] [PubMed]
- Manousou, P.; Kalambokis, G.; Grillo, F.; Watkins, J.; Xirouchakis, E.; Pleguezuelo, M.; Leandro, G.; Arvaniti, V.; Germani, G.; Patch, D.; et al. Serum ferritin is a discriminant marker for both fibrosis and inflammation in histologically proven non-alcoholic fatty liver disease patients. Liver Int. 2011, 31, 730–739. [Google Scholar] [CrossRef]
- Yi, K.H.; Hwang, J.S.; Lim, S.W.; Lee, J.A.; Kim, D.H.; Lim, J.S. Ferritin level is associated with metabolic syndrome and elevated alanine aminotransferase in children and adolescents. J. Pediatr. Endocrinol. Metab. 2016, 29, 1337–1344. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Ballantyne, C.M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, W.M.; Ayub, M.; Humayun, M.; Haroon, M. Ferritin Is a Marker of Inflammation rather than Iron Deficiency in Overweight and Obese People. J. Obes. 2016, 2016, 1937320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Sinclair, A.J.; Mann, N.J.; Turner, A.; Ball, M.J. Selected micronutrient intake and status in men with differing meat intakes, vegetarians and vegans. Asia Pac. J. Clin. Nutr. 2000, 9, 18–23. [Google Scholar] [CrossRef]
- Hua, N.W.; Stoohs, R.A.; Facchini, F.S. Low iron status and enhanced insulin sensitivity in lacto-ovo vegetarians. Br. J. Nutr. 2001, 86, 515–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oellgaard, J.; Abitz Winther, S.; Schmidt Hansen, T.; Rossing, P.; Johan von Scholten, B. Trimethylamine N-oxide (TMAO) as a New Potential Therapeutic Target for Insulin Resistance and Cancer. Curr. Pharm. Des. 2017, 23, 3699–3712. [Google Scholar] [CrossRef]
- Estadella, D.; da Penha Oller do Nascimento, C.; Oyama, L.M.; Ribeiro, E.B.; Damaso, A.R.; de Piano, A. Lipotoxicity: Effects of dietary saturated and transfatty acids. Mediat. Inflamm. 2013, 2013, 137579. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.K.; Ball, M.J. Nutrient intake and iron status of Australian male vegetarians. Eur. J. Clin. Nutr. 1999, 53, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.N.; Xu, C.; Xu, L.; Yu, C.; Miao, M.; Xie, J.; Li, Y. High serum ferritin levels increase the risk of hyperuricemia: A cross-sectional and longitudinal study. Ann. Nutr. Metab. 2014, 64, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.B.; Moen, I.W.; Mandrup-Poulsen, T. Iron: The hard player in diabetes pathophysiology. Acta Physiol. 2014, 210, 717–732. [Google Scholar] [CrossRef]
- Sasso, A.; Latella, G. Role of Heme Iron in the Association Between Red Meat Consumption and Colorectal Cancer. Nutr. Cancer 2018, 70, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.; Ball, M.J.; Mann, J. Nutrient intake and haematological status of vegetarians and age-sex matched omnivores. Eur. J. Clin. Nutr. 1994, 48, 538–546. [Google Scholar]
- Ball, M.J.; Bartlett, M.A. Dietary intake and iron status of Australian vegetarian women. Am. J. Clin. Nutr. 1999, 70, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Helman, A.D.; Darnton-Hill, I. Vitamin and iron status in new vegetarians. Am. J. Clin. Nutr. 1987, 45, 785–789. [Google Scholar] [CrossRef] [Green Version]
| Variable (Mean ± SD) | Men A | Women Who Do Not Menstruate B | Women Who Menstruate C | p-Value (A vs. B) | p-Value (A vs. C) | p-Value (B vs. C) |
|---|---|---|---|---|---|---|
| BMI (kg/m2) | 25.51 ± 4.4 | 25.17 ± 5.6 | 23.32 ± 4.3 | 0.1551 | <0.0001 | <0.0001 |
| hs-CRP (mg/dL) | 0.61 ± 2.1 | 0.85 ± 3.0 | 0.96 ± 3.7 | 0.0026 | 0.0005 | 0.6829 |
| HOMA-IR | 2.14 ± 1.6 | 2.10 ± 1.9 | 1.61 ± 1.2 | 0.4477 | <0.0001 | 0.0005 |
| Ferritin | 208.88 ± 162.29 | 89.35 ± 72.03 | 45.79 ± 39.59 | <0.0001 | <0.0001 | <0.0001 |
| Eating Habit | Nutritional Status—Men | Nutritional Status—Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low Weight n (%) | Eutrophy n (%) | Overweight n (%) | Obesity n (%) | Total | Low Weight n (%) | Eutrophy n (%) | Overweight n (%) | Obesity n (%) | Total | |
| Omnivore | 2 1.1% | 67 36.0% | 75 40.3% | 42 22.6% | 186 100.0% | 13 3.5% | 225 59.5% | 75 19.8% | 65 17.2% | 378 100.0% |
| Semi-vegetarian | 1 1.8% | 35 61.4% | 20 35.1% | 1 1.7% | 57 100.0% | 9 6.3% | 96 67.1% | 31 21.7% | 7 4.9% | 143 100.0% |
| Lacto-ovo vegetarian | 8 5.6% | 76 52.8% | 48 33.3% | 12 8.3% | 144 100.0% | 17 5.4% | 224 70.6% | 60 18.9% | 16 5.1% | 317 100.0% |
| Vegan | 2 5.7% | 20 57.1% | 10 28.6% | 3 8.6% | 35 100.0% | 11 13.8% | 49 61.2% | 13 16.3% | 7 8.7% | 80 100.0% |
| Total | 13 | 198 | 153 | 58 | 422 | 50 | 594 | 179 | 95 | 918 |
| Sample | Habit | Ferritin | Total | p-Value | |
|---|---|---|---|---|---|
| Normal (≥30 µg/L) | Altered (<30 µg/L) | ||||
| Men | Vegetarian | 225 | 7 | 232 | 0.0201 (2) |
| % | 96.88 | 3.02 | 100.00 | ||
| Omnivore | 180 | 0 | 180 | ||
| % | 100.00 | 0 | 100.00 | ||
| Total | 405 | 7 | 412 | ||
| Women who do not menstruate | Vegetarian | 95 | 19 | 114 | 0.0014 (1) X2(df = 1) = 10.1756 |
| % | 83.33 | 16.67 | 100.00 | ||
| Omnivore | 105 | 4 | 109 | ||
| % | 96.33 | 3.67 | 100.00 | ||
| Total | 200 | 23 | 223 | ||
| Women who menstruate | Vegetarian | 214 | 206 | 420 | <0.0001 (1) X2(df = 1) = 24.1562 |
| % | 50.95 | 49.05 | 100.00 | ||
| Omnivore | 179 | 76 | 225 | ||
| % | 70.20 | 29.80 | 100.00 | ||
| Total | 257 | 209 | 675 | ||
| Sample | Habit | Ferritin | Total | p-Value | |
|---|---|---|---|---|---|
| Normal (≥30 µg/L) | Altered (<30 µg/L) | ||||
| Men | Vegetarian | 124 | 4 | 128 | 0.3032 (2) |
| % | 96.88 | 3.13 | 100.00 | ||
| Omnivore | 64 | 0 | 64 | ||
| % | 100.00 | 0 | 100.00 | ||
| Total | 188 | 4 | 192 | ||
| Women who do not menstruate | Vegetarian | 49 | 8 | 57 | 0.1062 (1) X2(df = 1) = 2.6104 |
| % | 85.96 | 14.04 | 100.00 | ||
| Omnivore | 55 | 3 | 58 | ||
| % | 94.83 | 5.17 | 100.00 | ||
| Total | 104 | 11 | 115 | ||
| Women who menstruate | Vegetarian | 150 | 159 | 309 | <0.0001 (1) X2(df = 1) = 16.1839 |
| % | 48.54 | 51.46 | 100.00 | ||
| Omnivore | 107 | 50 | 157 | ||
| % | 68.15 | 31.85 | 100.00 | ||
| Total | 257 | 209 | 466 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slywitch, E.; Savalli, C.; Duarte, A.C.G.; Escrivão, M.A.M.S. Iron Deficiency in Vegetarian and Omnivorous Individuals: Analysis of 1340 Individuals. Nutrients 2021, 13, 2964. https://doi.org/10.3390/nu13092964
Slywitch E, Savalli C, Duarte ACG, Escrivão MAMS. Iron Deficiency in Vegetarian and Omnivorous Individuals: Analysis of 1340 Individuals. Nutrients. 2021; 13(9):2964. https://doi.org/10.3390/nu13092964
Chicago/Turabian StyleSlywitch, Eric, Carine Savalli, Antonio Cláudio Goulart Duarte, and Maria Arlete Meil Schimith Escrivão. 2021. "Iron Deficiency in Vegetarian and Omnivorous Individuals: Analysis of 1340 Individuals" Nutrients 13, no. 9: 2964. https://doi.org/10.3390/nu13092964
APA StyleSlywitch, E., Savalli, C., Duarte, A. C. G., & Escrivão, M. A. M. S. (2021). Iron Deficiency in Vegetarian and Omnivorous Individuals: Analysis of 1340 Individuals. Nutrients, 13(9), 2964. https://doi.org/10.3390/nu13092964






