Studies to Improve Perinatal Health through Diet and Lifestyle among South Asian Women Living in Canada: A Brief History and Future Research Directions
Abstract
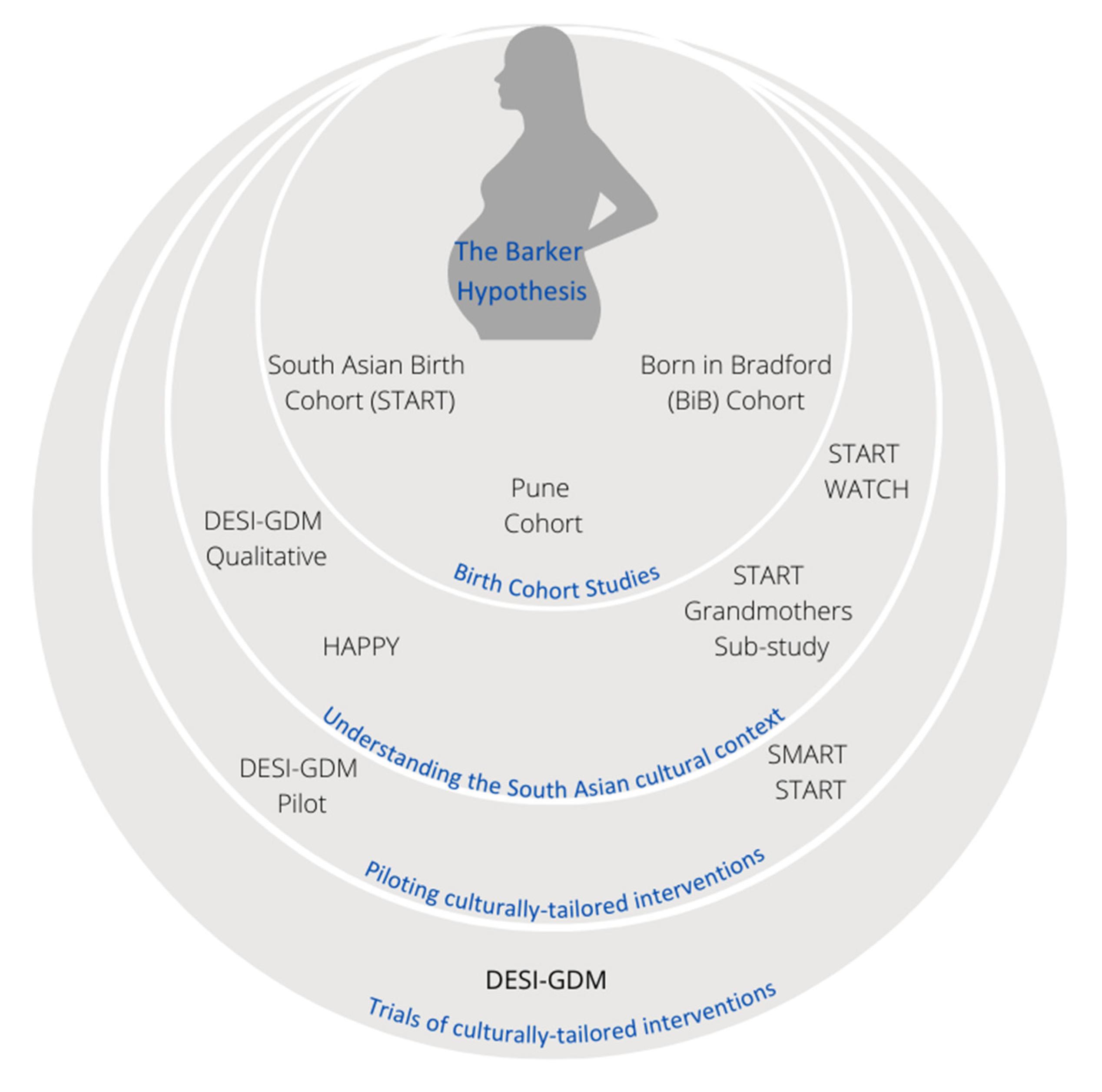
1. Introduction
2. Early Life Determinants of CVD
3. Gestational Diabetes and Related Complications to Mother and Offspring
4. Birth Cohort Studies for Understanding GDM in South Asian Populations
4.1. Pune Maternal Nutrition Study Cohort (PMNS)
4.2. Findings from the South Asian Birth Cohort (START)
4.3. Findings from the Born in Bradford (BiB) Cohort
5. Randomized Trials for GDM Prevention
6. Laying the Groundwork for Culturally Tailored Interventions: The Canadian Experience
6.1. Qualitative Studies
6.1.1. The START Grandmothers’ Study
- the pre-conception phase should emphasize the establishment of healthy habits (nutrition, physical activity, and mental wellness);
- the gestational period should encompass an enriched environment (positive relationships, healthy routines, nutritional enhancement) and;
- the postpartum period should focus on healing and restoration for the mother and newborn child (self-care, bonding, rebuilding healthy habits).
6.1.2. DESI-GDM Qualitative Study
6.1.3. The HAPPY Study
6.2. Mixed Methods Research Studies
6.2.1. START WATCH
6.2.2. SMART START
7. Design and Piloting an RCT
- Providing personalized food recommendations that consider a woman’s current dietary habits by identifying food choices and substitutions that will optimize her diet;
- Providing dietary advice that is sensitive to religious and regional culinary practices;
- Involving the household meal preparer, if this is not the participant herself, in the coaching contacts;
- Use of mobile health technology to support self-management and reduce the amount of in-office time a healthcare practitioner spends on dietary counselling.
8. The DESI-GDM Randomized Controlled Trial
8.1. The DESI-GDM RCT Pilot
8.2. The DESI-GDM RCT
- it was used to establish SA-specific diagnostic criteria for GDM, and thus our outcomes will be directly comparable [85];
- Diabetes Canada recognizes that the one-step strategy can identify a subset of women who would not otherwise be identified as having GDM and who may benefit with regards to certain perinatal outcomes [86].
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistics Canada. National Household Survey. Available online: https://www.peelregion.ca/planning/pdc/pdf/Ethicity_Religion_Bulletin.pdf (accessed on 20 August 2021).
- Morency, J.-D.; Malenfant, E.C.; MacIssac, S. Immigration and Diversity: Population Projections for Canada and Its Regions, 2011 to 2036; Statistics Canada: Ottawa, ON, Canada, 2017.
- Bainey, K.R.; Jugdutt, B.I. Increased burden of coronary artery disease in South-Asians living in North America. Need for an aggressive management algorithm. Atherosclerosis 2009, 204, 1–10. [Google Scholar] [CrossRef]
- Anand, S.S.; Yusuf, S.; Vuksan, V.; Devanesen, S.; Teo, K.K.; Montague, P.A.; Kelemen, L.; Yi, C.; Lonn, E.; Gerstein, H.; et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: The Study of Health Assessment and Risk in Ethnic groups (SHARE). Lancet 2000, 356, 279–284. [Google Scholar] [CrossRef]
- Chiu, M.; Austin, P.C.; Manuel, D.G.; Tu, J.V. Comparison of cardiovascular risk profiles among ethnic groups using population health surveys between 1996 and 2007. CMAJ Can. Med. Assoc. J. 2010, 182, E301–E310. [Google Scholar] [CrossRef]
- Chiu, M.; Austin, P.C.; Manuel, D.G.; Tu, J.V. Cardiovascular risk factor profiles of recent immigrants vs long-term residents of Ontario: A multi-ethnic study. Can. J. Cardiol. 2012, 28, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Grubisic, M.; Hemmelgarn, B.; Humphries, K.; King, K.M.; Quan, H. Outcomes after acute myocardial infarction in South Asian, Chinese, and white patients. Circulation 2010, 122, 1570–1577. [Google Scholar] [CrossRef]
- King, K.M.; Khan, N.A.; Quan, H. Ethnic variation in acute myocardial infarction presentation and access to care. Am. J. Cardiol. 2009, 103, 1368–1373. [Google Scholar] [CrossRef]
- Gupta, M.; Doobay, A.V.; Singh, N.; Anand, S.S.; Raja, F.; Mawji, F.; Kho, J.; Karavetian, A.; Yi, Q.; Yusuf, S. Risk factors, hospital management and outcomes after acute myocardial infarction in South Asian Canadians and matched control subjects. CMAJ Can. Med. Assoc. J. 2002, 166, 717–722. [Google Scholar]
- Barker, D.J.P.; Medical Research Council; Environmental Epidemiology Unit. Fetal and Infant Origins of Adult Disease. Br. Med. J. 1990, 301, 1111. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.N.; Barker, D.J. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. 1992. Int. J. Epidemiol. 2013, 42, 1215–1222. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 1, 1077–1081. [Google Scholar] [CrossRef]
- Barker, D.J.; Winter, P.D.; Osmond, C.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 2, 577–580. [Google Scholar] [CrossRef]
- Eriksson, J.G.; Forsen, T.; Tuomilehto, J.; Winter, P.D.; Osmond, C.; Barker, D.J. Catch-up growth in childhood and death from coronary heart disease: Longitudinal study. BMJ 1999, 318, 427–431. [Google Scholar] [CrossRef]
- Roseboom, T.; de Rooij, S.; Painter, R. The Dutch famine and its long-term consequences for adult health. Early Hum. Dev. 2006, 82, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Lumey, L.H.; Stein, A.D.; Kahn, H.S.; van der Pal-de Bruin, K.M.; Blauw, G.J.; Zybert, P.A.; Susser, E.S. Cohort profile: The Dutch Hunger Winter families study. Int. J. Epidemiol. 2007, 36, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, G.P.; Stein, Z.A.; Susser, M.W. Obesity in young men after famine exposure in utero and early infancy. N. Engl. J. Med. 1976, 295, 349–353. [Google Scholar] [CrossRef]
- Wu, L.; Feng, X.; He, A.; Ding, Y.; Zhou, X.; Xu, Z. Prenatal exposure to the Great Chinese Famine and mid-age hypertension. PLoS ONE 2017, 12, e0176413. [Google Scholar] [CrossRef]
- Wang, P.X.; Wang, J.J.; Lei, Y.X.; Xiao, L.; Luo, Z.C. Impact of fetal and infant exposure to the Chinese Great Famine on the risk of hypertension in adulthood. PLoS ONE 2012, 7, e49720. [Google Scholar] [CrossRef]
- Lumey, L.H.; Khalangot, M.D.; Vaiserman, A.M. Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932–33: A retrospective cohort study. Lancet Diabetes Endocrinol. 2015, 3, 787–794. [Google Scholar] [CrossRef]
- Gillman, M.W. Prenatal famine and developmental origins of type 2 diabetes. Lancet Diabetes Endocrinol. 2015, 3, 751–752. [Google Scholar] [CrossRef]
- Ezzati, M.; Riboli, E. Can noncommunicable diseases be prevented? Lessons from studies of populations and individuals. Science 2012, 337, 1482–1487. [Google Scholar] [CrossRef]
- Martinez, J.A.; Cordero, P.; Campion, J.; Milagro, F.I. Interplay of early-life nutritional programming on obesity, inflammation and epigenetic outcomes. Proc. Nutr. Soc. 2012, 71, 276–283. [Google Scholar] [CrossRef]
- Erkkola, M.; Nwaru, B.I.; Kaila, M.; Kronberg-Kippila, C.; Ilonen, J.; Simell, O.; Veijola, R.; Knip, M.; Virtanen, S.M. Risk of asthma and allergic outcomes in the offspring in relation to maternal food consumption during pregnancy: A Finnish birth cohort study. Pediatr. Allergy Immunol. 2012, 23, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann, F.; Demenais, F. Gene-environment interactions in asthma and allergic diseases: Challenges and perspectives. J. Allergy Clin. Immunol. 2012, 130, 1229–1240. [Google Scholar] [CrossRef]
- Hoffman, D.J.; Reynolds, R.M.; Hardy, D.B. Developmental origins of health and disease: Current knowledge and potential mechanisms. Nutr. Rev. 2017, 75, 951–970. [Google Scholar] [CrossRef]
- Ranucci, G.; Buccigrossi, V.; de Freitas, M.B.; Guarino, A.; Giannattasio, A. Early-Life Intestine Microbiota and Lung Health in Children. J. Immunol. Res. 2017, 2017, 8450496. [Google Scholar] [CrossRef] [PubMed]
- Nash, M.J.; Frank, D.N.; Friedman, J.E. Early Microbes Modify Immune System Development and Metabolic Homeostasis-The “Restaurant” Hypothesis Revisited. Front. Endocrinol. 2017, 8, 349. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. Gestational Diabetes. Available online: https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes/gestational (accessed on 20 August 2021).
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 1–19. [Google Scholar] [CrossRef]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational diabetes mellitus: Mechanisms, treatment, and complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Billionnet, C.; Mitanchez, D.; Weill, A.; Nizard, J.; Alla, F.; Hartemann, A.; Jacqueminet, S. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 2017, 60, 636–644. [Google Scholar] [CrossRef]
- Hapo Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Diabetes in Pregnancy: Management from Preceonception to the Postnatal Period. Available online: https://www.nice.org.uk/guidance/ng3/chapter/2-research-recommendations#postnatal-treatment-for-women-diagnosed-with-gestational-diabetes (accessed on 20 August 2021).
- Lee, A.J.; Hiscock, R.J.; Wein, P.; Walker, S.P.; Permezel, M. Gestational diabetes mellitus: Clinical predictors and long-term risk of developing type 2 diabetes: A retrospective cohort study using survival analysis. Diabetes Care 2007, 30, 878–883. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Chiang, V.; Pletcher, M.J.; Jacobs, D.R.; Quesenberry, C.P.; Sidney, S.; Lewis, C.E. History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: The Coronary Artery Risk Development in Young Adults study. J. Am. Heart Assoc. 2014, 3, e000490. [Google Scholar] [CrossRef]
- Kim, S.Y.; England, J.L.; Sharma, J.A.; Njoroge, T. Gestational diabetes mellitus and risk of childhood overweight and obesity in offspring: A systematic review. Exp. Diabetes Res. 2011, 2011, 541308. [Google Scholar] [CrossRef]
- Feig, D.S.; Zinman, B.; Wang, X.; Hux, J.E. Risk of development of diabetes mellitus after diagnosis of gestational diabetes. CMAJ Can. Med. Assoc. J. 2008, 179, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational Diabetes and the Incidence of Type 2 Diabetes. A systematic review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.D.; Mathiesen, E.R.; Hansen, T.; Pedersen, O.; Jensen, D.M.; Lauenborg, J.; Damm, P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: The role of intrauterine hyperglycemia. Diabetes Care 2008, 31, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Aceti, A.; Santhakumaran, S.; Logan, K.M.; Philipps, L.H.; Prior, E.; Gale, C.; Hyde, M.J.; Modi, N. The diabetic pregnancy and offspring blood pressure in childhood: A systematic review and meta-analysis. Diabetologia 2012, 55, 3114–3127. [Google Scholar] [CrossRef] [PubMed]
- Archambault, C.; Arel, R.; Filion, K.B. Gestational diabetes and risk of cardiovascular disease: A scoping review. Open Med. 2014, 8, e1–e9. [Google Scholar] [PubMed]
- Anand, S.S.; Gupta, M.; Teo, K.K.; Schulze, K.M.; Desai, D.; Abdalla, N.; Zulyniak, M.; de Souza, R.; Wahi, G.; Shaikh, M.; et al. Causes and consequences of gestational diabetes in South Asians living in Canada: Results from a prospective cohort study. CMAJ Open 2017, 5, E604–E611. [Google Scholar] [CrossRef]
- Simmons, D.; Jelsma, J.G.; Galjaard, S.; Devlieger, R.; van Assche, A.; Jans, G.; Corcoy, R.; Adelantado, J.M.; Dunne, F.; Desoye, G.; et al. Results From a European Multicenter Randomized Trial of Physical Activity and/or Healthy Eating to Reduce the Risk of Gestational Diabetes Mellitus: The DALI Lifestyle Pilot. Diabetes Care 2015, 38, 1650–1656. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Andersen, A.M.; Batty, G.D. Birth cohort studies: Past, present and future. Int. J. Epidemiol. 2009, 38, 897–902. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Sachdev, H.S.; Fall, C.H.; Osmond, C.; Lakshmy, R.; Barker, D.J.; Biswas, S.K.; Ramji, S.; Prabhakaran, D.; Reddy, K.S. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N. Engl. J. Med. 2004, 350, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, G.V.; Veena, S.R.; Hill, J.C.; Kehoe, S.; Karat, S.C.; Fall, C.H. Intrauterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diabetes Care 2010, 33, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, P.; Antonisamy, B.; Geethanjali, F.S.; Saperia, J.; Leary, S.D.; Priya, G.; Richard, J.; Barker, D.J.; Fall, C.H. Glucose tolerance, insulin resistance and insulin secretion in young south Indian adults: Relationships to parental size, neonatal size and childhood body mass index. Diabetes Res. Clin. Pract. 2010, 87, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, C.S.; Fall, C.H.; Coyaji, K.J.; Hirve, S.S.; Rao, S.; Barker, D.J.; Joglekar, C.; Kellingray, S. Neonatal anthropometry: The thin-fat Indian baby. The Pune Maternal Nutrition Study. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2003, 27, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.S.; Vasudevan, A.; Gupta, M.; Morrison, K.; Kurpad, A.; Teo, K.K.; Srinivasan, K. Rationale and design of south Asian birth cohort (START): A Canada-India collaborative study. BMC Public Health 2013, 13, 79. [Google Scholar] [CrossRef]
- Wright, J.; Small, N.; Raynor, P.; Tuffnell, D.; Bhopal, R.; Cameron, N.; Fairley, L.; Lawlor, D.A.; Parslow, R.; Petherick, E.S.; et al. Cohort Profile: The Born in Bradford multi-ethnic family cohort study. Int. J. Epidemiol. 2013, 42, 978–991. [Google Scholar] [CrossRef]
- Yajnik, C.S. Transmission of obesity-adiposity and related disorders from the mother to the baby. Ann. Nutr. Metab. 2014, 64 (Suppl. 1), 8–17. [Google Scholar] [CrossRef]
- Yajnik, C.S.; Deshpande, S.S.; Jackson, A.A.; Refsum, H.; Rao, S.; Fisher, D.J.; Bhat, D.S.; Naik, S.S.; Coyaji, K.J.; Joglekar, C.V.; et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: The Pune Maternal Nutrition Study. Diabetologia 2008, 51, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, G.V.; Yajnik, C.S. Developmental origins of diabetes-an Indian perspective. Eur. J. Clin. Nutr. 2017, 71, 865–869. [Google Scholar] [CrossRef]
- Yajnik, C.S.; Fall, C.H.; Vaidya, U.; Pandit, A.N.; Bavdekar, A.; Bhat, D.S.; Osmond, C.; Hales, C.N.; Barker, D.J. Fetal growth and glucose and insulin metabolism in four-year-old Indian children. Diabet. Med. J. Br. Diabet. Assoc. 1995, 12, 330–336. [Google Scholar] [CrossRef]
- Anand, S.S.; Gupta, M.K.; Schulze, K.M.; Desai, D.; Abdalla, N.; Wahi, G.; Wade, C.; Scheufler, P.; McDonald, S.D.; Morrison, K.M.; et al. What accounts for ethnic differences in newborn skinfold thickness comparing South Asians and White Caucasians? Findings from the START and FAMILY Birth Cohorts. Int. J. Obes. 2016, 40, 239–244. [Google Scholar] [CrossRef]
- West, J.; Kelly, B.; Collings, P.J.; Santorelli, G.; Mason, D.; Wright, J. Is small size at birth associated with early childhood morbidity in white British and Pakistani origin UK children aged 0-3? Findings from the born in Bradford cohort study. BMC Pediatrics 2018, 18, 22. [Google Scholar] [CrossRef]
- Walsh, J.M.; McGowan, C.A.; Mahony, R.; Foley, M.E.; McAuliffe, F.M. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): Randomised control trial. BMJ 2012, 345, e5605. [Google Scholar] [CrossRef] [PubMed]
- Tanentsapf, I.; Heitmann, B.L.; Adegboye, A.R. Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy Childbirth 2011, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Streuling, I.; Beyerlein, A.; Rosenfeld, E.; Hofmann, H.; Schulz, T.; Von Kries, R. Physical activity and gestational weight gain: A meta-analysis of intervention trials. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 278–284. [Google Scholar] [CrossRef]
- Ronnberg, A.K.; Nilsson, K. Interventions during pregnancy to reduce excessive gestational weight gain: A systematic review assessing current clinical evidence using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Tuffnell, D.J.; West, J.; Walkinshaw, S.A. Treatments for Gestational Diabetes and Impaired Glucose Tolerance in Pregnancy; The Cochrane Library: Hoboken, NJ, USA, 2008. [Google Scholar]
- Oostdam, N.; van Poppel, M.N.; Wouters, M.G.; van Mechelen, W. Interventions for preventing gestational diabetes mellitus: A systematic review and meta-analysis. J. Womens Health 2011, 20, 1551–1563. [Google Scholar] [CrossRef] [PubMed]
- Goveia, P.; Canon-Montanez, W.; Santos, D.P.; Lopes, G.W.; Ma, R.C.W.; Duncan, B.B.; Ziegelman, P.K.; Schmidt, M.I. Lifestyle Intervention for the Prevention of Diabetes in Women With Previous Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2018, 9, 583. [Google Scholar] [CrossRef] [PubMed]
- Madhuvrata, P.; Govinden, G.; Bustani, R.; Song, S.; Farrell, T.A. Prevention of gestational diabetes in pregnant women with risk factors for gestational diabetes: A systematic review and meta-analysis of randomised trials. Obstet. Med. 2015, 8, 68–85. [Google Scholar] [CrossRef]
- Rogozinska, E.; Chamillard, M.; Hitman, G.A.; Khan, K.S.; Thangaratinam, S. Nutritional manipulation for the primary prevention of gestational diabetes mellitus: A meta-analysis of randomised studies. PLoS ONE 2015, 10, e0115526. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Li, J.; Leng, J.; Ma, R.C.; Yang, X. Lifestyle intervention can reduce the risk of gestational diabetes: A meta-analysis of randomized controlled trials. Obes. Rev. 2016, 17, 960–969. [Google Scholar] [CrossRef]
- Ha, V.; Bonner, A.J.; Jadoo, J.K.; Beyene, J.; Anand, S.S.; de Souza, R.J. The effects of various diets on glycemic outcomes during pregnancy: A systematic review and network meta-analysis. PLoS ONE 2017, 12, e0182095. [Google Scholar] [CrossRef] [PubMed]
- Thangaratinam, S.; Rogozinska, E.; Jolly, K.; Glinkowski, S.; Roseboom, T.; Tomlinson, J.W.; Kunz, R.; Mol, B.W.; Coomarasamy, A.; Khan, K.S. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: Meta-analysis of randomised evidence. BMJ 2012, 344, e2088. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, M.A.; Ainscough, K.; Lindsay, K.L.; O’Sullivan, E.; Gibney, E.R.; McCarthy, M.; Segurado, R.; DeVito, G.; Maguire, O.; Smith, T.; et al. Pregnancy Exercise and Nutrition With Smartphone Application Support: A Randomized Controlled Trial. Obstet. Gynecol. 2018, 131, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Assaf-Balut, C.; Garcia de la Torre, N.; Duran, A.; Fuentes, M.; Bordiu, E.; Del Valle, L.; Familiar, C.; Ortola, A.; Jimenez, I.; Herraiz, M.A.; et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLoS ONE 2017, 12, e0185873. [Google Scholar] [CrossRef]
- Sahariah, S.A.; Potdar, R.D.; Gandhi, M.; Kehoe, S.H.; Brown, N.; Sane, H.; Coakley, P.J.; Marley-Zagar, E.; Chopra, H.; Shivshankaran, D.; et al. A Daily Snack Containing Leafy Green Vegetables, Fruit, and Milk before and during Pregnancy Prevents Gestational Diabetes in a Randomized, Controlled Trial in Mumbai, India. J. Nutr. 2016, 146, 1453S–1460S. [Google Scholar] [CrossRef]
- Kandasamy, S.; Anglin, R.; Gaind, L.; Desai, D.; Wahi, G.; Gupta, M.; Anand, S.S. A qualitative investigation of optimal perinatal health: The perspectives of south Asian grandmothers living in southern Ontario, Canada. BMC Pregnancy Childbirth 2020, 20, 113. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Clinch, M.; Afsar, N.; Choudhury, Y.; Sudra, R.; Campbell-Richards, D.; Claydon, A.; Hitman, G.A.; Hanson, P.; Finer, S. Socio-cultural influences on the behaviour of South Asian women with diabetes in pregnancy: Qualitative study using a multi-level theoretical approach. BMC Med. 2015, 13, 120. [Google Scholar] [CrossRef]
- Bandyopadhyay, M.; Small, R.; Davey, M.A.; Oats, J.J.; Forster, D.A.; Aylward, A. Lived experience of gestational diabetes mellitus among immigrant South Asian women in Australia. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 360–364. [Google Scholar] [CrossRef]
- Green, J.; Thorogood, N. Qualitative Methods for Health Research, 2nd ed.; Sage Publications: London, UK, 2009. [Google Scholar]
- Kandasamy, S.; Nguyen, L.; Desai, D.; Anand, S.S.; Sherifali, D.; de Souza, R.J. Barriers to, and Facilitators and barriers to lifestyle changes during pregnancy faced by South Asian women living in Canada: An interpretive descriptive study of women and healthcare providers. Can. J. Diabetes 2021, 45, 144–154. [Google Scholar] [CrossRef] [PubMed]
- The Social Planning Council of Peel. An Exploratory Study of Diabetes among South Asians in Peel; The Social Planning Council of Peel: Peel, ON, Canada, 2015. [Google Scholar]
- de Souza, R.J. A Culturally-Tailored Personalized Nutrition Intervention in South Asian Women at Risk of Gestational Diabetes. Available online: https://ClinicalTrials.gov/show/NCT03607799 (accessed on 20 August 2021).
- Anand, S.S.; Samaan, Z.; Middleton, C.; Irvine, J.; Desai, D.; Schulze, K.M.; Sothiratnam, S.; Husdain, F.; Shah, B.R.; Pare, G.; et al. A digital health intervention to lower cardiovascular risk: A randomized clinical trial. JAMA Cardiol. 2016, 1, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.K.; Redfern, J.; Hillis, G.S.; Thakkar, J.; Santo, K.; Hackett, M.L.; Jan, S.; Graves, N.; de Keizer, L.; Barry, T.; et al. Effect of Lifestyle-Focused Text Messaging on Risk Factor Modification in Patients With Coronary Heart Disease: A Randomized Clinical Trial. JAMA 2015, 314, 1255–1263. [Google Scholar] [CrossRef]
- Allison, D.B.; Paultre, F.; Maggio, C.; Mezzitis, N.; Pi-Sunyer, F.X. The use of areas under curves in diabetes research. Diabetes Care 1995, 18, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, K.; Takeda, K.; Maeda, M.; Ogawa, W.; Sato, T.; Okada, S.; Ohnishi, Y.; Nakajima, H.; Kashiwagi, A. Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetol. Int. 2016, 7, 53–58. [Google Scholar] [CrossRef]
- Farrar, D.; Fairley, L.; Santorelli, G.; Tuffnell, D.; Sheldon, T.A.; Wright, J.; van Overveld, L.; Lawlor, D.A. Association between hyperglycaemia and adverse perinatal outcomes in south Asian and white British women: Analysis of data from the Born in Bradford cohort. Lancet Diabetes Endocrinol. 2015, 3, 795–804. [Google Scholar] [CrossRef]
- Diabetes Canada Clinical Practice Guidelines Expert Committee; Feig, D.S.; Berger, H.; Donovan, L.; Godbout, A.; Kader, T.; Keely, E.; Sanghera, R. Diabetes and Pregnancy. Can. J. Diabetes 2018, 42 (Suppl. 1), S255–S282. [Google Scholar] [CrossRef]
- Thompson, D.; Berger, H.; Feig, D.; Gagnon, R.; Kader, T.; Keely, E.; Kozak, S.; Ryan, E.; Sermer, M.; Vinokuroff, C. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Diabetes and Pregnancy. Can. J. Diabetes 2013, 37, S1–S212. [Google Scholar] [CrossRef]
- Mohan, V.; Mahalakshmi, M.M.; Bhavadharini, B.; Maheswari, K.; Kalaiyarasi, G.; Anjana, R.M.; Uma, R.; Usha, S.; Deepa, M.; Unnikrishnan, R.; et al. Comparison of screening for gestational diabetes mellitus by oral glucose tolerance tests done in the non-fasting (random) and fasting states. Acta Diabetol. 2014, 51, 1007–1013. [Google Scholar] [CrossRef]
- Duran, A.; Saenz, S.; Torrejon, M.J.; Bordiu, E.; Del Valle, L.; Galindo, M.; Perez, N.; Herraiz, M.A.; Izquierdo, N.; Rubio, M.A.; et al. Introduction of IADPSG criteria for the screening and diagnosis of gestational diabetes mellitus results in improved pregnancy outcomes at a lower cost in a large cohort of pregnant women: The St. Carlos Gestational Diabetes Study. Diabetes Care 2014, 37, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Lapolla, A.; Dalfra, M.G.; Ragazzi, E.; De Cata, A.P.; Fedele, D. New International Association of the Diabetes and Pregnancy Study Groups (IADPSG) recommendations for diagnosing gestational diabetes compared with former criteria: A retrospective study on pregnancy outcome. Diabet. Med. J. Br. Diabet. Assoc. 2011, 28, 1074–1077. [Google Scholar] [CrossRef]
- Mission, J.F.; Ohno, M.S.; Cheng, Y.W.; Caughey, A.B. Gestational diabetes screening with the new IADPSG guidelines: A cost-effectiveness analysis. Am. J. Obstet. Gynecol. 2012, 207, 326.e1–326.e9. [Google Scholar] [CrossRef] [PubMed]
- Trico, D.; Filice, E.; Baldi, S.; Frascerra, S.; Mari, A.; Natali, A. Sustained effects of a protein and lipid preload on glucose tolerance in type 2 diabetes patients. Diabetes Metab. 2016, 42, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Numao, S.; Kawano, H.; Endo, N.; Yamada, Y.; Konishi, M.; Takahashi, M.; Sakamoto, S. Short-term low carbohydrate/high-fat diet intake increases postprandial plasma glucose and glucagon-like peptide-1 levels during an oral glucose tolerance test in healthy men. Eur. J. Clin. Nutr. 2012, 66, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; de Souza, R.J.; Kandasamy, S.; Lear, S.A.; Anand, S.S. Cardiovascular risk among South Asians living in Canada: A systematic review and meta-analysis. CMAJ Open 2014, 2, E183–E191. [Google Scholar] [CrossRef]
- Edvardsson, K.; Ivarsson, A.; Eurenius, E.; Garvare, R.; Nystrom, M.E.; Small, R.; Mogren, I. Giving offspring a healthy start: Parents’ experiences of health promotion and lifestyle change during pregnancy and early parenthood. BMC Public Health 2011, 11, 936. [Google Scholar] [CrossRef]
- Guo, X.Y.; Shu, J.; Fu, X.H.; Chen, X.P.; Zhang, L.; Ji, M.X.; Liu, X.M.; Yu, T.T.; Sheng, J.Z.; Huang, H.F. Improving the effectiveness of lifestyle interventions for gestational diabetes prevention: A meta-analysis and meta-regression. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 311–320. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desai, D.; Kandasamy, S.; Limbachia, J.; Zulyniak, M.A.; Ritvo, P.; Sherifali, D.; Wahi, G.; Anand, S.S.; de Souza, R.J. Studies to Improve Perinatal Health through Diet and Lifestyle among South Asian Women Living in Canada: A Brief History and Future Research Directions. Nutrients 2021, 13, 2932. https://doi.org/10.3390/nu13092932
Desai D, Kandasamy S, Limbachia J, Zulyniak MA, Ritvo P, Sherifali D, Wahi G, Anand SS, de Souza RJ. Studies to Improve Perinatal Health through Diet and Lifestyle among South Asian Women Living in Canada: A Brief History and Future Research Directions. Nutrients. 2021; 13(9):2932. https://doi.org/10.3390/nu13092932
Chicago/Turabian StyleDesai, Dipika, Sujane Kandasamy, Jayneel Limbachia, Michael A. Zulyniak, Paul Ritvo, Diana Sherifali, Gita Wahi, Sonia S. Anand, and Russell J. de Souza. 2021. "Studies to Improve Perinatal Health through Diet and Lifestyle among South Asian Women Living in Canada: A Brief History and Future Research Directions" Nutrients 13, no. 9: 2932. https://doi.org/10.3390/nu13092932
APA StyleDesai, D., Kandasamy, S., Limbachia, J., Zulyniak, M. A., Ritvo, P., Sherifali, D., Wahi, G., Anand, S. S., & de Souza, R. J. (2021). Studies to Improve Perinatal Health through Diet and Lifestyle among South Asian Women Living in Canada: A Brief History and Future Research Directions. Nutrients, 13(9), 2932. https://doi.org/10.3390/nu13092932





