Abstract
There is growing evidence that bone health may be programmed in the first years of life. Factors during the prenatal period, especially maternal nutrition, may have an influence on offspring’s skeletal development and thus the risk of osteoporosis in further life, which is an increasing societal, health and economic burden. However, it is still inconclusive which early life factors are the most important and to what extent they may affect bone health. We searched through three databases (PubMed, Google Scholar, Cochrane Library) and after eligibility criteria were met, the results of 49 articles were analyzed. This narrative review is an overall summary of up-to-date studies on maternal diet, nutritional status, and birth-related factors that may affect offspring bone development, particularly bone mineral density (BMD). Maternal vitamin D status and diet in pregnancy, anthropometry and birth weight seem to influence BMD, however other factors such as subsequent growth may mediate these associations. Due to the ambiguity of the results in the analyzed studies, future, well-designed studies are needed to address the limitations of the present study.
1. Introduction
The first years of life are crucial for proper child development. For the first time, this theory was proposed by Baker and his hypothesis gave the grounds for the following concepts of metabolic programming, such as the first 1000 days of life or the Developmental Origins of the Health and Disease hypothesis (DOHaD) [1,2,3,4]. According to them, factors during periconceptual, fetal and early infant phases play an important role in a child’s growth and development. By consequence, if their influence is adverse, they may increase the risk of poorer neurocognitive development, metabolic disorders and related conditions, such as obesity, diabetes or cardiovascular diseases later in life [1,2,3,4,5]. Besides, it is also a very crucial period in bone development, which may influence bone health, including further risk of osteoporosis [4,6,7].
The human skeleton starts to develop in the early prenatal phase, when cells differentiate into chondrocytes and osteoblasts [8]. During the third trimester the majority of a fetus’ bone is gained, so this time seems to be very important in the terms of skeletal development [9]. The skeleton grows intensively in the utero environment and the rate of growth is still high immediately after birth, then slows and increases again in infancy. This continuous development lasts until about age 20 when peak bone mass is reached, but this time is dependent on skeletal site and sex, among others [8,9].
Genetic and epigenetic processes, as well as accessibility of nutrients, seems to play an important role in bone development and affect peak bone mass, whose lower levels are a crucial risk factor for osteoporosis [7,10,11]. Among dietary factors, vitamin D and calcium are the most often mentioned [11,12,13,14,15,16,17,18], however, the role of macronutrients [19,20,21] and other nutrients, such as vitamin A [22,23], folate [19,24] or magnesium [19,21] have also been raised in studies regarding bone health.
In addition, peak bone mass may be regulated by two components, skeletal envelope size and bone mineral density [25]. Bone mineral density (BMD) increases during childhood and adolescence and, according to the study of Foley et al. [26], BMD levels at age 8 may determine those at age 16. This is important from a long-term perspective, as it suggests that risk factors for osteoporosis may be identified in adolescence, when it is still possible to take actions aimed at improving skeletal health.
In the available studies several factors, both maternal (especially nutrition in pregnancy, particularly vitamin D status, maternal anthropometry), and birth-related (i.a. birth parameters) were analyzed in relation to offspring bone health. Also during the postnatal period, factors such as children nutrition (especially sufficient calcium intake and vitamin D status) or physical activity have a great contribution to offspring bone health and are considered as a part of primary prevention of osteoporosis [27]. Nevertheless, in the presented review we focus on the selected prenatal and birth-related factors.
The most frequently assessed parameters were: BMD, areal BMD (aBMD), bone mineral apparent density (BMAD), volumetric BMD (vBMD), and speed of sound (SOS). BMD is usually defined as bone mineral content (BMC)/bone surface area ratio [28]. However, it remains inconclusive whether and to what extent those factors may influence bone mineral density in children and if those changes are still apparent later in life. In addition, a recent systematic review [29] has raised a similar subject, but the authors included studies assessing BMD in young adults aged 16–30 years, so less is known about those associations in younger population. Moreover, the results of foregoing studies have been less consistent.
Taking into consideration the abovementioned background, the aim of this review was to identify and synthesize the best available evidence from observational, cohort, and randomized controlled trials on the association between maternal factors, especially nutritional and anthropometric, as well as birth-related factors influencing offspring bone health in the childhood up to young adulthood, particularly BMD—the main parameter analyzed in this review.
2. Methods
2.1. Search Strategy
In this narrative review a comprehensive literature search was performed until the 31 August 2020, with no starting date specified, using three databases, PubMed, Google Scholar and Cochrane Library, with no restrictions on type of studies, publication date, or country. Search terms were defined by two senior researchers (J.H., M.A.Z.-P.) and included the following keywords in the title and/or abstract: maternal, pregnancy, vitamin D, supplementation, status, diet, offspring, children, bone mass, BMI (body mass index), smoking, in various combinations. Due to the large number of results obtained in Google Scholar, in this database only the first few (10–15) pages were screened.
The search and eligibility process is presented in detail in Table 1 and Figure 1. After first screening by titles and further by titles and abstracts and removing duplicates, papers were screened in the full-text versions. Then, the exclusion criteria were applied (Table 1).

Table 1.
Inclusion and exclusion criteria for screening studies.
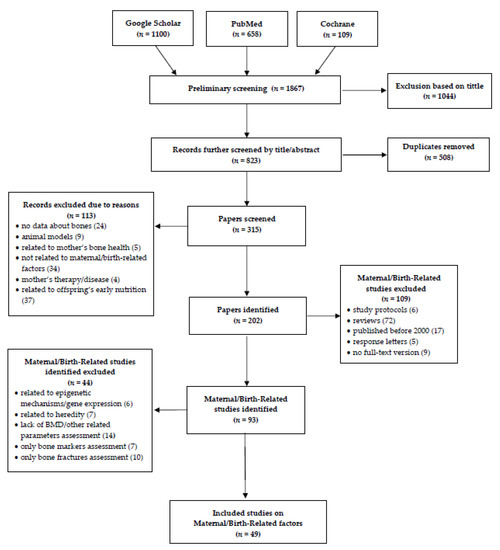
Figure 1.
Flow diagram of the records included in the narrative review.
2.2. Analysis of Included Studies
In the assessment of the results we considered fully adjusted, multivariate models of conducted analyses. When such analysis had not been conducted, we focused on the available results of univariate models, correlation coefficients, or in intervention studies, comparisons between groups. Moreover, in the presented tables, if a particular factor/nutrient was mentioned in the column with the assessed factors but not in the column with the outcome, it means that this factor was not significant in the considered analysis.
3. Findings and Discussion
In total, 1867 studies were identified through the literature search up to 31 August 2020. Among these, 1044 were excluded in the first screening based on the title and 508 duplicates were removed. In the further eligibility process (Figure 1), we included 49 articles in the final review. We did not apply any criteria for the offspring’s age, thus the results of the analyzed studies include a wide age range, from neonates to young adults.
3.1. Maternal Factors
3.1.1. Dietary Intake
The results of studies concerning maternal dietary intakes are presented in Table 2. In total, eight studies aimed to identify possible associations between maternal macronutrients, vitamins, mineral intake, as well as specified dietary patterns in pregnancy and offspring BMD. Furthermore, Table S1 provides detailed information about maternal dietary intakes reported in those studies, as well as methodological details about dietary assessment methods. In all of the assessed studies, the analyses focused on the assessment of the associations between quantitative data, expressed as daily intakes, quintiles [19] or tertiles [20,21]. In two studies [20,21], the authors converted dietary variables to nutrient/food density by dividing estimated daily nutrient/food intake by estimated total daily energy intake. Data about inadequate or excessive intake were not reported.

Table 2.
Maternal dietary intake in pregnancy and offspring bone outcomes.
Macronutrients
Four studies investigated associations between BMD and dietary intake of components such as macronutrients [19,20,21,30]. Maternal energy from carbohydrate (quintiles) was negatively associated with offspring BMD [19]. In one study energy from protein (quintile) was positively related to BMD in offspring [19]. However, in two other studies, no association between protein intake was found [20,30]. Results regarding fat intake, expressed as quantiles of energy from fat [19] or fat density (divided estimated nutrient intake by estimated total daily energy intake) [20,21] were ambiguous: one study found a negative association between fat density and offspring lumbar spine (LS) and femoral neck (FN) BMD (with no changes in whole body (WB) BMD) [21], one study found a positive association [20], and one study reported no association [19].
Minerals
A possible link between children’s BMD and maternal dietary intake of minerals was assessed in six studies [19,20,21,30,31,32]. No association was found between offspring BMD and maternal potassium nor zinc intake in pregnancy [20,30]. In some studies, offspring BMD (in at least one site) was associated with higher maternal intakes of magnesium [21], phosphorus [19,20], calcium [19,21,31], or folate [32]. However, it remains inconclusive whether intake of those nutrients is associated with children’s bone outcomes, as the above results have not been confirmed in other studies [19,20,30].
Group Products
Associations between BMD and specific dietary pattern [33] or intake of group products like green vegetables [31], milk and milk products [24], or milk density (divided estimated daily food intake by estimated total daily energy intake) [21] were assessed in four studies. Interestingly, higher maternal green vegetable intake in early pregnancy was related to lower neonatal spine BMD [31]. Maternal milk and milk products intake as well as milk density in late pregnancy was positively associated with BMD in offspring [21,24]. In addition, Cole et al. [33] demonstrated that a high prudent diet score in late pregnancy was associated with higher offspring WB BMD.
Intervention Studies
Table 3 summarizes the results of dietary interventions during pregnancy. Three studies involved vitamin D supplementation [11,12,13]. Two studies did not find any associations between maternal vitamin D supplementation during pregnancy and neonatal BMD [11,12]. Contrary to those studies, the study conducted by Brustad et al. [13] demonstrated higher head BMD, with no difference in total body less head (TBLH) BMD or total BMD in the group of children (combined analysis at 3 and 6 years old) whose mothers received higher vitamin D3 doses.

Table 3.
Maternal dietary intervention studies and offspring bone outcomes.
In turn, in studies where intervention included supplementation of vitamin D and calcium, the results were conflicting [15,16]. In a study from India, WB BMD was higher in offspring whose mothers were in the placebo group, compared to groups that received high doses of vitamin D [15]. In another study no association was found.
Studies on the effect of supplementation with calcium [14] and n-3 long chain polyunsaturated fatty acids (LC PUFAs) [34] showed no association with offspring vBMD and TBLH BMD, respectively.
3.1.2. Nutritional Status
Vitamin D Status
Maternal vitamin D status during pregnancy was assessed in eleven studies (Table 4), however the results are inconclusive. One study reported a negative association with offspring total BMD (with no difference in lumbar BMD) [17]. Six studies found no association between maternal vitamin D status and bone parameters from the neonatal period even to the age of 26 years [23,35,36,37,38,39]. In the four remaining studies, positive associations were found between maternal vitamin D status and offspring BMD [18,40], with sex-specific association in one study [41], as well as neonatal bone SOS [42].

Table 4.
Maternal vitamin D status in pregnancy and offspring bone outcomes.
Other Nutrients
Table 5 presents the results of studies whose aim was to assess maternal nutritional status in categories other than vitamin D. In one study, maternal vitamin B12 and homocysteine concentrations in pregnancy were assessed [19]. Vitamin B12 concentrations were positively associated with offspring BMD, whereas in relation to homocysteine no association was found [19]. Two studies investigated maternal folate concentrations but the results varied [19,24]. In one study no association was found [19], whereas in another study a positive association with offspring BMD was noticed [24]. In a study by Javaid et al. [18], calcium concentrations in cord blood were positively related to offspring LS aBMD but not WB aBMD. Two studies assessed maternal vitamin A status in relation to offspring BMD [22,23]. When samples were taken at around weeks 33/34 of gestation, no association was found in both studies [22,23]. However, retinol concentrations in samples taken in week 17 and 37 of gestation showed a positive association with WB, LS BMD and LS, total hip BMD, respectively [23]. Harvey et al. [43] investigated maternal LC PUFAs status. Late pregnancy concentrations of n-3 LC PUFAs, especially docosapentaenoic acid (DPA) and eicosapentaenoic acid (EPA), were positively associated with offspring aBMD/vBMD at several sites, whereas arachidonic acid (AA) was inversely related to WBMH aBMD, which suggests the significant role of n-6 to n-3 balance [43].

Table 5.
Maternal nutrients status in pregnancy and offspring bone outcomes.
- Potential mechanisms of associations between maternal dietary intake, nutritional status and offspring bone outcomes
There are several potential mechanisms that could explain associations between maternal dietary intake of the analyzed nutrients and offspring bone health. Higher protein intake may increase bone mineral accrual and insulin-like growth factor 1 (IGF-1) concentrations. IGF-1 is an osteotrophic factor, whose levels have been related to a higher BMD [19,44]. Saturated fat intake has been inversely related to bone density in adults [45], besides in animal studies a high intake of fat decreased intestinal calcium absorption and thus could have a negative impact on offspring bones [21]. Magnesium deficiency may have both a direct and indirect effect on bone health, through an influence on bone cells (reducing osteoblast and enhancing osteoclast activity) and changes in calcium homeostasis regulators (parathyroid hormone and 1,25(OH)2D, leading to hypocalcemia), as shown in in vitro and in vivo studies [46].
In all of the included studies on maternal vitamin D status, authors assessed 25(OH)D concentrations [17,18,23,35,36,37,38,39,40,41,42]. In addition, in one study, 1,25(OH)2D concentrations were also assessed and its levels were similar across different levels of 25(OH)D [23]. According to the guidelines, recommended marker for assessing vitamin D status is 25(OH)D, which is the most abundant metabolite of vitamin D [47,48]. Possible mechanisms given in the analyzed studies suggest an effect of vitamin D on fetal skeletal development and mineralization, which may be impaired because of this relevant vitamin deficiency [23,40]. Moreover vitamin D is essential for proper placental calcium transport, so its insufficient levels may affect the trajectory of bone mineral accrual in intrauterine environments [18]. However, since some of the studies did not show an association between maternal vitamin D status and children’s bone outcomes [23,35,36,37,38,39], there may be compensatory mechanisms that enable proper skeletal growth in the children of mothers with vitamin D deficiency during pregnancy [23,38].
Maternal LC PUFAs status/intake in pregnancy seems to have a positive influence on children’s bone outcomes, but the potential mechanism is uncertain. Essential n-6 and n-3 are substrates for the production of eicosanoids and thus may have a variety of effects in human bone cell culture, like inhibited osteoclast formation and enhanced osteoblast formation, which is optimal, as well as osteoblast apoptosis, which is adverse [49]. Vitamin A plays the role of modulator in epigenetic processes, moreover retinol is an important nutrient in embryogenesis, when the axial skeleton is shaped [23,50]. Carotenoids may be beneficial for bone health, as some of them are provitamin A precursors, they have antioxidant functions and reduce bone resorption [50]. However, some studies have observed that high intake of supplements or fortified foods with preformed vitamin A (retinol and retinyl esters) may be related to higher bone loss [50]. Folate and vitamin B12 may have an indirect impact on bone health through epigenetic changes, as they are important methyl donors for deoxyribonucleic acid (DNA) methylation, but also a direct effect related to osteoblast function or homocysteine metabolism is possible [19,51].
3.1.3. Maternal Anthropometry
Regarding maternal anthropometry, the included studies are presented in Table 6 and Table 7. Maternal height was related to offspring bone parameters, but the results of five analyzed studies are inconclusive [24,31,52,53,54]. In one study, this maternal factor was inversely associated with spine BMAD, but no association was observed in relation to TB and spine BMD [53]. Three studies suggested no effects regarding offspring BMD [24,52,54]. Godfrey et al. [31], in turn, showed a positive association between maternal stature and neonatal spine BMD.

Table 6.
Maternal anthropometry and offspring bone outcomes.

Table 7.
Maternal prepregnancy weight and offspring bone outcomes.
In one study, higher maternal early pregnancy fat stores, assessed by triceps skinfold thickness, were associated with higher neonatal WB BMD [31].
Maternal BMI in early pregnancy was analyzed in two studies. In one study, no association was found [31], whereas in another study a positive association was noticed but only in relation to one of the measured sites (LS BMD) [53]. The influence of pregnancy weight gain, which was investigated in three studies [53,55,56], was also ambiguous. In a study by Xu et al. [56], pregnancy weight gain was positively related to offspring BMD. In another study, a positive influence was observed only in relation to one measured site (TB BMD) [53]. Monjardino et al. [55] found that maternal gestational weight gain (GWG) was associated with higher aBMD in children, but only if the mothers were under/normal weight at the beginning of pregnancy.
Four studies assessed the possible link between maternal prepregnancy weight and offspring BMD (Table 7). In three of them, authors found no association [57,58,59], whereas Rudang et al. [54] found a positive association.
- Potential Mechanisms of Associations between Maternal Anthropometry and Offspring Bone Outcomes
Maternal height may have an influence on children’s BMD through genetics. Moreover, larger pelvic diameter in mothers with a higher stature may be a factor that influences fetal growth because of a greater capacity to supply the fetus with nutrients [52]. Both triceps skinfold thickness and BMI are indicators of maternal fat stores, which may reflect overall maternal nutritional status and the availability of nutrients in the uterus, which contributes to fetal growth [31,59]. Nonetheless, it needs to be considered that in the Macdonald-Wallis et al. [59] study, the results between maternal and paternal BMI and bone outcomes were similar, so this effect might arise from genetics and the postnatal environment, rather than the environment in the uterus. Furthermore, in a study where DXA assessments were made several times over six years, maternal prepregnancy weight was not related to offspring BMD at each individual assessment [57]. This may indicate that maternal prepregnancy weight does not affect BMD in offspring in the first six years.
3.1.4. Maternal Demographic and Socioeconomic Factors
Other maternal factors, such as age [54], education [60], socioeconomic status (SES) [61] and parity [24,52,54,62], were also analyzed regarding offspring BMD (Table 8).

Table 8.
Maternal demographic and socioeconomic factors and offspring bone outcomes.
Advancing maternal age was associated with lower spine aBMD in sons [54]. No influence of maternal education levels [60] nor parity [24,52,54,62] on childhood bone outcomes has been confirmed. One study investigated maternal SES and this factor was associated with higher BMD at all measured sites in daughters [61]. Identical results were obtained in sons with the exception of LS BMD (no effect) [61].
- Potential Mechanisms of Associations between Maternal Demographic, Socioeconomic Factors and Offspring Bone Outcomes
A possible reason for the association between maternal age and offspring aBMD is epigenetic causes, related to DNA methylation and histone modification in utero [54], whereas maternal SES may be a factor that influences nutritional behaviors as well as anthropometric measures and thus affects bone growth [61].
3.1.5. Maternal Smoking in Pregnancy
Eight studies analyzed maternal smoking in pregnancy [31,52,54,63,64,65,66,67] (Table 9). However, its influence was confirmed only in one study, where the authors have observed lower neonatal WB BMD [31]. Two studies suggested a positive association between maternal smoking and BMD, but those associations were no longer significant after adjusting several factors, such as current weight [66,67].

Table 9.
Maternal smoking in pregnancy and offspring bone outcomes.
- Potential Mechanisms of Associations between Maternal Smoking in Pregnancy and Offspring Bone Outcomes
Smoking in pregnancy has extremely harmful effects on a fetus, even if the negative effect on BMD was not observed in several studies [52,54,64,65]. Moreover, maternal smoking has been related to adverse effects on bone structure, so the negative influence of smoking in utero may be independent of BMD [63]. Adverse effects of maternal smoking are mostly caused by impaired placental functions and calcium transport, as well as the toxic effect of cadmium [31,52,68,69]. It is worth mentioning that according to some authors maternal smoking affects birth weight or weight gain later in life, and thus has an adverse indirect effect on bone parameters in offspring [65,66]. Moreover, a similar assumption was made in a recent systematic review by Jensen et al. [29].
3.2. Birth-Related Factors
3.2.1. Birth Anthropometry
Birth anthropometry might be another factor that contributes to bone outcomes and was analyzed in fifteen studies, presented in Table 10 [10,23,24,31,42,53,54,56,62,63,64,65,70,71,72].

Table 10.
Birth anthropometry and offspring bone outcomes.
The ponderal index was analyzed in one study and was positively related to WB, but not spine BMD [31].
Four studies assessed birth length [24,56,62,70]. In two studies it was not related to offspring BMD [24,56], in another study a positive association was observed with neonatal bone SOS [62] and in the final study birth length was positively related to BMD at only one site [70].
Fourteen studies investigated birth weight [10,23,31,42,53,54,56,62,63,64,65,70,71,72]. In one study, neonatal bone SOS was lower in infants with a lower birth weight in comparison to those with a higher one [42]. In addition, in the study by Heppe et al. [10], BMD was also lower in children with a lower birth weight, but authors found no association between weight to gestational age and BMD. Six studies found no association [23,54,63,64,70,72]. In five studies, birth weight was positively associated with offspring bone parameters (in one study only at one site [53]) [31,56,62,65]. Moreover, Akcakus et al. [71] found that children that were born large for gestational age (LGA) had higher BMD in comparison to those that were born small for gestational age (SGA).
- Potential Mechanisms of Associations between Birth Anthropometry and Offspring Bone Outcomes
A possible mechanism of the association between birth anthropometry and bone outcomes may be the result of hormonal changes. Fetal growth restriction probably has a negative effect on the growth hormone/insulin-like growth factor 1 (GH/IGF-1) axis, which has been deemed an important determinant of bone mass acquisition [73]. Higher IGF-1 levels are related to higher bone mass and this may be a reason of lower bone mass in children who were born with a lower birth weight [74]. Moreover, neonates born with a lower birth weight had higher serum cortisol levels [75]. This hormone has been negatively associated with bone mass and its levels may be a determinant of prospectively determined bone loss [10,76]. However, the influence of birth anthropometry, especially birth weight, might be of less importance than subsequent growth, as after adjustment for this factor, associations were no longer significant [63,64].
3.2.2. Gestational Age
The influence of gestational age was investigated in seven studies, presented in Table S2. In the study conducted by Bas et al. [77], children born preterm had lower BMD in comparison to those born at term. In two other studies, no association was found [10,24]. In the remaining four studies, gestational age was positively related to offspring bone parameters [31,42,60,62].
3.3. Ambiguity in Analyzed Studies
In total, the results of 49 studies were summarized in the presented review (Table S3). Maternal vitamin D status and birth anthropometry were assessed in the most studies, 11 [17,18,23,35,36,37,38,39,40,41,42] and 15 [10,23,24,31,42,53,54,56,62,63,64,65,70,71,72], respectively. However, factors that seem to be the most relevant in terms of BMD are dietary intake, other than vitamin D nutrients status, and gestational age, as the plurality of the studies showed positive associations. Despite our major efforts, this review does not cover the entirety and complexity of research in this field and their conflicting results. Overall, this review demonstrates that the first years of life are important for proper bone development and that the analyzed early life factors may influence a child’s bone mineral density to some extent.
Inconclusive results in the studies related to maternal vitamin D status may arise from the fact that both bone measurements and maternal vitamin D levels were performed in different age ranges or week of gestation, respectively. It might be misleading, because 25(OH)D may have a different effect on bone parameters depending on the moment of pregnancy. On the one hand, the third trimester seems to be the most important in terms of bone development [9]. Essential nutrients like calcium and vitamin D are supplied to the fetus intensely in this period [6,12]. On the other hand, the results of Hyde et al. [30] suggest that maternal 25(OH)D levels in early pregnancy may also be very important. Nonetheless, the second trimester is also crucial, as the long bones accelerate their growth during this time [76].
The fact that that maternal vitamin D supplementation in pregnancy did not affect offspring BMD in some of the included studies may be explained in several ways. First of all, studies with null effect on BMD were performed with neonatal patients [11,12,16]. In a study that assessed BMD in older children, aged 3 and 6 years old, a higher head BMD was observed in the group of children whose mothers received 2800 IU of vitamin D [13]. As mentioned in the study by Diogenes et al. [16], infant BMD may decrease in the first months of life, which is a physiological effect, therefore the influence of maternal vitamin D supplementation in pregnancy might not be significant in the youngest children. In addition, this dependence may be supported by a recent systematic review, which reported that maternal 25(OH)D status may increase BMD in young adult offspring [29]. Another explanation is that the groups of mothers in the studies were not equal in terms of vitamin D status at the moment of enrollment to the trials. In addition, the doses of vitamin D and periods of interventions were different [11,12,13,15,16].
Inconsistency in the results of studies regarding maternal dietary intakes may arise from different methodologies (e.g., various FFQ) and the reference period of food intake assessment in the analyzed studies [19,20,21,24,30,31,32,33]. Admittedly, all of the included studies used FFQ, however questionnaires were adjusted to examined populations, thus there might be differences in obtained data, depending on the type of applied FFQ. Nonetheless, FFQs that were applied were in majority validated (Table S1). In some studies [19,20,21,24,31,32,33] semiquantitative FFQs were used, thus authors could estimate quantities of foods eaten and/or nutrients intakes [78]. FFQ is a commonly used method to estimate selected food items usually eaten, which is characterized by the low cost of processing, respondent burden, and requires little time [78,79]. Moreover, FFQ is suitable for studies on large populations [78]. Nevertheless, it has limitations, including the possibility of inaccurate quantification of food intake, and requires memory of food patterns in the past. Taken together, results regarding maternal food intake during pregnancy should be interpreted cautiously.
It also remains inconclusive through which mechanisms maternal diet in pregnancy may play a role in offspring bone development. On the one hand, it may be due to individual nutrients, on the other hand, the effect of an overall healthier diet. Moreover, it has been suggested that maternal diet in pregnancy is more likely to have an impact on bone health by long-term metabolic programming than specific mechanisms in utero, as a very small amount of bone is laid down during pregnancy [20,21]. In addition, it needs to be considered that in the analyzed studies only quantitative data were assessed (Table S1). Both inadequate and excessive nutrients intake may have adverse effects on bone health [17,50], nonetheless included studies aimed at the assessment of the association between dietary intake, not sufficient/insufficient intakes.
The influence of birth anthropometry on bone outcomes seems to be temporal, as changes were observed more often in neonates and young children than in older offspring [53,64,70,71]. Moreover, after adjusting for weight gain, differences were no longer significant [63,64]. For example, in the study by Ay et al. [53], catch-up weight in the first six weeks decreased the possibility of low BMD. So, even if a low birth weight may reflect an adverse intrauterine environment, and thus limited bone development in utero, this effect might be observed only in early childhood and may not be independent of weight gain later in life [63]. Furthermore, the results of systematic reviews suggest that birth weight affects adult BMD to a much lesser extent than BMC [80,81]. All things considered, it seems that weight and height gain later in life, as well as environmental factors, may be more important in terms of bone development, rather than birth anthropometry. In addition, it might not be possible to estimate the effects of birth weight, growth, and current weight independently of each other, because they are related [70]. However, there is still considerable ambiguity with regard to the influence of later weight and height, as Foley et al. [26] found that bone mass tracks largely independently of linear growth.
In addition, Choi et al. [82] showed an association between parental and offspring BMD, suggesting that peak bone mass acquisition may be influenced by genetic factors rather than environmental. Therefore, poor bone health may cause negative implications for further generations, as children of adults with lower BMD may also be at risk of lower BMD. This review has raised the need for further observations, especially longitudinal studies to assess the trajectory of changes in BMD levels during childhood and adolescence in regard to the analyzed factors.
3.4. Future Directions
The results of the included studies have several possible implications that should be taken into consideration. Because BMD levels in childhood may be related to those in adolescence [26], considerable attention should be paid to improving BMD levels in the first years of life. Especially considering that according to Jones et al. [70], bone growth trajectory is determined in this early life period. Moreover, BMD is one of the factors that contributes to peak bone mass, which has been suggested to be a predictor of osteoporosis risk in further life [7,25]. Taken together, the early life period may have a long-term influence on bone health. Because some of the assessed factors may be modifiable (i.a. nutrition or smoking during pregnancy), education programs directed at pregnant women would be beneficial, as those factors may also influence other aspects of a child’s development [2,83,84]. Hence, further attention should be paid to prevention strategies. These should focus on the improvement of nutritional status in women in the preconceptional and prenatal periods as well as on proper health care during pregnancy.
Future research is therefore required, especially well-designed studies. The obtained results of further high-quality studies would enable us to create recommendations aimed at improving bone health from the very first years of life, and in turn, to lower the risk of osteoporosis in the elderly.
Strengths and Limitations
The main strength of the presented review is that we analyzed many factors that have been suggested in previous studies to influence BMD. Moreover, we included studies that assessed not only BMD but also related parameters, such as aBMD, vBMD, and SOS, thus it was possible to obtain a broader picture of those associations. The fact that the presented study did not include criteria regarding age or method of bone assessment may be considered both a strength and a limitation. Nonetheless, our work clearly has some limitations, which need to be considered. First, our review does not meet the criteria for a full systematic review, which is why, despite our major effort in the search process, some studies might not have been included. Second, BMD may be underestimated in children because of their short stature and should be corrected for bone size (BMAD) [53]. Thus, BMAD may be more appropriate parameter than aBMD, which is derived from the bone area and may limit the use of DXA for example in children with abnormal growth patterns [85,86]. Nonetheless, in the study by Kalkwarf et al. [87], the authors demonstrated the practicability of measuring aBMD in children aged 1–3 years old, and they provided values for this parameter that can be used in the evaluation of bone deficits. Anyway, DXA measurements do not allow us to assess “true” density, as it would require the consideration of tridimensional bone depth. BMD value is in fact the BMC/bone area (BA) index and in DXA it is possible to obtain only a bidimensional BA [88]. Nevertheless, as BMD demonstrated a low variability during childhood, children with a low BMD are more likely to also have a low BMD later in life [86]. Taking into consideration the above, as well as that BMD may be related to fracture risk in healthy children and contributes to peak bone strength, BMD seems to be an important and evolving field in the children bone health assessment [8,86]. More interestingly, the results in one study have also suggested that TB BMD may be mostly associated with prenatal factors, whereas LS BMD with postnatal factors [53], and in our review we included various parameters. It should also be emphasized that some factors may influence bone development via other parameters than bone density. For example, the mechanism of the association between birth anthropometry may be through bone size rather than density, as birth anthropometry made significant contributions to BMD but not BMAD after adjustment for subsequent growth [70].
4. Conclusions
This review revealed that maternal and birth-related factors may influence children’s bone mineral density. Nonetheless, our paper has highlighted the inconsistencies in the foregoing studies. The findings of this review suggest that factors during prenatal period, such as maternal nutritional status, which may be a reflection of overall healthier diet, as well as factors related to gestation or birth, like gestational age and birth parameters, through direct and indirect mechanisms may contribute to offspring’s bone health. This paper has underlined the importance of early life period in bone development. Although some studies reported no association with bone outcomes, factors affecting prenatal period may have long-term consequences on children’s bone health, as they may act through indirect mechanisms.
In 2010, more than 20 million women and five million men were estimated to have osteoporosis in the European Union and the number of fractures caused by this disease has been estimated as 3.5 million. Moreover, the economic burden of incidents and prior fragility fractures was estimated at € 37 billion in the European Union and a worrying finding is that the costs are expected to increase by 25% by 2025 [89]. This would appear to indicate that osteoporosis should be widely considered as a growing social, economic and health problem. Thus, a decrease in the number of patients with osteoporosis would enable us to save resources in health care and, more importantly, increase the quality of life in the elderly.
This review has underlined the importance of early bone health prophylaxis and actions aimed at ensuring optimal bone development. We identified the need to reinforce communications to young women aimed at emphasizing the importance of the prenatal period for a child’s optimal development.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13072302/s1, Table S1: Detailed data about dietary assessment method, maternal dietary intake in pregnancy and type of data used for statistical analysis. Table S2: Gestational age and offspring bone outcomes. Table S3: The summary of included studies. References [90,91,92,93,94] are cited in the Supplementary Materials.
Author Contributions
D.M.-K. conceptualized the paper and conducted the literature search. D.M.-K. and M.A.Z.-P. were responsible for data extraction. D.M.-K. wrote the original paper. D.M.-K. and M.A.Z.-P. were responsible for visualization. J.H. and M.A.Z.-P. reviewed and edited the paper. J.H. was responsible for revising the manuscript critically for important intellectual content and obtained the funding. The manuscript was revised by all co-authors. All authors have read and agreed to the published version of the manuscript.
Funding
The study was financially supported by sources of the Polish Ministry of Sciences and Higher Education within funds of the Institute of Human Nutrition Sciences, Warsaw University of Life Sciences (WULS), for scientific research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AA | arachidonic acid |
| aBMD | areal bone mineral density |
| AGA | appropriate for gestational age |
| ALSPAC | Avon Longitudinal Study of Parents and Children |
| BA | bone area |
| BMAD | bone mineral apparent density |
| BMC | bone mineral content |
| BMD | bone mineral density |
| BMI | body mass index |
| COPSAC2010 | Copenhagen Prospective Studies on Asthma in Childhood |
| d | days |
| DNA | deoxyribonucleic acid |
| DOHaD | Developmental Origins of the Health and Disease hypothesis |
| DPA | docosapentaenoic acid |
| DXA | dual-energy X-ray absorptiometry |
| EPA | eicosapentaenoic acid |
| F | female |
| FFQ | food frequency questionnaire |
| FN | femoral neck |
| FT | full-term |
| GH | growth hormone |
| GOOD | Gothenburg Osteoporosis and Obesity Determinants study |
| GWG | gestational weight gain |
| HRpQCT | high-resolution peripheral quantitative computed tomography |
| IGF-1 | insulin-like growth factor 1 |
| IU | International Units |
| JKB | Japan Kids Body-composition Study |
| LC PUFAs | long chain polyunsaturated fatty acids |
| LGA | large for gestational age |
| LP | late preterm |
| LS | lumbar spine |
| M | males |
| m | months |
| MAVIDOS | Maternal Vitamin D Osteoporosis Study |
| PDS | Prudent Diet Score |
| PMNS | Pune Maternal Nutrition Study |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PT | preterm |
| pQCT | peripheral quantitative computed tomography |
| QUS | quantitative ultrasound |
| SES | socioeconomic status |
| SGA | small for gestational age |
| SOS | speed of sound |
| SWS | Southampton Women’s Survey |
| TB | total body |
| TBLH | total body less head |
| WB | whole body |
| WBLH | whole body less head |
| wk | week |
| vBMD | volumetric bone mineral density |
| VIP | Vitamin D in Pregnancy study |
| y | years |
| 1,25(OH)2D | 1,25-dihydroxyvitamin D |
| 25(OH)D | 25-hydroxyvitamin D |
References
- Barker, D.J.P. In utero programming of chronic disease. Clin. Sci. 1998, 95, 115–128. [Google Scholar] [CrossRef]
- Schwarzenberg, S.J.; Georgieff, M.K. Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health. Pediatrics 2018, 141, 1–10. [Google Scholar] [CrossRef]
- Lacagnina, S. The Developmental Origins of Health and Disease (DOHaD). Am. J. Lifestyle Med. 2020, 14, 47–50. [Google Scholar] [CrossRef]
- Zhu, Z.; Cao, F.; Li, X. Epigenetic programming and fetal metabolic programming. Front. Endocrinol. 2019, 10, 764. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Brands, B.; Grote, V.; Kirchberg, F.F.; Prell, C.; Rzehak, P.; Uhl, O.; Weber, M. Long-term health impact of early nutrition: The power of programming. Ann. Nutr. Metab. 2017, 70, 161–169. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, H.; McGowan, A.; Ahmed, S.F. Establishing good bone health in children. Paediatr. Child Health. 2014, 24, 78–82. [Google Scholar] [CrossRef]
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: A lifecourse approach. J. Bone Miner. Res. 2014, 29, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.; Schoenmakers, I.; Ann Laskey, M.; de Bono, S.; Ginty, F.; Goldberg, G.R. Symposium on „Nutrition and health in children and adolescents” Session 1: Nutrition in growth and development. Nutrition and bone growth and development. Proc. Nutr. Soc. 2006, 65, 348–360. [Google Scholar] [CrossRef]
- Dennison, E.M.; Cooper, C.; Cole, Z.A. Early development and osteoporosis and bone health. J. Dev. Orig. Health Dis. 2010, 1, 142–149. [Google Scholar] [CrossRef]
- Heppe, D.H.M.; Medina-Gomez, C.; de Jongste, J.C.; Raat, H.; Steegers, E.A.P.; Hofman, A.; Rivadeneira, F.; Jaddoe, V.W.V. Fetal and childhood growth patterns associated with bone mass in school-age children: The Generation R study. J. Bone Miner. Res. 2014, 29, 2584–2593. [Google Scholar] [CrossRef]
- Cooper, C.; Harvey, N.C.; Bishop, N.J.; Kennedy, S.; Papageorghiou, A.T.; Schoenmakers, I.; Fraser, R.; Gandhi, S.V.; Carr, A.; D’Angelo, S.; et al. Maternal gestational vitamin D supplementation and offspring bone health: A multicentre, double-blind, randomised placebo-controlled trial (MAVIDOS). Lancet Diabetes Endocrinol. 2016, 4, 393–402. [Google Scholar] [CrossRef]
- Vaziri, F.; Dabbaghmanesh, M.H.; Samsami, A.; Nasiri, S.; Shirazi, P.T. Vitamin D supplementation during pregnancy on infant anthropometric measurements and bone mass of mother-infant pairs: A randomized placebo clinical trial. Early Hum. Dev. 2016, 103, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Brustad, N.; Garland, J.; Thorsen, J.; Sevelsted, A.; Krakauer, M.; Vinding, R.K.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Chawes, B.L. Effect of high-dose vs standard-dose vitamin D supplementation in pregnancy on bone mineralization in offspring until age 6 years: A prespecified secondary analysis of a double-blinded, randomized clinical trial. JAMA Pediatr. 2020, 174, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.A.; Jarjou, L.; Prentice, A. Long-term effects of maternal calcium supplementation on childhood growth differ between males and females in a population accustomed to a low calcium intake. Bone 2017, 103, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Katam, K.K.; Das, V.; Agarwal, A.; Bhatia, V. Maternal vitamin D supplementation in pregnancy and offspring outcomes: A double-blind randomized placebo-controlled trial. J. Bone Miner. Metab. 2017, 35, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Diogenes, M.E.L.; Bezerra, F.F.; Rezende, E.P.; Donangelo, C.M. Calcium plus vitamin d supplementation during third trimester of pregnancy in adolescents accustomed to low calcium diets did not affect infant bone mass at early lactation in a randomized controlled trial. J. Nutr. 2015, 145, 1515–1523. [Google Scholar] [CrossRef]
- Boghossian, N.S.; Koo, W.; Liu, A.; Mumford, S.L.; Tsai, M.Y.; Yeung, E.H. Longitudinal measures of maternal vitamin D and neonatal body composition. Eur. J. Clin. Nutr. 2019, 73, 424–431. [Google Scholar] [CrossRef]
- Javaid, M.K.; Crozier, S.R.; Harvey, N.C.; Gale, C.R.; Dennison, E.M.; Boucher, B.J.; Arden, N.K.; Godfrey, K.M.; Cooper, C. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: A longitudinal study. Lancet 2006, 367, 36–43. [Google Scholar] [CrossRef]
- Heppe, D.H.M.; Medina-Gomez, C.; Hofman, A.; Franco, O.H.; Rivadeneira, F.; Jaddoe, V.W.V. Maternal first-trimester diet and childhood bone mass: The Generation R Study. Am. J. Clin. Nutr. 2013, 98, 224–232. [Google Scholar] [CrossRef]
- Jones, G.; Riley, M.D.; Dwyer, T. Maternal diet during pregnancy is associated with bone mineral density in children: A longitudinal study. Eur. J. Clin. Nutr. 2000, 54, 749–756. [Google Scholar] [CrossRef]
- Yin, J.; Dwyer, T.; Riley, M.; Cochrane, J.; Jones, G. The association between maternal diet during pregnancy and bone mass of the children at age 16. Eur. J. Clin. Nutr. 2010, 64, 131–137. [Google Scholar] [CrossRef]
- Händel, M.N.; Moon, R.J.; Titcombe, P.; Abrahamsen, B.; Heitmann, B.L.; Calder, P.C.; Dennison, E.M.; Robinson, S.M.; Godfrey, K.M.; Inskip, H.M.; et al. Maternal serum retinol and β-carotene concentrations and neonatal bone mineralization: Results from the Southampton Women’s Survey cohort. Am. J. Clin. Nutr. 2016, 104, 1183–1188. [Google Scholar] [CrossRef]
- Balasuriya, C.N.D.; Larose, T.L.; Mosti, M.P.; Evensen, K.A.I.; Jacobsen, G.W.; Thorsby, P.M.; Stunes, A.K.; Syversen, U. Maternal serum retinol, 25(OH)D and 1,25 (OH)2D concentrations during pregnancy and peak bone mass and trabecular bone score in adult offspring at 26-year follow-up. PLoS ONE 2019, 14, 1–15. [Google Scholar] [CrossRef]
- Ganpule, A.; Yajnik, C.S.; Fall, C.H.D.; Rao, S.; Fisher, D.J.; Kanade, A.; Cooper, C.; Naik, S.; Joshi, N.; Lubree, H.; et al. Bone mass in Indian children—Relationships to maternal nutritional status and diet during pregnancy: The Pune Maternal Nutrition Study. J. Clin. Endocrinol. Metab. 2006, 91, 2994–3001. [Google Scholar] [CrossRef] [PubMed]
- Weiler, H.A.; Yuen, C.K.; Seshia, M.M. Growth and bone mineralization of young adults weighing less than 1500 g at birth. Early Hum. Dev. 2002, 67, 101–112. [Google Scholar] [CrossRef]
- Foley, S.; Quinn, S.; Jones, G. Tracking of bone mass from childhood to adolescence and factors that predict deviation from tracking. Bone 2009, 44, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Vierucci, F.; Saggese, G.; Cimaz, R. Osteoporosis in childhood. Curr. Opin. Rheumatol. 2017, 29, 535–546. [Google Scholar] [CrossRef]
- Baroncelli, G.I. Quantitative ultrasound methods to assess bone mineral status in children: Technical characteristics, performance, and clinical application. Pediatr. Res. 2008, 63, 220–228. [Google Scholar] [CrossRef]
- Jensen, K.H.; Riis, K.R.; Abrahamsen, B.; Händel, M.N. Nutrients, diet, and other factors in prenatal life and bone health in young adults: A systematic review of longitudinal studies. Nutrients 2020, 12, 2866. [Google Scholar] [CrossRef]
- Hyde, N.K.; Brennan-Olsen, S.L.; Wark, J.D.; Hosking, S.M.; Pasco, J.A. Maternal dietary nutrient intake during pregnancy and offspring linear growth and bone: The Vitamin D in Pregnancy Cohort Study. Calcif. Tissue Int. 2017, 100, 47–54. [Google Scholar] [CrossRef]
- Godfrey, K.; Walker-Bone, K.; Robinson, S.; Taylor, P.; Shore, S.; Wheeler, T.; Cooper, C. Neonatal bone mass: Influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J. Bone Miner. Res. 2001, 16, 1694–1703. [Google Scholar] [CrossRef]
- Tobias, J.H.; Steer, C.D.; Emmett, P.M.; Tonkin, R.J.; Cooper, C.; Ness, A.R. Bone mass in childhood is related to maternal diet in pregnancy. Osteoporos. Int. 2005, 16, 1731–1741. [Google Scholar] [CrossRef]
- Cole, Z.A.; Gale, C.R.; Javaid, M.K.; Robinson, S.M.; Law, C.; Boucher, B.J.; Crozier, S.R.; Godfrey, K.M.; Dennison, E.M.; Cooper, C. Maternal dietary patterns during pregnancy and childhood bone mass: A longitudinal study. J. Bone Miner. Res. 2009, 24, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Vinding, R.K.; Stokholm, J.; Sevelsted, A.; Sejersen, T.; Chawes, B.L.; Bønnelykke, K.; Thorsen, J.; Howe, L.D.; Krakauer, M.; Bisgaard, H. Effect of fish oil supplementation in pregnancy on bone, lean, and fat mass at six years: Randomised clinical trial. BMJ 2018, 362, 3312. [Google Scholar] [CrossRef] [PubMed]
- Velkavrh, M.; Paro-Panjan, D.; Benedik, E.; Mis, N.F.; Godnov, U.; Salamon, A.S. The influence of maternal levels of vitamin D and adiponectin on anthropometrical measures and bone health in offspring. Pril. Makedon. Akad. Nauk. Umet. Odd. Med. Nauk. 2019, 40, 91–98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prentice, A.; Jarjou, L.M.A.; Goldberg, G.R.; Bennett, J.; Cole, T.J.; Schoenmakers, I. Maternal plasma 25-hydroxyvitamin D concentration and birthweight, growth and bone mineral accretion of Gambian infants. Acta Paediatr. 2009, 98, 1360–1362. [Google Scholar] [CrossRef] [PubMed]
- Viljakainen, H.T.; Korhonen, T.; Hytinantti, T.; Laitinen, E.K.A.; Andersson, S.; Mäkitie, O.; Lamberg-Allardt, C. Maternal vitamin D status affects bone growth in early childhood—A prospective cohort study. Osteoporos. Int. 2011, 22, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.H.; Erler, N.S.; Jaddoe, V.W.V.; Tiemeier, H.; van den Hooven, E.H.; Franco, O.H.; Rivadeneira, F.; Voortman, T. 25-hydroxyvitamin D concentrations during fetal life and bone health in children aged 6 years: A population-based prospective cohort study. Lancet Diabetes Endocrinol. 2017, 5, 367–376. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Wills, A.K.; Fraser, A.; Sayers, A.; Fraser, W.D.; Tobias, J.H. Association of maternal vitamin D status during pregnancy with bone-mineral content in offspring: A prospective cohort study. Lancet 2013, 381, 2176–2183. [Google Scholar] [CrossRef]
- Zhu, K.; Whitehouse, A.J.O.; Hart, P.H.; Kusel, M.; Mountain, J.; Lye, S.; Pennell, C.; Walsh, J.P. Maternal vitamin D status during pregnancy and bone mass in offspring at 20 years of age: A prospective cohort study. J. Bone Miner. Res. 2014, 29, 1088–1095. [Google Scholar] [CrossRef]
- Hyde, N.K.; Brennan-Olsen, S.L.; Mohebbi, M.; Wark, J.D.; Hosking, S.M.; Pasco, J.A. Maternal vitamin D in pregnancy and offspring bone measures in childhood: The Vitamin D in Pregnancy study. Bone 2019, 124, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.P.; Zhang, W.L.; Yan, C.H.; Zhou, X.J.; Wang, P.; Sun, J.H.; Yu, X.D.; Wu, M.Q. Reduced tibial speed of sound in Chinese infants at birth compared with Caucasian peers: The effects of race, gender, and vitamin D on fetal bone development. Osteoporos. Int. 2010, 21, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.; Dhanwal, D.; Robinson, S.; Kim, M.; Inskip, H.; Godfrey, K.; Dennison, E.; Calder, P.; Cooper, C. Does maternal long chain polyunsaturated fatty acid status in pregnancy influence the bone health of children? The Southampton Women’s Survey. Osteoporos. Int. 2012, 23, 2359–2367. [Google Scholar] [CrossRef]
- Javaid, M.K.; Godfrey, K.M.; Taylor, P.; Shore, S.R.; Breier, B.; Arden, N.K.; Cooper, C. Umbilical venous IGF-1 concentration, neonatal bone mass, and body composition. J. Bone Miner. Res. 2004, 19, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Corwin, R.L.; Hartman, T.J.; Maczuga, S.A.; Graubard, B.I. Dietary Saturated fat intake is inversely associated with bone density in humans: Analysis of NHANES III. J. Nutr. 2006, 136, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A.M. Magnesium and osteoporosis: Current state of knowledge and future research directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Rusińska, A.; Płudowski, P.; Walczak, M.; Borszewska-Kornacka, M.K.; Bossowski, A.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dobrzańska, A.; Franek, E.; Helwich, E.; et al. Vitamin D supplementation guidelines for general population and groups at risk of vitamin D deficiency in Poland—Recommendations of the Polish Society of Pediatric Endocrinology and Diabetes and the Expert Panel with participation of national specialist consultans and representatives of scientific societes—2018 update. Front. Endocrinol. 2018, 9, 1–21. [Google Scholar] [CrossRef]
- Longo, A.B.; Ward, W.E. PUFAs, bone mineral density, and fragility fracture: Findings from human studies. Adv. Nutr. 2016, 7, 299–312. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A. Vitamin A and bone health: The balancing act. J. Clin. Densitom. 2013, 16, 414–419. [Google Scholar] [CrossRef]
- Fratoni, V.; Brandi, M.L. B vitamins, Homocysteine and Bone Health. Nutrients 2015, 7, 2176–2192. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.C.; Javaid, M.K.; Arden, N.K.; Poole, J.R.; Crozier, S.R.; Robinson, S.M.; Inskip, H.M.; Godfrey, K.M.; Dennison, E.M.; Cooper, C. Maternal predictors of neonatal bone size and geometry: The Southampton Women’s Survey. J. Dev. Orig. Health Dis. 2010, 1, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ay, L.; Jaddoe, V.W.V.; Hofman, A.; Moll, H.A.; Raat, H.; Steegers, E.A.P.; Hokken-Koelega, A.C.S. Foetal and postnatal growth and bone mass at 6 months: The Generation R Study. Clin. Endocrinol. 2011, 74, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Rudäng, R.; Mellström, D.; Clark, E.; Ohlsson, C.; Lorentzon, M. Advancing maternal age is associated with lower bone mineral density in young adult male offspring. Osteoporos. Int. 2012, 23, 475–482. [Google Scholar] [CrossRef]
- Monjardino, T.; Henriques, A.; Moreira, C.; Rodrigues, T.; Adubeiro, N.; Nogueira, L.; Cooper, C.; Santos, A.C.; Lucas, R. Gestational weight gain and offspring bone mass: Different associations in healthy weight versus overweight women. J. Bone Miner. Res. 2019, 34, 38–48. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, Z.; Wang, H.; Ding, M.; Zhou, A.; Wang, X.; Zhang, P.; Duggan, C.; Hu, F.B. Bone mineral density of the spine in 11,898 Chinese infants and young children: A cross-sectional study. PLoS ONE 2013, 8, 1–6. [Google Scholar] [CrossRef]
- Andres, A.; Hull, H.R.; Shankar, K.; Casey, P.H.; Cleves, M.A.; Badger, T.M. Longitudinal body composition of children born to mothers with normal weight, overweight, and obesity. Obesity 2015, 23, 1252–1258. [Google Scholar] [CrossRef]
- Fujita, Y.; Kouda, K.; Ohara, K.; Nakamura, H.; Iki, M. Maternal pre-pregnancy underweight is associated with underweight and low bone mass in school-aged children. J. Bone Miner. Metab. 2020, 38, 878–884. [Google Scholar] [CrossRef]
- Macdonald-Wallis, C.; Tobias, J.H.; Smith, G.D.; Lawlor, D.A. Relation of maternal prepregnancy body mass index with offspring bone mass in childhood: Is there evidence for an intrauterine effect? Am. J. Clin. Nutr. 2010, 92, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Abou Samra, H.; Stevens, D.; Binkley, T.; Specker, B. Determinants of bone mass and size in 7-year-old former term, late-preterm, and preterm boys. Osteoporos. Int. 2009, 20, 1903–1910. [Google Scholar] [CrossRef]
- Nabulsi, M.; Mahfoud, Z.; Maalouf, J.; Arabi, A.; Fuleihan, G.E.H. Impact of maternal veiling during pregnancy and socioeconomic status on offspring’s musculoskeletal health. Osteoporos. Int. 2008, 19, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Pereda, L.; Ashmeade, T.; Zaritt, J.; Carver, J.D. The use of quantitative ultrasound in assessing bone status in newborn preterm infants. J. Perinatol. 2003, 23, 655–659. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, F.; Dwyer, T.; Antony, B.; Winzenberg, T.; Jones, G. Associations of breastfeeding, maternal smoking, and birth weight with bone density and microarchitecture in young adulthood: A 25-year birth-cohort study. J. Bone Miner. Res. 2020, 35, 1652–1659. [Google Scholar] [CrossRef]
- Jones, G.; Hynes, K.L.; Dwyer, T. The association between breastfeeding, maternal smoking in utero, and birth weight with bone mass and fractures in adolescents: A 16-year longitudinal study. Osteoporos. Int. 2013, 24, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mesa, J.; Menezes, A.M.B.; Howe, L.D.; Wehrmeister, F.C.; Muniz, L.C.; González-Chica, D.A.; Assunção, M.C.; Gonçalves, H.; Barros, F.C. Lifecourse relationship between maternal smoking during pregnancy, birth weight, contemporaneous anthropometric measurements and bone mass at 18 years old. The 1993 Pelotas Birth Cohort. Early Hum. Dev. 2014, 90, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Heppe, D.H.M.; Medina-Gomez, C.; Hofman, A.; Rivadeneira, F.; Jaddoe, V.W.V. Does fetal smoke exposure affect childhood bone mass? The Generation R Study. Osteoporos. Int. 2015, 26, 1319–1329. [Google Scholar] [CrossRef]
- Macdonald-Wallis, C.; Tobias, J.H.; Smith, G.D.; Lawlor, D.A. Parental smoking during pregnancy and offspring bone mass at age 10 years: Findings from a prospective birth cohort. Osteoporos. Int. 2011, 22, 1809–1819. [Google Scholar] [CrossRef]
- Shiverick, K.T.; Salafia, C. Cigarette smoking and pregnancy. I: Ovarian, uterine and placental effects. Placenta 1999, 20, 265–272. [Google Scholar] [CrossRef]
- Lin, F.J.; Fitzpatrick, J.W.; Iannotti, C.A.; Martin, D.S.; Mariani, B.D.; Tuan, R.S. Effects of cadmium on trophoblast calcium transport. Placenta 1997, 18, 341–356. [Google Scholar] [CrossRef]
- Jones, G.; Dwyer, T. Birth weight, birth length, and bone density in prepubertal children: Evidence for an association that may be mediated by genetic factors. Calcif. Tissue Int. 2000, 67, 304–308. [Google Scholar] [CrossRef]
- Akcakus, M.; Koklu, E.; Kurtoglu, S.; Kula, M.; Koklu, S.S. The relationship among intrauterine growth, insulinlike growth factor I (IGF-I), IGF-Binding Protein-3, and bone mineral status in newborn infants. Am. J. Perinatol. 2006, 23, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Micklesfield, L.; Levitt, N.; Dhansay, M.; Norris, S.; van der Merwe, L.; Lambert, E. Maternal and early life influences on calcaneal ultrasound parameters and metacarpal morphometry in 7- to 9-year-old children. J. Bone Miner. Metab. 2006, 24, 235–242. [Google Scholar] [CrossRef]
- Setia, S.; Sridhar, M.G. Changes in GH/IGF-1 axis in intrauterine growth retardation: Consequences of fetal programming? Horm. Metab. Res. 2009, 41, 791–798. [Google Scholar] [CrossRef]
- Jones, I.E.; Williams, S.M.; Goulding, A. Associations of birth weight and length, childhood size, and smoking with bone fractures during growth: Evidence from a birth cohort study. Am. J. Epidemiol. 2004, 159, 343–350. [Google Scholar] [CrossRef]
- van Montfoort, N.; Finken, M.J.J.; le Cessie, S.; Dekker, F.W.; Wit, J.M. Could cortisol explain the association between birth weight and cardiovascular disease in later life? A meta-analysis. Eur. J. Endocrinol. 2005, 153, 811–817. [Google Scholar] [CrossRef]
- Cooper, C.; Westlake, S.; Harvey, N.; Javaid, K.; Dennison, E.; Hanson, M. Review: Developmental origins of osteoporotic fracture. Osteoporos. Int. 2006, 17, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Baş, E.K.; Bülbül, A.; Şirzai, H.; Arslan, S.; Uslu, S.; Baş, V.; Zubarioglu, U.; Celik, M.; Dursun, M.; Güran, Ö.; et al. The long-term impacts of preterm birth and associated morbidities on bone health in preschool children: A prospective cross-sectional study from Turkey. J. Matern. Neonatal Med. 2020, 1–8. [Google Scholar] [CrossRef]
- Biró, G.; Hulsof, K.F.A.M.; Ovesen, L.; Amorim Cruz, J.A. Selection of methodology to assess food intake. Eur. J. Clin. Nutr. 2002, 56, 25–32. [Google Scholar] [CrossRef]
- Illner, A.K.; Freisling, H.; Boeing, H.; Huybrechts, I.; Crispim, S.P.; Slimani, N. Review and evaluation of innovative technologies for measuring diet in nutritional epidemiology. Int. J. Epidemiol. 2012, 41, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Schlüssel, M.M.; dos Santos Vaz, J.; Kac, G. Birth weight and adult bone mass: A systematic literature review. Osteoporos. Int. 2010, 21, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.; Kurshid, M.A.; Kim, M.; Harvey, N.; Dennison, E.; Cooper, C. Does birthweight predict bone mass in adulthood? A systematic review and meta-analysis. Osteoporos. Int. 2011, 22, 1323–1334. [Google Scholar] [CrossRef]
- Choi, H.S.; Park, J.H.; Kim, S.H.; Shin, S.; Park, M.J. Strong familial association of bone mineral density between parents and offspring: KNHANES 2008–2011. Osteoporos. Int. 2017, 28, 955–964. [Google Scholar] [CrossRef]
- Borge, T.C.; Aase, H.; Brantsæter, A.L.; Biele, G. The importance of maternal diet quality during pregnancy on cognitive and behavioural outcomes in children: A systematic review and meta-analysis. BMJ Open 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- McGrath-Morrow, S.A.; Gorzkowski, J.; Groner, J.A.; Rule, A.M.; Wilson, K.; Tanski, S.E.; Collaco, J.M.; Klein, J.D. The effects of nicotine on development. Pediatrics 2020, 145, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shalof, H.; Dimitri, P.; Shuweihdi, F.; Offiah, A.C. Which skeletal imaging modality is best for assessing bone health in children and young adults compared to DXA? A systematic review and meta-analysis. Bone 2021, 150, 116013. [Google Scholar] [CrossRef] [PubMed]
- Khalatbari, H.; Binkovitz, L.A.; Parisi, M.T. Dual-energy X-ray absorptiometry bone densitometry in pediatrics: A practical review and update. Pediatr. Radiol. 2021, 51, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Kalkwarf, H.J.; Zemel, B.S.; Yolton, K.; Heubi, J.E. Bone mineral content and density of the lumbar spine of infants and toddlers: Influence of age, sex, race, growth and human milk feeding. J. Bone Miner. Res. 2013, 28, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Avila-Díaz, M.; Flores-Huerta, S.; Martínez-Muñiz, I.; Amato, D. Increments in whole body bone mineral content associated with weight and length in pre-term and full-term infants during the first 6 months of life. Arch. Med. Res. 2001, 32, 288–292. [Google Scholar] [CrossRef]
- Svedbom, A.; Hernlund, E.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: A compendium of country-specific reports. Arch. Osteoporos. 2013, 8, 1–218. [Google Scholar] [CrossRef]
- Klipstein-Grobusch, K.; den Breeijen, J.H.; Goldbohm, R.A.; Geleijnse, J.M.; Hofman, A.; Grobbee, D.E.; Witteman, J.C.M. Dietary assessment in the elderly: Validation of a semiquantitative food frequency questionnaire. Eur. J. Clin. Nutr. 1998, 52, 588–596. [Google Scholar] [CrossRef]
- Rogers, I.; Emmett, P. and the ALPSAC Study Team. Diet during pregnancy in a population of pregnant women in South West England. Eur. J. Clin. Nutr. 1998, 52, 246–250. [Google Scholar] [CrossRef]
- Robinson, S.; Godfrey, K.; Osmond, C.; Cox, V.; Barker, D. Evaluation of a food frequency questionnaire used to assess nutrient intakes in pregnant women. Eur. J. Clin. Nutr. 1996, 50, 302–308. [Google Scholar] [PubMed]
- Rao, S.; Yajnik, C.S.; Kanade, A.; Fall, C.H.D.; Margetts, B.M.; Jackson, A.A.; Shier, R.; Joshi, S.; Rege, S.; Lubree, H.; et al. Intake of micronutrient-rich foods in rural Indian mothers is associated with the size of their babies at birth: Pune Maternal Nutrition Study. J. Nutr. 2001, 131, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Cancer Council Victoria. Dietary Questionnaires. Available online: https://www.cancervic.org.au/research/epidemiology/nutritional_assessment_services (accessed on 13 June 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).