Temporal Dynamics of T Helper Populations in the Proximal Small Intestine after Oral Bovine Lactoferrin Administration in BALB/c Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Animal Diet
2.3. Bovine Lactoferrin
2.4. Experimental Design
2.5. Collection of Samples
2.6. Immunoglobulin Measurement
2.7. Peyer Patches and Lamina Propria Lymphocytes Isolation
2.8. Flow Cytometry Analysis
2.9. Statistical Analysis
3. Results
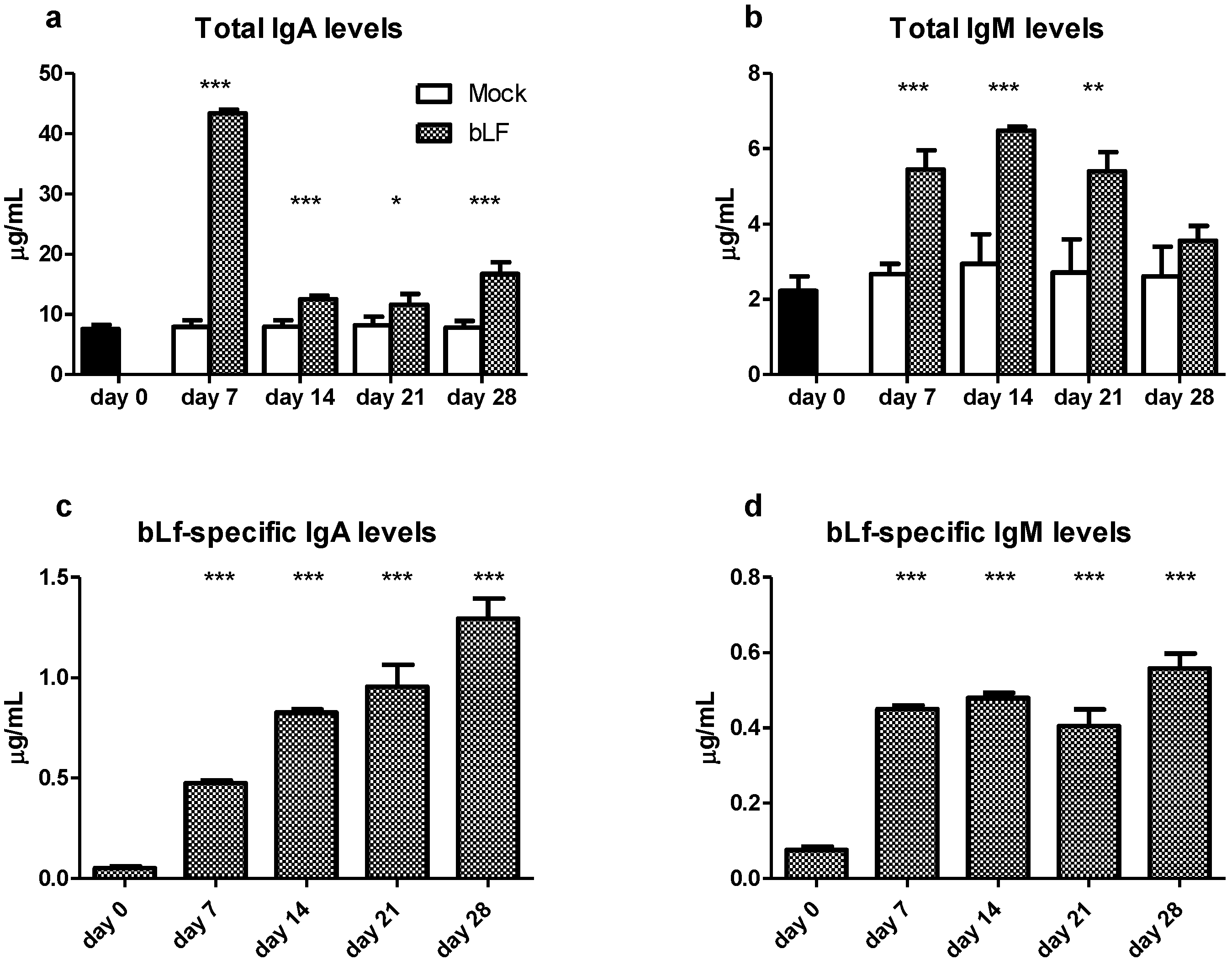
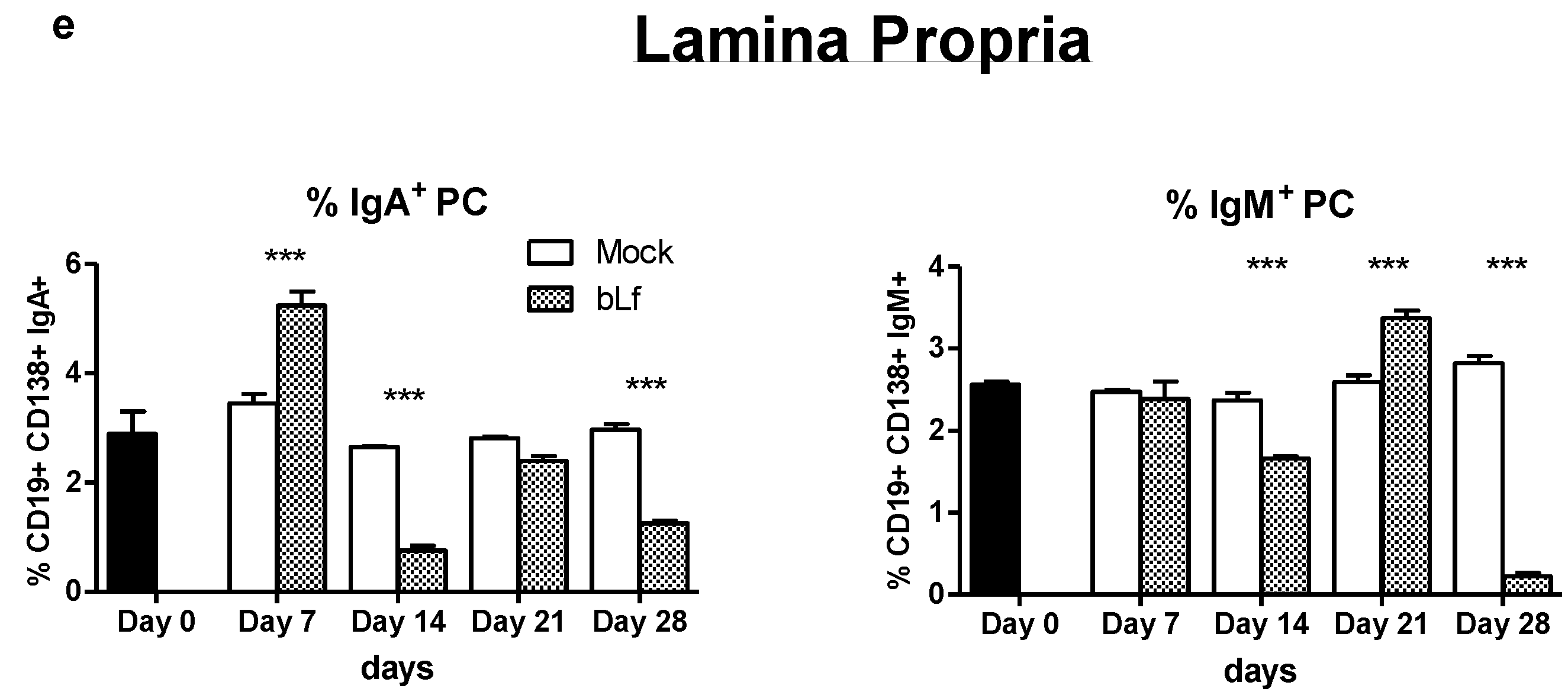
3.1. Oral bLf Increases the Total and Specific IgA and IgM Levels and Modulates the IgA+ and IgM+ PC Populations in the Proximal Small Intestine
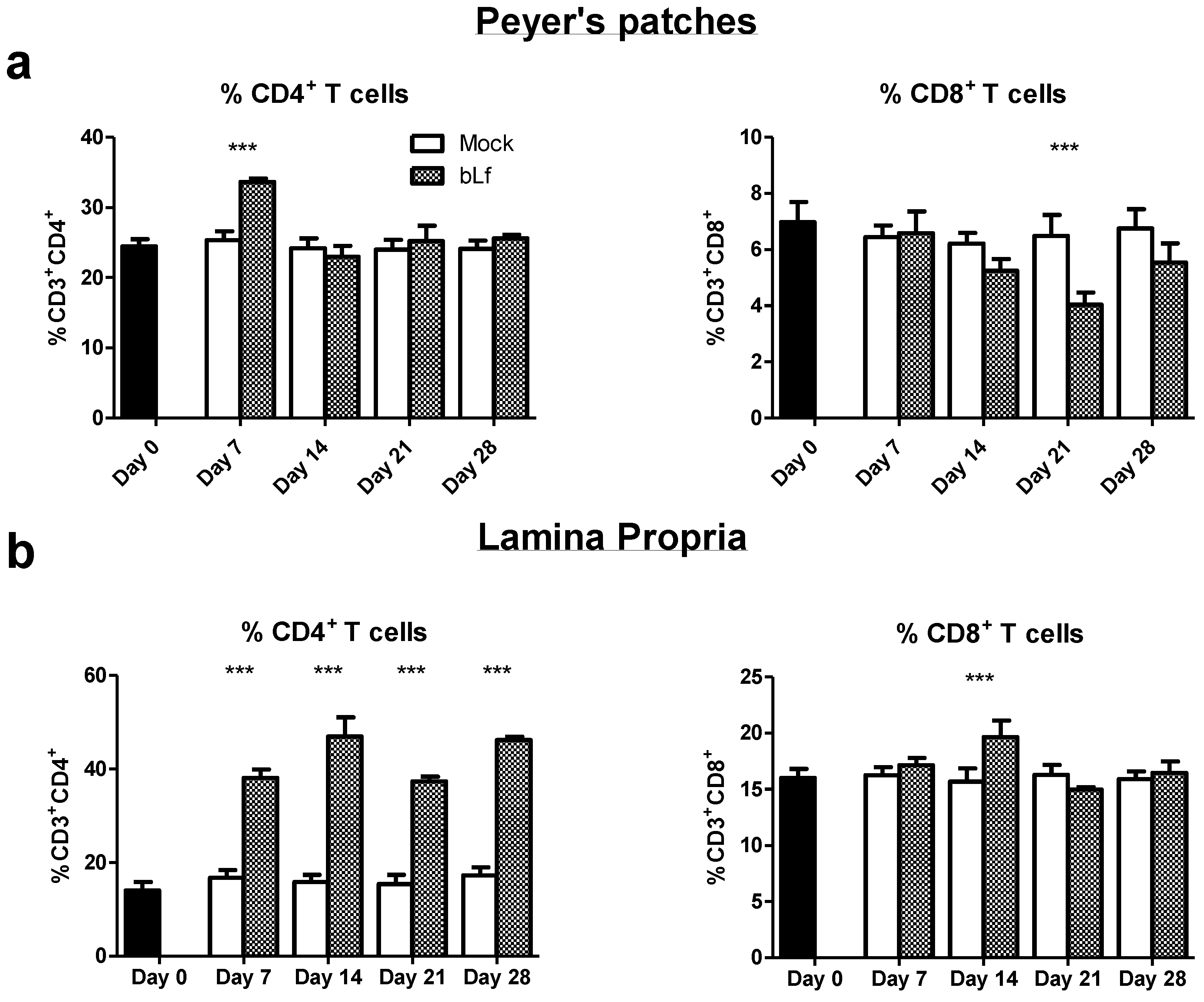
3.2. Oral bLf Modulates the Percentage of T Populations in the Effector and Inductor Compartments of the Proximal Small Intestine
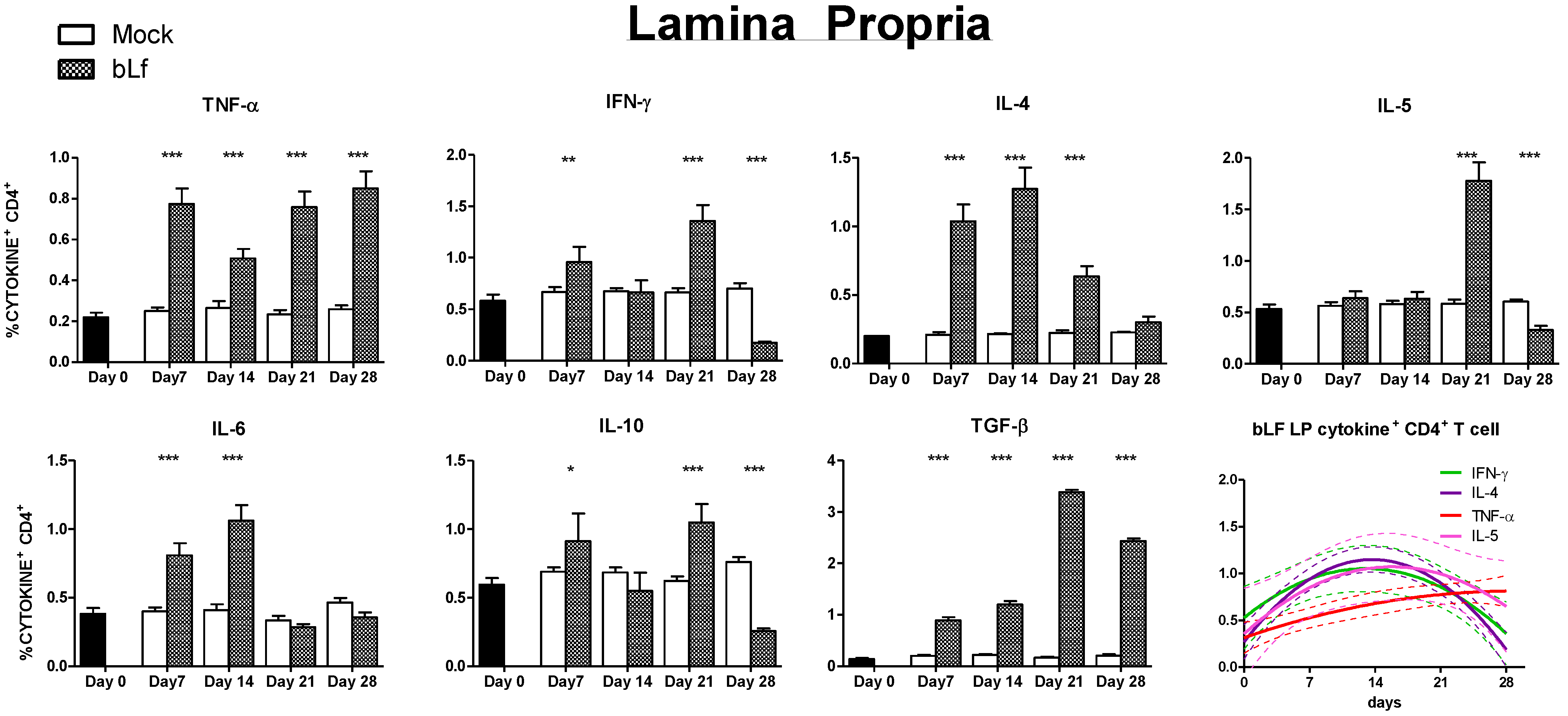
3.3. Oral bLf Modulates the Cytokine-Producing CD4+ T Cell Populations in the Effector and Inductor Compartments of the Proximal Small Intestine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, E.N.; Baker, H.M. Molecular structure, binding properties and dynamics of lactoferrin. Cell Mol. Life Sci. 2005, 62, 2531–2539. [Google Scholar] [CrossRef]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Actor, J.K.; Hwang, S.-A.; Kruzel, M. Lactoferrin as a Natural Immune Modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legrand, D. Overview of Lactoferrin as a Natural Immune Modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimecki, M.; Mazurier, J.; Spik, G.; Kapp, J.A. Lactoferrin inhibits proliferative response and cytokine production of TH1 but not TH2 cell lines. Arch. Immunol. Ther. Exp. 1996, 44, 51–56. [Google Scholar]
- Sfeir, R.M.; Dubarry, M.; Boyaka, P.; Rautureau, M.; Tomé, D. The Mode of Oral Bovine Lactoferrin Administration Influences Mucosal and Systemic Immune Responses in Mice. J. Nutr. 2004, 134, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pena-Juarez, M.C.; Campos-Rodriguez, R.; Godinez-Victoria, M.; Cruz-Hernandez, T.R.; Reyna-Garfias, H.; Barbosa-Cabrera, R.E.; Drago-Serrano, M.E. Effect of Bovine Lactoferrin Treatment Followed by Acute Stress on the IgA-Response in Small Intestine of BALB/c Mice. Immunol. Investig. 2016, 45, 652–667. [Google Scholar] [CrossRef]
- Arciniega-Martinez, I.M.; Campos-Rodriguez, R.; Drago-Serrano, M.E.; Sánchez-Torres, L.E.; Hernandez, T.R.C.; Reséndiz-Albor, A.A. Modulatory Effects of Oral Bovine Lactoferrin on the IgA Response at Inductor and Effector Sites of Distal Small Intestine from BALB/c Mice. Arch. Immunol. Ther. Exp. 2015, 64, 57–63. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Rivera-Aguilar, V.; Reséndiz-Albor, A.A.; Campos-Rodriguez, R. Lactoferrin increases both resistance to Salmonella typhimurium infection and the production of antibodies in mice. Immunol. Lett. 2010, 134, 35–46. [Google Scholar] [CrossRef]
- Debbabi, H.; Dubarry, M.; Rautureau, M.; Tomé, D. Bovine lactoferrin induces both mucosal and systemic immune response in mice. J. Dairy Res. 1998, 65, 283–293. [Google Scholar] [CrossRef]
- Takakura, N.; Wakabayashi, H.; Yamauchi, K.; Takase, M. Influences of orally administered lactoferrin on IFN-gamma and IL-10 production by intestinal intraepithelial lymphocytes and mesenteric lymph-node cells. Biochem. Cell Biol. 2006, 84, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Kuhara, T.; Iigo, M.; Itoh, T.; Ushida, Y.; Sekine, K.; Terada, N.; Okamura, H.; Tsuda, H. Orally Administered Lactoferrin Exerts an Antimetastatic Effect and Enhances Production of IL-18 in the Intestinal Epithelium. Nutr. Cancer 2000, 38, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Iigo, M.; Alexander, D.B.; Long, N.; Xu, J.; Fukamachi, K.; Futakuchi, M.; Takase, M.; Tsuda, H. Anticarcinogenesis pathways activated by bovine lactoferrin in the murine small intestine. Biochimie 2009, 91, 86–101. [Google Scholar] [CrossRef]
- Togawa, J.; Nagase, H.; Tanaka, K.; Inamori, M.; Umezawa, T.; Nakajima, A.; Naito, M.; Sato, S.; Saito, T.; Sekihara, H. Lac-toferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. Am. J. Physiol. Gastrointest. Liver Physiol 2002, 283, G187–G195. [Google Scholar] [CrossRef] [PubMed]
- Agace, W.W.; McCoy, K.D. Regionalized Development and Maintenance of the Intestinal Adaptive Immune Landscape. Immunity 2017, 46, 532–548. [Google Scholar] [CrossRef] [Green Version]
- Senda, T.; Dogra, P.; Granot, T.; Furuhashi, K.; Snyder, M.E.; Carpenter, D.J.; Szabo, P.A.; Thapa, P.; Miron, M.; Farber, D.L. Microanatomical dissection of human intestinal T-cell immunity reveals site-specific changes in gut-associated lymphoid tissues over life. Mucosal Immunol. 2018, 12, 378–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godínez-Victoria, M.; Hernandez, T.R.C.; Reyna-Garfias, H.; Barbosa-Cabrera, R.E.; Drago-Serrano, M.E.; Sánchez-Gómez, M.C.; Campos-Rodríguez, R. Modulation by bovine lactoferrin of parameters associated with the IgA response in the proximal and distal small intestine of BALB/c mice. Immunopharmacol. Immunotoxicol. 2017, 39, 66–73. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pr. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; de Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Mutwiri, G.; Watts, T.; Lew, L.; Beskorwayne, T.; Papp, Z.; Baca-Estrada, M.E.; Griebel, P. Ileal and jejunal Peyer’s patches play distinct roles in mucosal immunity of sheep. Immunology 1999, 97, 455–461. [Google Scholar] [CrossRef]
- McGhee, J.R.; Fujihashi, K. Inside the Mucosal Immune System. PLoS Biol. 2012, 10, e1001397. [Google Scholar] [CrossRef] [Green Version]
- Reséndiz-Albor, A.A.; Esquivel, R.; López-Revilla, R.; Verdín, L.; Moreno-Fierros, L. Striking phenotypic and functional differences in lamina propria lymphocytes from the large and small intestine of mice. Life Sci. 2005, 76, 2783–2803. [Google Scholar] [CrossRef]
- Wang, X.; Hao, G.-L.; Wang, B.-Y.; Gao, C.-C.; Wang, Y.-X.; Li, L.-S.; Xu, J.-D. Function and dysfunction of plasma cells in intestine. Cell Biosci. 2019, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Tindemans, I.; Joosse, M.E.; Samsom, J.N. Dissecting the Heterogeneity in T-Cell Mediated Inflammation in IBD. Cells 2020, 9, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giansanti, F.; Panella, G.; Leboffe, L.; Antonini, G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals 2016, 9, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Superti, F. Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients 2020, 12, 2562. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Campos-Rodriguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin and Peptide-derivatives: Antimi-crobial Agents with Potential Use in Nonspecific Immunity Modulation. Curr. Pharm. Des. 2018, 24, 1067–1078. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakajima, A. New aspects of IgA synthesis in the gut. Int. Immunol. 2014, 26, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Mesin, L.; Di Niro, R.; Thompson, K.M.; Lundin, K.E.A.; Sollid, L.M. Long-Lived Plasma Cells from Human Small Intestine Biopsies Secrete Immunoglobulins for Many Weeks In Vitro. J. Immunol. 2011, 187, 2867–2874. [Google Scholar] [CrossRef] [Green Version]
- Brucklacher-Waldert, V.; Carr, E.; Linterman, M.; Veldhoen, M. Cellular Plasticity of CD4+ T Cells in the Intestine. Front. Immunol. 2014, 5, 488. [Google Scholar] [CrossRef] [Green Version]
- Bauche, D.; Marie, J.C. Transforming growth factor beta: A master regulator of the gut microbiota and immune cell interac-tions. Clin. Transl. Immunol. 2017, 6, e136. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Debbabi, H.; Dubarry, M.; Boyaka, P.; Tome, D. Regulation of physiological and pathological Th1 and Th2 re-sponses by lactoferrin. Biochem. Cell Biol. 2006, 84, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Deng, Y.Q.; Ren, J.; Xiao, B.K.; Chen, Z.; Tao, Z.Z. Lactoferrin Administration into the Nostril Alleviates Murine Allergic Rhinitis and its Mechanisms. Scand. J. Immunol. 2013, 78, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Herbert, D.R.; Douglas, B.; Zullo, K. Group 2 Innate Lymphoid Cells (ILC2): Type 2 Immunity and Helminth Immunity. Int. J. Mol. Sci. 2019, 20, 2276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leon-Sicairos, N.; Martinez-Pardo, L.; Sanchez-Hernandez, B.; de la Garza, M.; Carrero, J.C. Oral lactoferrin treatment re-solves amoebic intracecal infection in C3H/HeJ mice. Biochem. Cell Biol. 2012, 90, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Takamura, N.; Shinohara, M.; Wakui, N.; Shin, H.; Sumino, Y.; Ohmoto, Y.; Teraguchi, S.; Yamauchi, K. Long-term follow-up of chronic hepatitis C patients treated with oral lactoferrin for 12 months. Hepatol. Res. 2003, 25, 226–233. [Google Scholar] [CrossRef]
- Sherman, M.P. Lactoferrin and Necrotizing Enterocolitis. Clin. Perinatol. 2013, 40, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Ulloa, P.E.; Solís, C.J.; De La Paz, J.F.; Alaurent, T.G.S.; Caruffo, M.; Hernández, A.J.; Dantagnan, P.; Feijóo, C.G. Lactoferrin Decreases the Intestinal Inflammation Triggered by a Soybean Meal-Based Diet in Zebrafish. J. Immunol. Res. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Harari, Y.; Chen, C.-Y.; Castro, G.A. Lactoferrin Protects Gut Mucosal Integrity During Endotoxemia Induced by Lipopolysaccharide in Mice. Inflammation 2000, 24, 33–44. [Google Scholar] [CrossRef]
- Doursout, M.-F.; Horton, H.; Hoang, L.; Liang, Y.; Hwang, S.-A.; Boyd, S.; Actor, J.K.; Kruzel, M.L. Lactoferrin moderates LPS-induced hypotensive response and gut injury in rats. Int. Immunopharmacol. 2013, 15, 227–231. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, J.; Xiao, D.; Shu, G.; Gu, L. Bovine Lactoferrin Protects Dextran Sulfate Sodium Salt Mice Against Inflammation and Impairment of Colonic Epithelial Barrier by Regulating Gut Microbial Structure and Metabolites. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef]
- Shouval, D.S.; Ouahed, J.; Biswas, A.; Goettel, J.A.; Horwitz, B.H.; Klein, C.; Muise, A.M.; Snapper, S.B. Interleukin 10 receptor signaling: Master regulator of intestinal mucosal homeostasis in mice and humans. Adv. Immunol. 2014, 122, 177–210. [Google Scholar]
- Jankovic, D.; Kugler, D.G.; Sher, A. IL-10 production by CD4+ effector T cells: A mechanism for self-regulation. Mucosal Immunol. 2010, 3, 239–246. [Google Scholar] [CrossRef]
- Sofi, M.H.; Li, W.; Kaplan, M.H.; Chang, C.H. Elevated IL-6 expression in CD4 T cells via PKCtheta and NF-kappaB induces Th2 cytokine production. Mol. Immunol. 2009, 46, 1443–1450. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, J.; Ren, F.; Zhang, W.; Zhang, H.; Zhao, L.; Zhang, M.; Cui, W.; Wang, X.; Guo, H. Lactoferrin Promotes Oste-ogenesis through TGF-beta Receptor II Binding in Osteoblasts and Activation of Canonical TGF-beta Signaling in MC3T3-E1 Cells and C57BL/6J Mice. J. Nutr. 2018, 148, 1285–1292. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.S.; Seo, G.Y.; Lee, J.M.; Seo, H.Y.; Han, H.J.; Kim, S.J.; Jin, B.R.; Kim, H.J.; Park, S.R.; Rhee, K.J.; et al. Lactoferrin causes IgA and IgG2b isotype switching through betaglycan binding and activation of canonical TGF-beta signaling. Mucosal Immunol. 2015, 8, 906–917. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, W.; Ren, F.; Guo, H. Activation of TGF-beta Canonical and Noncanonical Signaling in Bovine Lactofer-rin-Induced Osteogenic Activity of C3H10T1/2 Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 2880. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Gunasekaran, S.; Saleh, D.M.; Alexander, W.T.; Alexander, D.B.; Ohara, H.; Tsuda, H. Effects of oral bovine lac-toferrin on a mouse model of inflammation associated colon cancer. Biochem. Cell Biol. 2021, 99, 159–165. [Google Scholar] [CrossRef]
- Brockmann, L.; Soukou, S.; Steglich, B.; Czarnewski, P.; Zhao, L.; Wende, S.; Bedke, T.; Ergen, C.; Manthey, C.; Agalioti, T.; et al. Molecular and functional heterogeneity of IL-10-producing CD4+ T cells. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kiner, E.; Willie, E.; Vijaykumar, B.; Chowdhary, K.; Schmutz, H.; Chandler, J.; Schnell, A.; Thakore, P.I.; LeGros, G.; Mo-stafavi, S.; et al. Gut CD4(+) T cell phenotypes are a continuum molded by microbes, not by TH archetypes. Nat. Immunol. 2021, 22, 216–228. [Google Scholar] [CrossRef]
- Tsuji, M.; Suzuki, K.; Kitamura, H.; Maruya, M.; Kinoshita, K.; Ivanov, I.I.; Itoh, K.; Littman, D.R.; Fagarasan, S. Requirement for Lymphoid Tissue-Inducer Cells in Isolated Follicle Formation and T Cell-Independent Immunoglobulin A Generation in the Gut. Immunity 2008, 29, 261–271. [Google Scholar] [CrossRef] [Green Version]
- European Commission. 2012/725/EU: Commission Implementing Decision of 22 November 2012 Authorising the Placing on the Market of Bovine Lactoferrin as a Novel Food Ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council (Morinaga); Official Journal of the European Union 2012, L 327/46; European Commission: Brussels, Belgium, 2012. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ynga-Durand, M.; Tapia-Pastrana, G.; Rebollar-Ruíz, X.A.; Yépez-Ortega, M.; Nieto-Yañez, O.; Arciniega-Martínez, I.M.; Reséndiz-Albor, A.A. Temporal Dynamics of T Helper Populations in the Proximal Small Intestine after Oral Bovine Lactoferrin Administration in BALB/c Mice. Nutrients 2021, 13, 2852. https://doi.org/10.3390/nu13082852
Ynga-Durand M, Tapia-Pastrana G, Rebollar-Ruíz XA, Yépez-Ortega M, Nieto-Yañez O, Arciniega-Martínez IM, Reséndiz-Albor AA. Temporal Dynamics of T Helper Populations in the Proximal Small Intestine after Oral Bovine Lactoferrin Administration in BALB/c Mice. Nutrients. 2021; 13(8):2852. https://doi.org/10.3390/nu13082852
Chicago/Turabian StyleYnga-Durand, Mario, Gabriela Tapia-Pastrana, Xóchitl Abril Rebollar-Ruíz, Mariazell Yépez-Ortega, Oscar Nieto-Yañez, Ivonne Maciel Arciniega-Martínez, and Aldo Arturo Reséndiz-Albor. 2021. "Temporal Dynamics of T Helper Populations in the Proximal Small Intestine after Oral Bovine Lactoferrin Administration in BALB/c Mice" Nutrients 13, no. 8: 2852. https://doi.org/10.3390/nu13082852
APA StyleYnga-Durand, M., Tapia-Pastrana, G., Rebollar-Ruíz, X. A., Yépez-Ortega, M., Nieto-Yañez, O., Arciniega-Martínez, I. M., & Reséndiz-Albor, A. A. (2021). Temporal Dynamics of T Helper Populations in the Proximal Small Intestine after Oral Bovine Lactoferrin Administration in BALB/c Mice. Nutrients, 13(8), 2852. https://doi.org/10.3390/nu13082852





