Pregnancy and Lactation Alter Vitamin A Metabolism and Kinetics in Rats under Vitamin A-Adequate Dietary Conditions
Abstract
1. Introduction
2. Materials and Methods
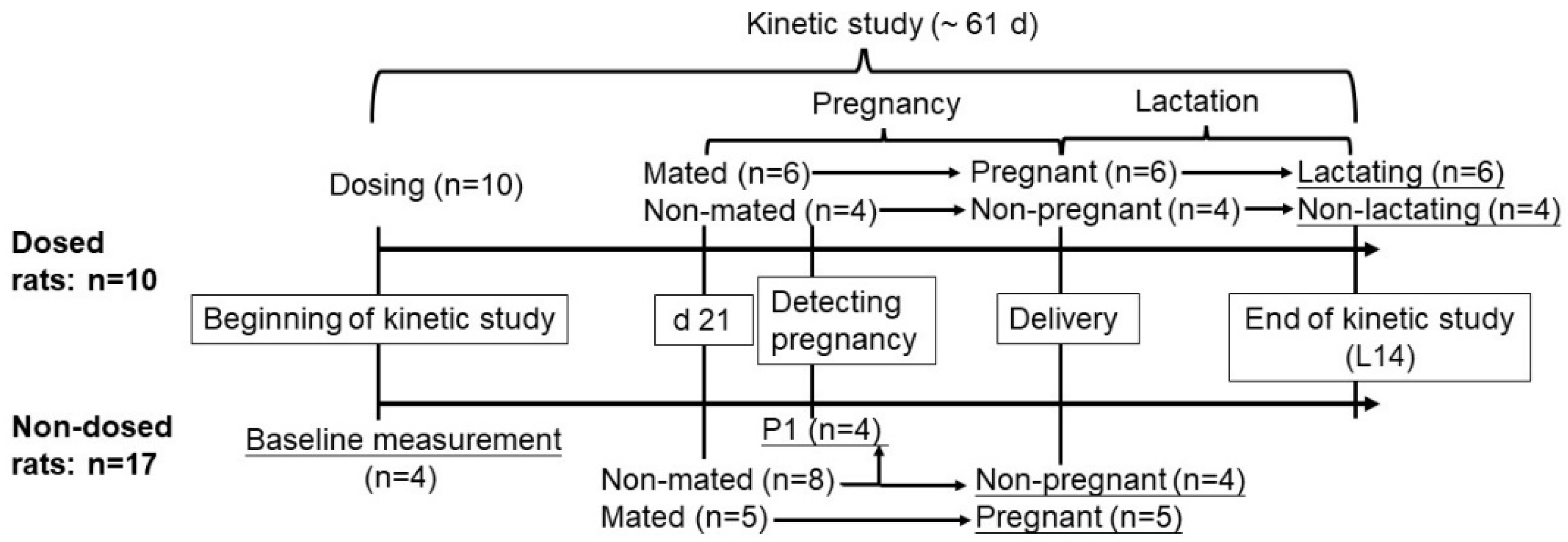
2.1. Animals and Diets
2.2. Preparation of 11,12-3H-Retinol-Labeled Oral Dose
2.3. Kinetic Studies
2.4. VA Mass Quantification
2.5. Radioactivity Measurement
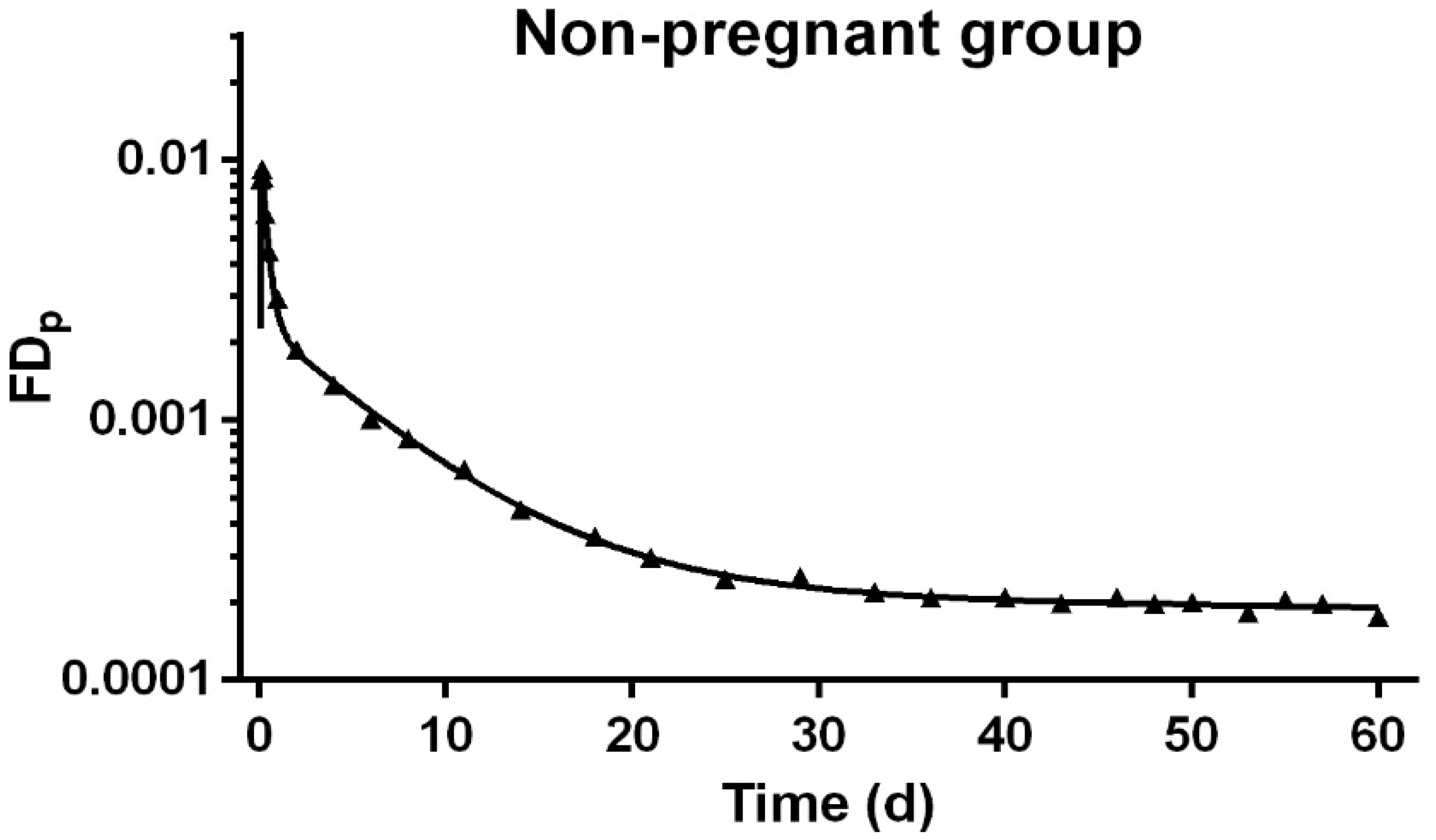
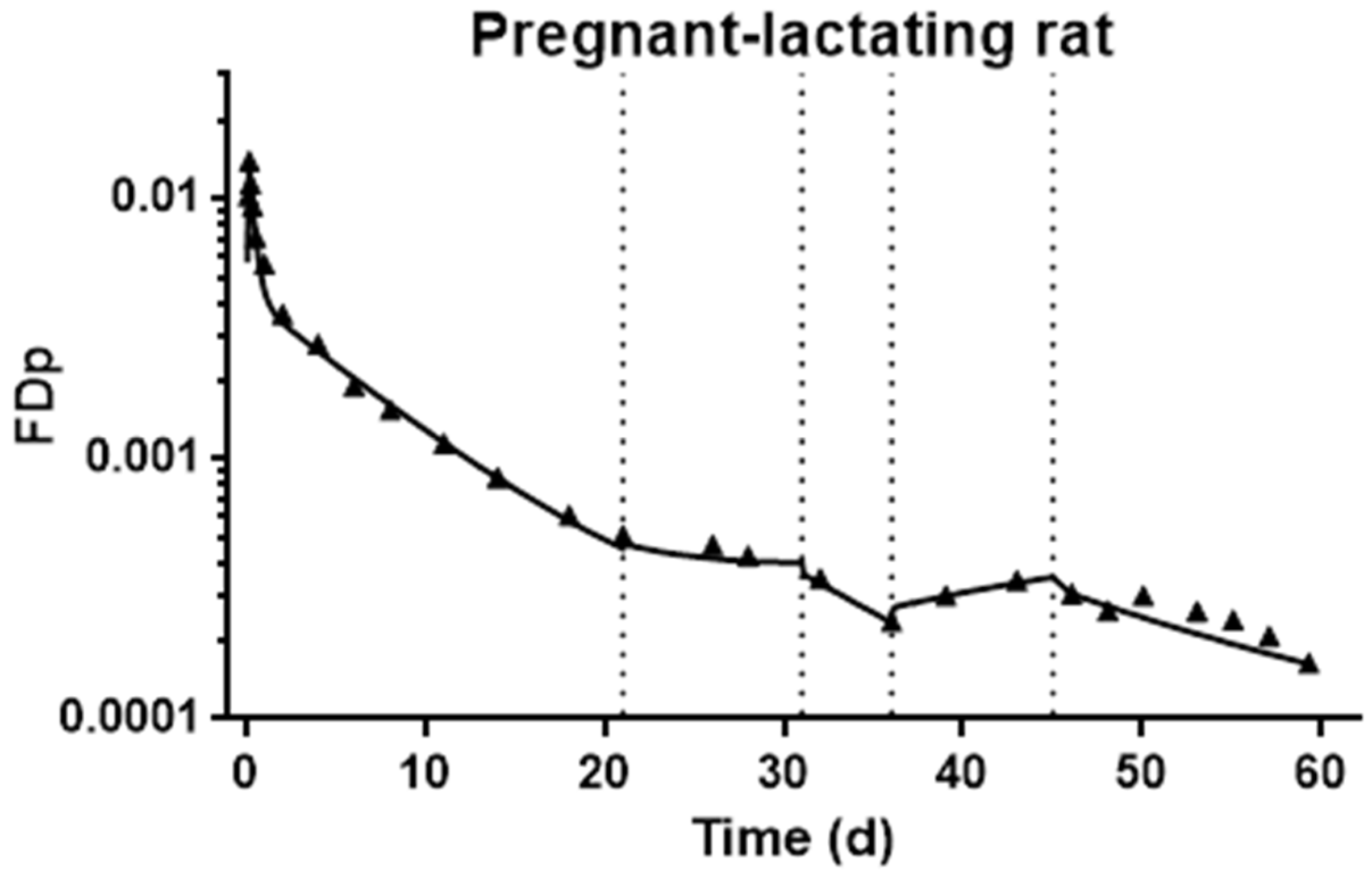
2.6. Kinetic Data Calculations
2.7. Model Development and Kinetic Parameters
2.8. Statistical Analysis
3. Results
3.1. Physiological Outcomes
3.2. Changes in VA Tissue Status during Pregnancy and Lactation
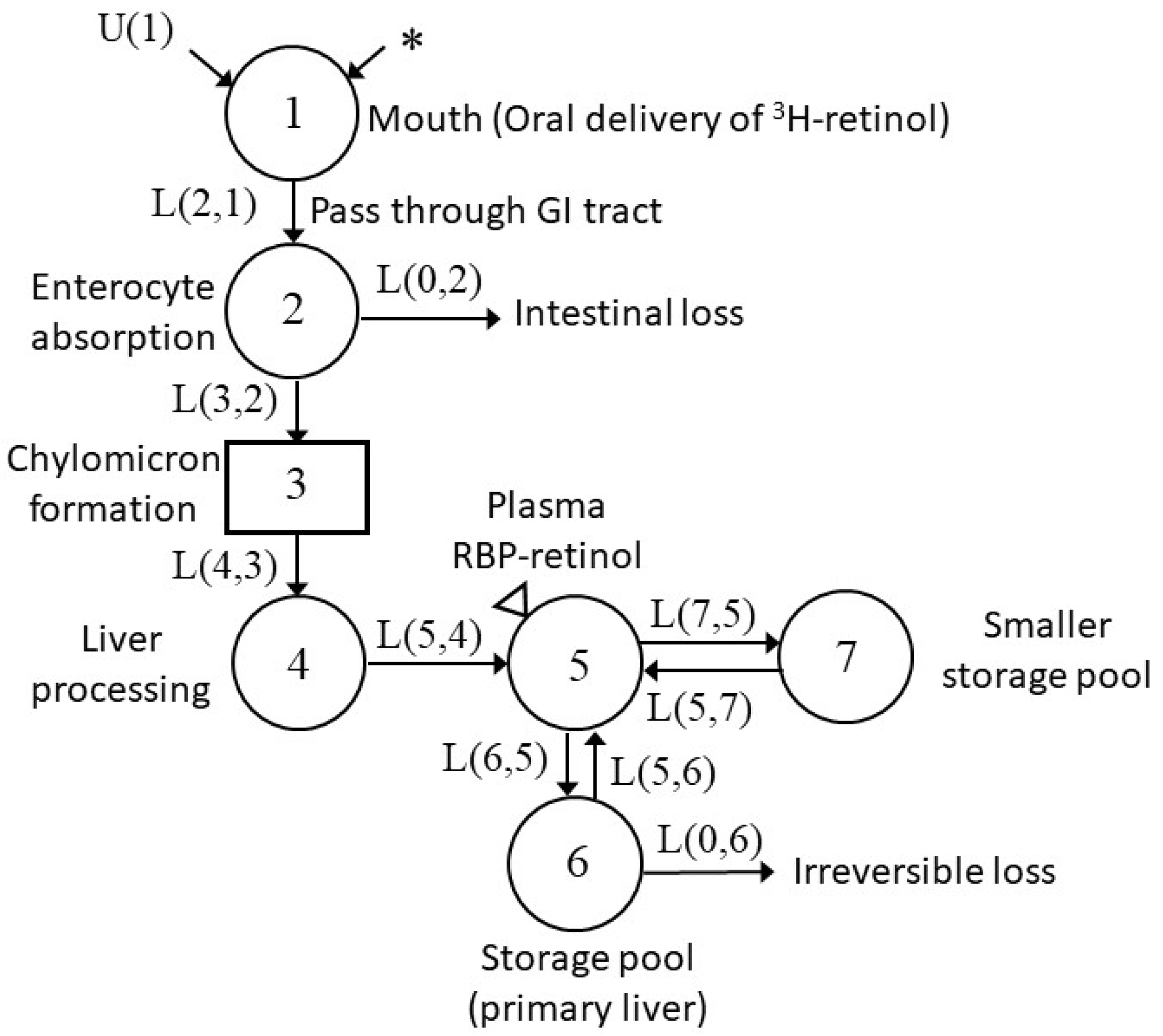
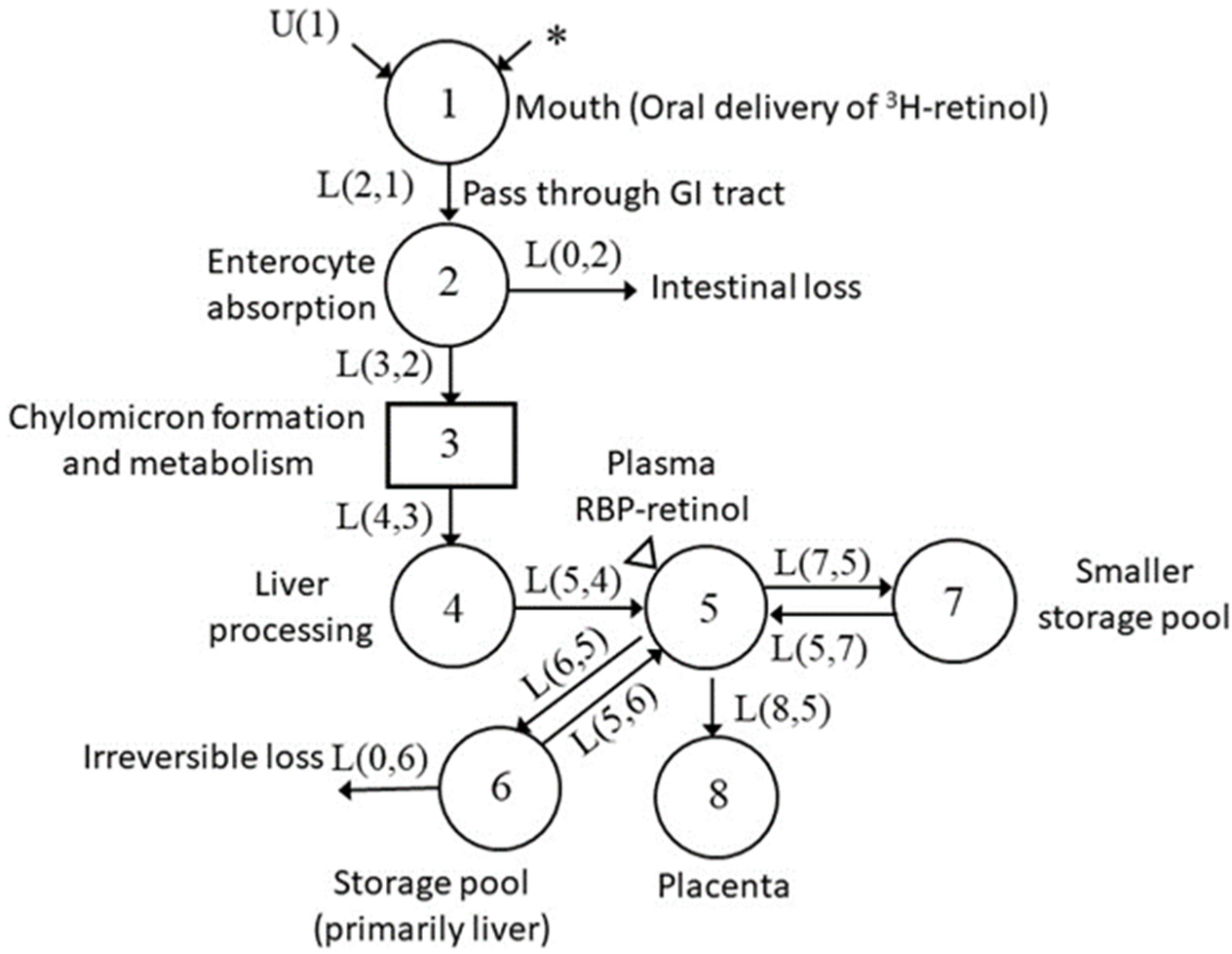
3.3. Development of Compartmental Model for VA Kinetics in Non-Pregnant Rats
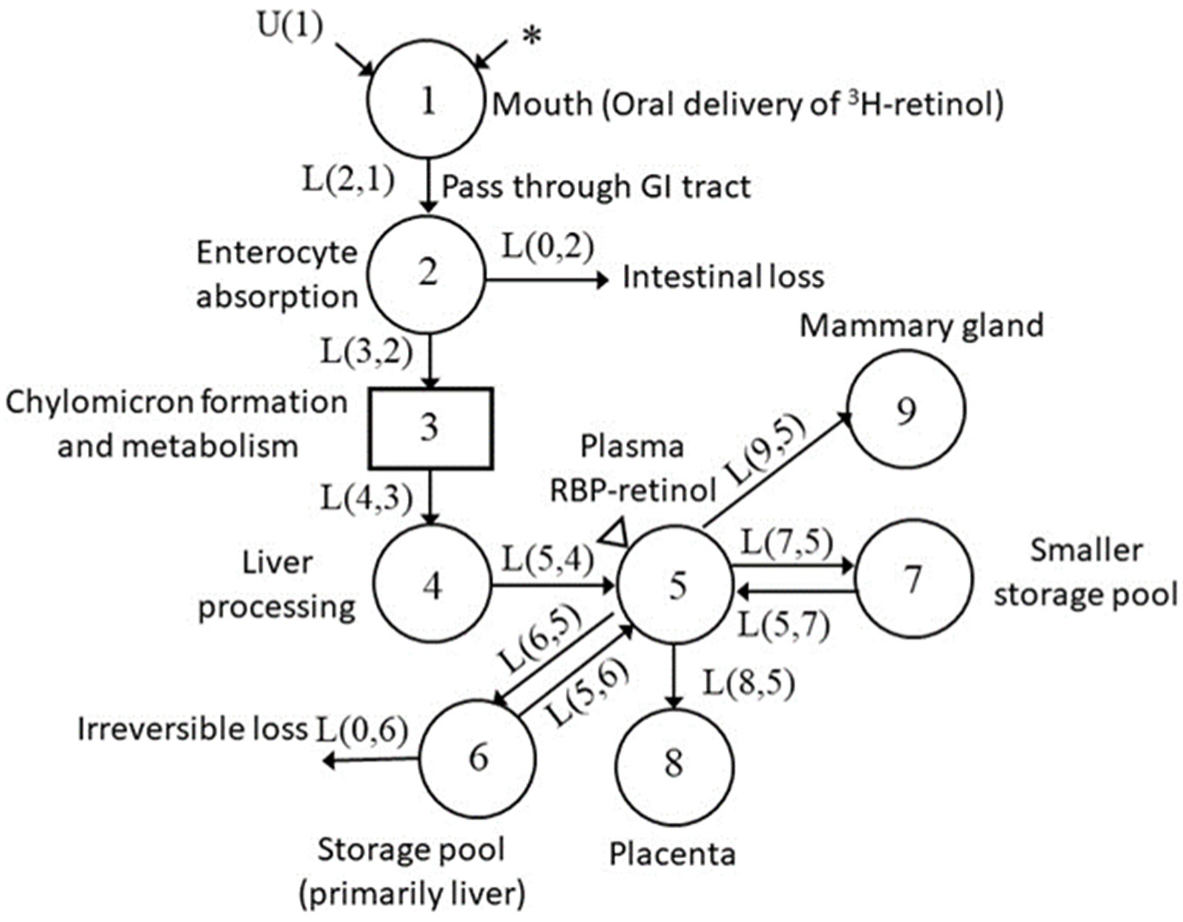
3.4. Development of Compartmental Model for VA Kinetics in Pregnant–Lactating Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ross, A.C.; Gardner, E.M. The function of vitamin A in cellular growth and differentiation, and its roles during pregnancy and lactation. Adv. Exp. Med. Biol. 1994, 352, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Warkany, J.; Schraffenberger, E. Congenital malformations induced in rats by maternal vitamin A deficiency; defects of the eye. Arch. Ophthal. 1946, 35, 150–169. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.G.; Warkany, J. Anomalies of the genito-urinary tract induced by maternal vitamin A deficiency in fetal rats. Anat. Rec. 1947, 97, 376. [Google Scholar]
- Cohlan, S.Q. Excessive intake of vitamin A as a cause of congenital anomalies in the rat. Science 1953, 117, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.G.; Roth, C.B.; Warkany, J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am. J. Anat. 1953, 92, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Nolen, G.A. Variations in teratogenic response to hypervitaminosis A in three strains of the albino rat. Food Cosmet. Toxicol. 1969, 7, 209–214. [Google Scholar] [CrossRef]
- Takahashi, Y.I.; Smith, J.E.; Winick, M.; Goodman, D.S. Vitam A deficiency and fetal growth and development in the rat. J. Nutr. 1975, 105, 1299–1310. [Google Scholar] [CrossRef]
- Geelen, J.A. Hypervitaminosis A induced teratogenesis. CRC Crit. Rev. Toxicol. 1979, 6, 351–375. [Google Scholar] [CrossRef]
- Rothman, K.J.; Moore, L.L.; Singer, M.R.; Nguyen, U.S.; Mannino, S.; Milunsky, A. Teratogenicity of high vitamin A intake. N. Engl. J. Med. 1995, 333, 1369–1373. [Google Scholar] [CrossRef]
- White, J.C.; Shankar, V.N.; Highland, M.; Epstein, M.L.; DeLuca, H.F.; Clagett-Dame, M. Defects in embryonic hindbrain development and fetal resorption resulting from vitamin A deficiency in the rat are prevented by feeding pharmacological levels of all-trans-retinoic acid. Proc. Natl. Acad. Sci. USA 1998, 95, 13459–13464. [Google Scholar] [CrossRef]
- Antipatis, C.; Grant, G.; Ashworth, C.J. Moderate maternal vitamin A deficiency affects perinatal organ growth and development in rats. Br. J. Nutr. 2000, 84, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.I.; Smith, J.E.; Goodman, D.S. Vitamin A and retinol-binding protein metabolism during fetal development in the rat. Am. J. Physiol. 1977, 233, E263–E272. [Google Scholar] [CrossRef] [PubMed]
- Wallingford, J.C.; Underwood, B.A. Vitamin A status needed to maintain vitamin A concentrations in nonhepatic tissues of the pregnant rat. J. Nutr. 1987, 117, 1410–1415. [Google Scholar] [CrossRef]
- Satre, M.A.; Ugen, K.E.; Kochhar, D.M. Developmental changes in endogenous retinoids during pregnancy and embryogenesis in the mouse. Biol. Reprod. 1992, 46, 802–810. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hummler, H.; Hendrickx, A.G.; Nau, H. Maternal toxicokinetics, metabolism, and embryo exposure following a teratogenic dosing regimen with 13-cis-retinoic acid (isotretinoin) in the cynomolgus monkey. Teratology 1994, 50, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.G.; Ku, H.O.; Jeong, S.H.; Cho, J.H.; Son, S.W. Evaluation of embryotoxic potential of olaquindox and vitamin a in micromass culture and in rats. Toxicol. Res. 2010, 26, 209–216. [Google Scholar] [CrossRef]
- Lewis, K.C.; Green, M.H.; Underwood, B.A. Vitamin A turnover in rats as influenced by vitamin A status. J. Nutr. 1981, 111, 1135–1144. [Google Scholar] [CrossRef]
- Gieng, S.H.; Green, M.H.; Green, J.B.; Rosales, F.J. Model-based compartmental analysis indicates a reduced mobilization of hepatic vitamin A during inflammation in rats. J. Lipid Res. 2007, 48, 904–913. [Google Scholar] [CrossRef]
- Tan, L.; Wray, A.E.; Green, M.H.; Ross, A.C. Retinol kinetics in unsupplemented and vitamin A-retinoic acid supplemented neonatal rats: A preliminary model. J. Lipid Res. 2014, 55, 1077–1086. [Google Scholar] [CrossRef]
- Tan, L.; Green, M.H.; Ross, A.C. Vitamin A kinetics in neonatal rats vs. adult rats: Comparisons from model-based compartmental analysis. J. Nutr. 2015, 145, 403–410. [Google Scholar] [CrossRef]
- Hodges, J.K.; Tan, L.; Green, M.H.; Ross, A.C. Vitamin A supplementation redirects the flow of retinyl esters from peripheral to central organs of neonatal rats raised under vitamin A-marginal conditions. Am. J. Clin. Nutr. 2017, 105, 1110–1121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ford, J.L.; Green, J.B.; Green, M.H. Addition of vitamin A intake data during compartmental modeling of retinol kinetics in theoretical humans leads to accurate prediction of vitamin A total body stores and kinetic parameters in studies of reasonable duration. J. Nutr. 2019, 149, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Green, M.H.; Ford, J.L.; Green, J.B. Inclusion of vitamin A intake data provides improved compartmental model-derived estimates of vitamin A total body stores and disposal rate in older adults. J. Nutr. 2019, 149, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.L.; Green, J.B.; Haskell, M.J.; Ahmad, S.M.; Mazariegos Cordero, D.I.; Oxley, A.; Engle-Stone, R.; Lietz, G.; Green, M.H. Use of model-based compartmental analysis and a super-child design to study whole-body retinol kinetics and vitamin A total body stores in children from 3 lower-income countries. J. Nutr. 2020, 150, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, C.H.; Green, M.H.; Ross, A.C. Dietary iron repletion stimulates hepatic mobilization of vitamin A in previously iron-deficient rats as determined by model-based compartmental analysis. J. Nutr. 2020, 150, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Kelley, S.K.; Nilsson, C.B.; Green, M.H.; Green, J.B.; Hakansson, H. Mobilization of vitamin A stores in rats after administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin: A kinetic analysis. Toxicol. Sci. 2000, 55, 478–484. [Google Scholar] [CrossRef][Green Version]
- Ross, A.C.; Ambalavanan, N.; Zolfaghari, R.; Li, N.Q. Vitamin A combined with retinoic acid increases retinol uptake and lung retinyl ester formation in a synergistic manner in neonatal rats. J. Lipid Res. 2006, 47, 1844–1851. [Google Scholar] [CrossRef]
- Li, Y.; Wei, C.H.; Xiao, X.; Green, M.H.; Ross, A.C. Perturbed vitamin A status induced by iron deficiency is corrected by iron repletion in rats with pre-existing iron deficiency. J. Nutr. 2020, 150, 1989–1995. [Google Scholar] [CrossRef]
- Cifelli, C.J.; Green, J.B.; Green, M.H. Use of model-based compartmental analysis to study vitamin A kinetics and metabolism. Vitam. Horm. 2007, 75, 161–195. [Google Scholar] [CrossRef]
- Green, M.H.; Green, J.B.; Akohoue, S.A.; Kelley, S.K. Vitamin A intake affects the contribution of chylomicrons vs. retinol-binding protein to milk vitamin A in lactating rats. J. Nutr. 2001, 131, 1279–1282. [Google Scholar] [CrossRef]
- Blomhoff, R.; Helgerud, P.; Rasmussen, M.; Berg, T.; Norum, K.R. In vivo uptake of chylomicron [3H]retinyl ester by rat liver: Evidence for retinol transfer from parenchymal to nonparenchymal cells. Proc. Natl. Acad. Sci. USA 1982, 79, 7326–7330. [Google Scholar] [CrossRef]
- Ross, A.C. Retinoid production and catabolism: Role of diet in regulating retinol esterification and retinoic Acid oxidation. J. Nutr. 2003, 133, 291S–296S. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Zolfaghari, R. Regulation of hepatic retinol metabolism: Perspectives from studies on vitamin A status. J. Nutr. 2004, 134, 269S–275S. [Google Scholar] [CrossRef]
- Sklan, D.; Ross, A.C. Synthesis of retinol-binding protein and transthyretin in yolk sac and fetus in the rat. J. Nutr. 1987, 117, 436–442. [Google Scholar] [CrossRef]
- Ross, A.C. Retinol esterification by mammary gland microsomes from the lactating rat. J. Lipid Res. 1982, 23, 133–144. [Google Scholar] [CrossRef]
- Ross, A.C.; Pasatiempo, A.M.; Green, M.H. Chylomicron margination, lipolysis, and vitamin a uptake in the lactating rat mammary gland: Implications for milk retinoid content. Exp. Biol. Med. 2004, 229, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Akohoue, S.A.; Green, J.B.; Green, M.H. Dietary vitamin A has both chronic and acute effects on vitamin A indices in lactating rats and their offspring. J. Nutr. 2006, 136, 128–132. [Google Scholar] [CrossRef]

| Period of Study | Intake | Non-Pregnant Group | Pregnant–Lactating Group |
|---|---|---|---|
| Before mating | Diet, g/d | 19.7 (2.69) | 20.0 (2.67) |
| VA, nmol/d | 82.6 (11.3) | 83.8 (11.2) | |
| During pregnancy | Diet, g/d | 18.9 (2.23) | 24.5 (2.85) * |
| VA, nmol/d | 79.0 (9.35) | 103 (11.9) * | |
| During lactation | Diet, g/d | 18.1 (3.23) | 41.4 (2.59) * |
| VA, nmol/d | 76.0 (13.5) | 173 (10.9) * |
| Time of Study | VA Mass | Non-Pregnant Group | Pregnant–Lactating Group |
|---|---|---|---|
| Detection of pregnancy | Plasma, nmol | 9.38 (1.51) a | |
| Liver, nmol | 5289 (376) | ||
| Parturition | Plasma, nmol | 10.4 (1.60) | 14.6 (0.586) *b |
| Liver, nmol | 5715 (835) | 4702 (1507) | |
| End of lactation | Plasma, nmol | 11.6 (3.60) | 11.3 (1.74) a |
| Liver, nmol | 5726 (1004) | 5503 (912) | |
| Model Estimates (FSD) | ||
|---|---|---|
| L(I,J) 2 | Non-Pregnant Group (Day−1) | Pregnant–lactating Group (Day−1) |
| L(5,4) | 3.04 (0.09) | 3.05 (0.1) |
| L(6,5) | 8.01 (0.1) | 4.33 (0.04) |
| L(5,6) | 0.0117 (0.04) | 0.00558 (0.09) |
| L(0,6) | 0.00237 (0.2) | 0.00251 (0.1) |
| L(7,5) | 33.0 (0.1) | 22.8 (0.1) |
| L(5,7) | 0.744 (0.08) | 0.895 (0.1) |
| Parameters | Before Mating | Mating | Early Pregnancy | Late Pregnancy | Lactation |
|---|---|---|---|---|---|
| UF(1), nmol/day 2 | 80.2 (9.74) | 17.2 (27.9) | 93.7 (5.99) | 93.7 (5.99) | 157 (10.4) |
| R(5,6), nmol/day 3 | 27.7 (5.40) | 44.7 (8.69) | 18.9 (7.58) | 57.2 (11.1) | 11.7 (7.42) |
| L(8,5) 4, day−1 | 0 | 0 | 0.463 (0.180) | 0.724 (0.388) | 0 |
| L(9,5), day−1 | 0 | 0 | 0 | 0.0108 (0.0) | 0.0844 (0.132) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Tajima, A.; Mattie, F.J.; Green, M.H.; Ross, A.C. Pregnancy and Lactation Alter Vitamin A Metabolism and Kinetics in Rats under Vitamin A-Adequate Dietary Conditions. Nutrients 2021, 13, 2853. https://doi.org/10.3390/nu13082853
Li Y, Tajima A, Mattie FJ, Green MH, Ross AC. Pregnancy and Lactation Alter Vitamin A Metabolism and Kinetics in Rats under Vitamin A-Adequate Dietary Conditions. Nutrients. 2021; 13(8):2853. https://doi.org/10.3390/nu13082853
Chicago/Turabian StyleLi, Yaqi, Ayasa Tajima, Floyd J. Mattie, Michael H. Green, and A. Catharine Ross. 2021. "Pregnancy and Lactation Alter Vitamin A Metabolism and Kinetics in Rats under Vitamin A-Adequate Dietary Conditions" Nutrients 13, no. 8: 2853. https://doi.org/10.3390/nu13082853
APA StyleLi, Y., Tajima, A., Mattie, F. J., Green, M. H., & Ross, A. C. (2021). Pregnancy and Lactation Alter Vitamin A Metabolism and Kinetics in Rats under Vitamin A-Adequate Dietary Conditions. Nutrients, 13(8), 2853. https://doi.org/10.3390/nu13082853







