Administration of Lactobacillus reuteri Combined with Clostridium butyricum Attenuates Cisplatin-Induced Renal Damage by Gut Microbiota Reconstitution, Increasing Butyric Acid Production, and Suppressing Renal Inflammation
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals
2.2. Probiotic Treatment
2.3. Study Design
2.4. Measurement of Serum Renal Function and Biochemical Parameters
2.5. Preparation of Tissue Homogenates
2.6. Oxidative Stress Biomarker Analysis
2.7. Serum Endotoxin, Myeloperoxidase, Kidney Homogenate Myeloperoxidase, and Hydroxyproline Measurements
2.8. Indoxyl Sulfate (IS) Assay
2.9. Protein Extraction and Western Blot Analysis
2.10. Real-Time PCR
2.11. Histopathological Examination
2.12. Immunohistochemistry (IHC) Analysis
2.13. Measurement of Brush Border Membrane (BBM) Enzyme
2.14. Short-Chain Fatty Acid Measurement
2.15. 16s rDNA Sequencing and Bioinformatics Analysis
3. Statistical Analyses
4. Results
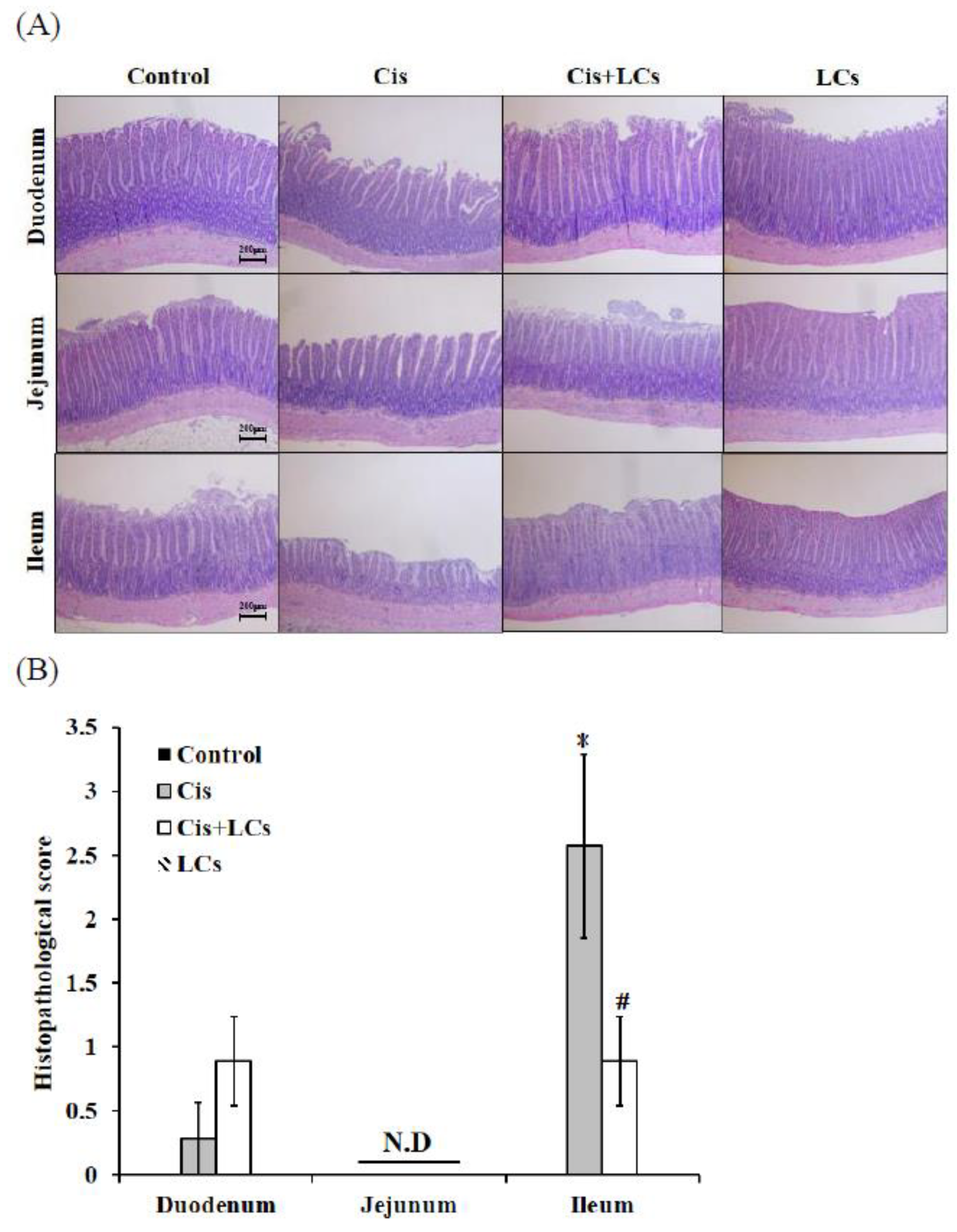
4.1. Pre-Administration of LCs Ameliorates Cisplatin-Induced Gastrointestinal Toxicity
4.2. LCs Ameliorated Cisplatin-Induced Nephrotoxicity
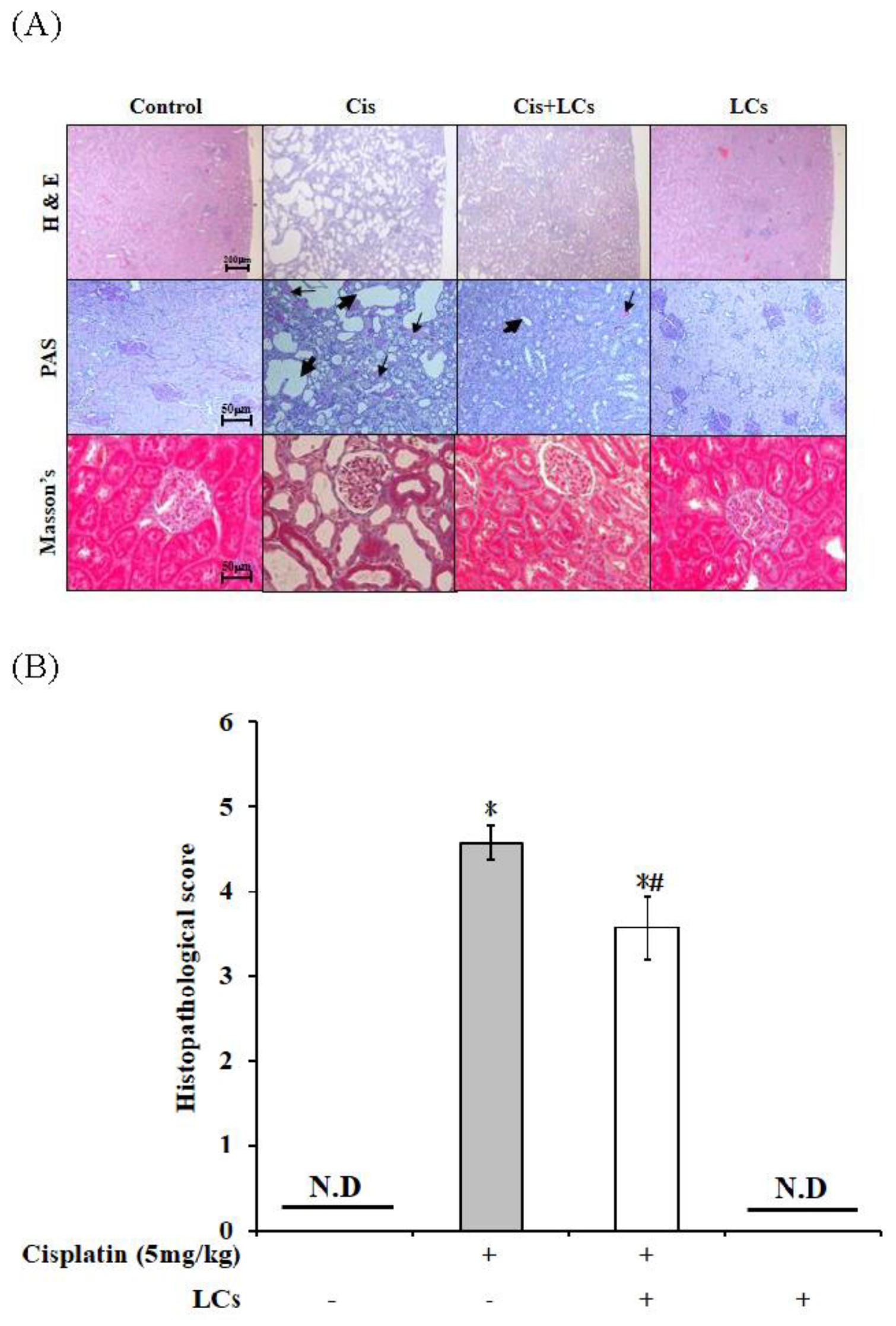
4.3. Effects of LCs on Histopathological Changes in the Kidneys of Cisplatin-Induced Nephrotoxicity Rats
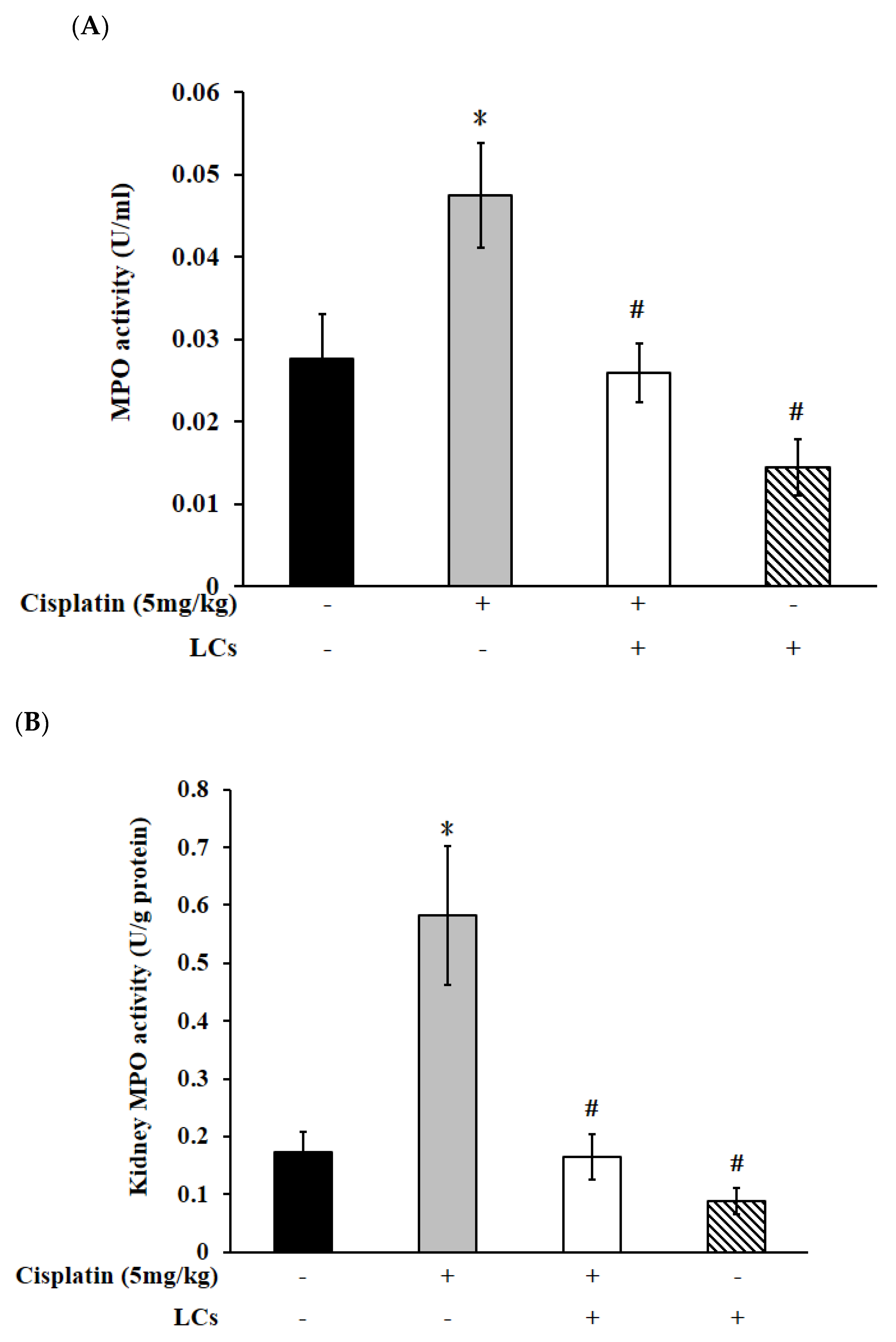
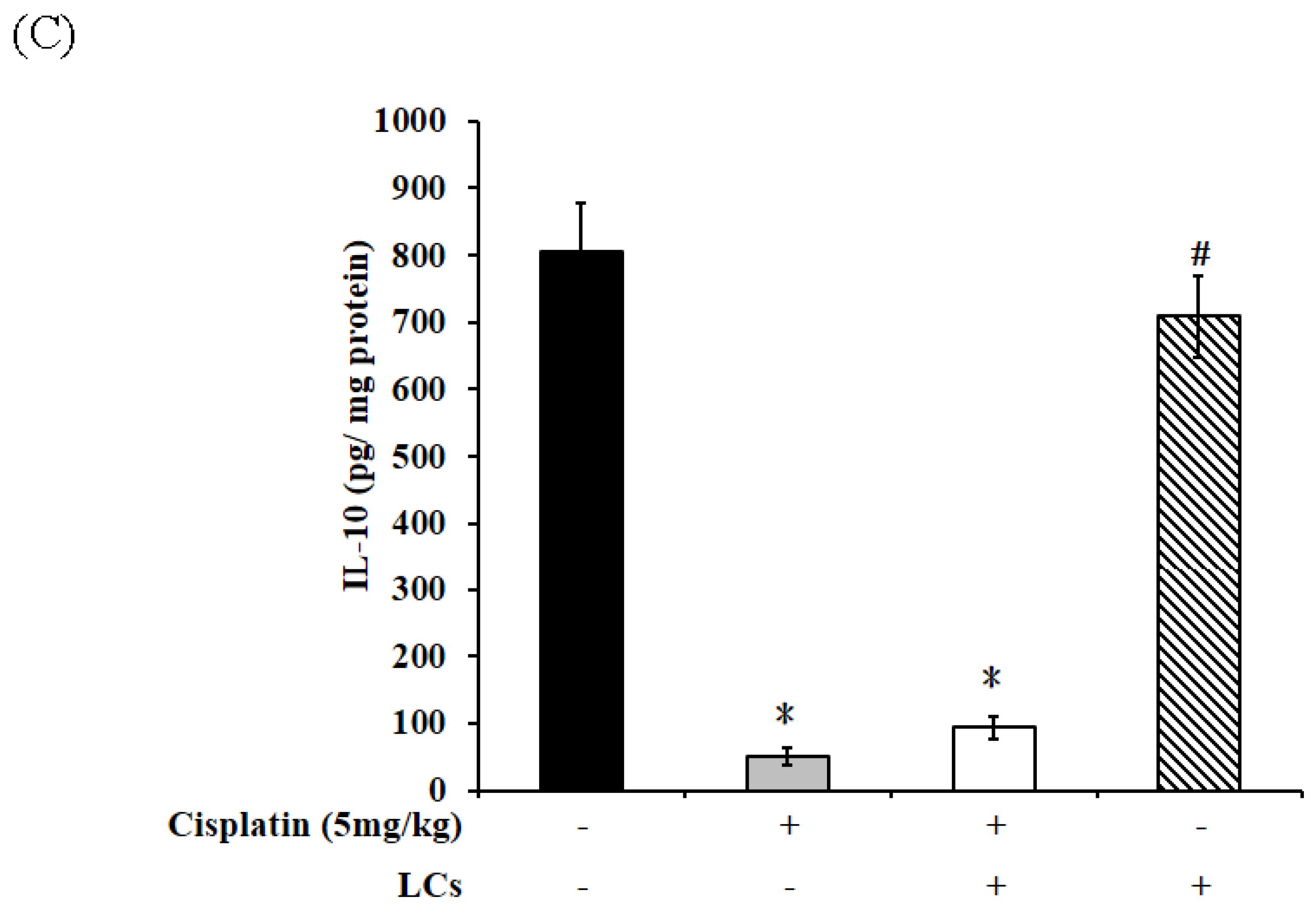
4.4. Effects of LCs on Renal Oxidative Stress Parameters, MPO, and IL-10 Level in the Cisplatin-Induced Nephrotoxicity Model
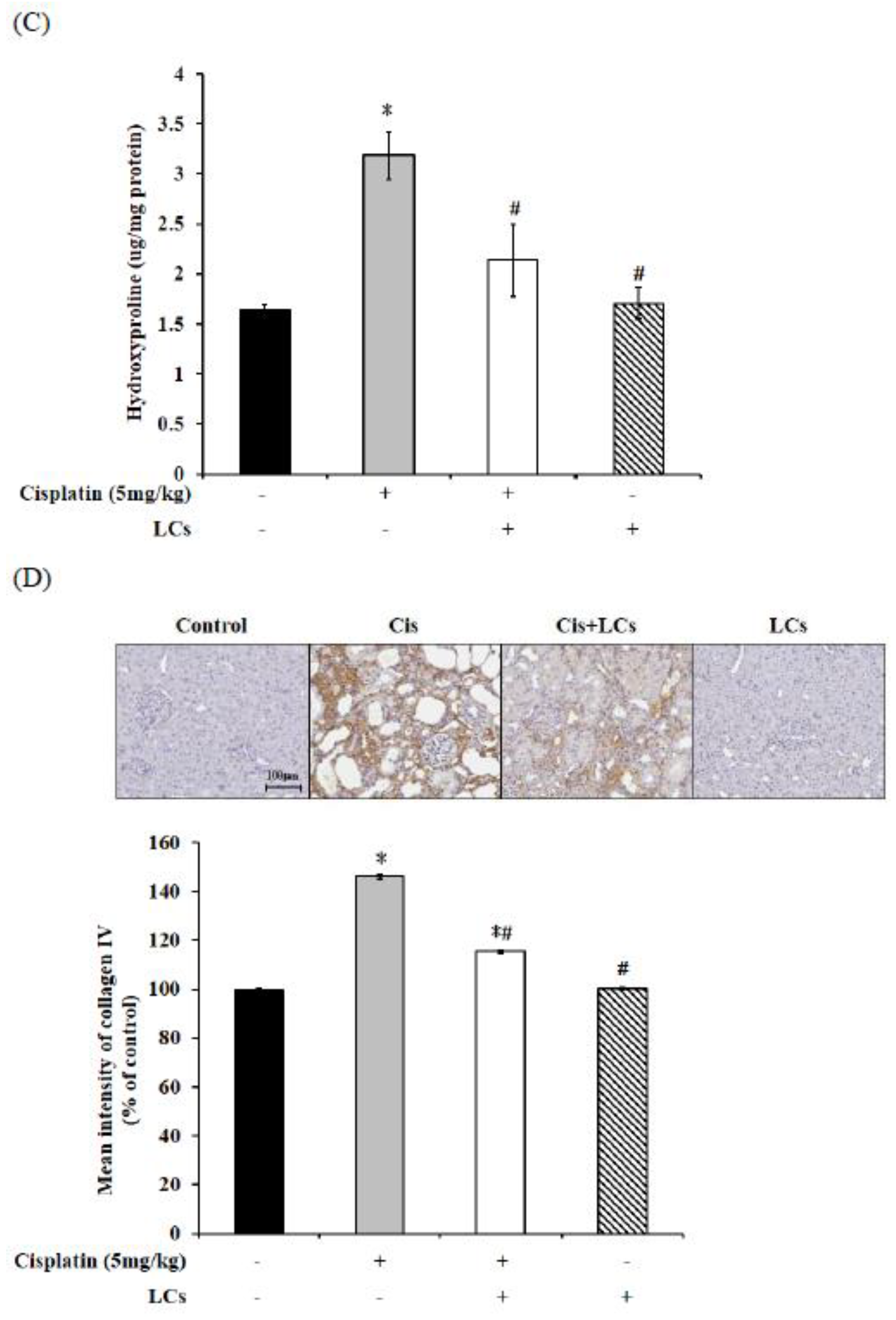
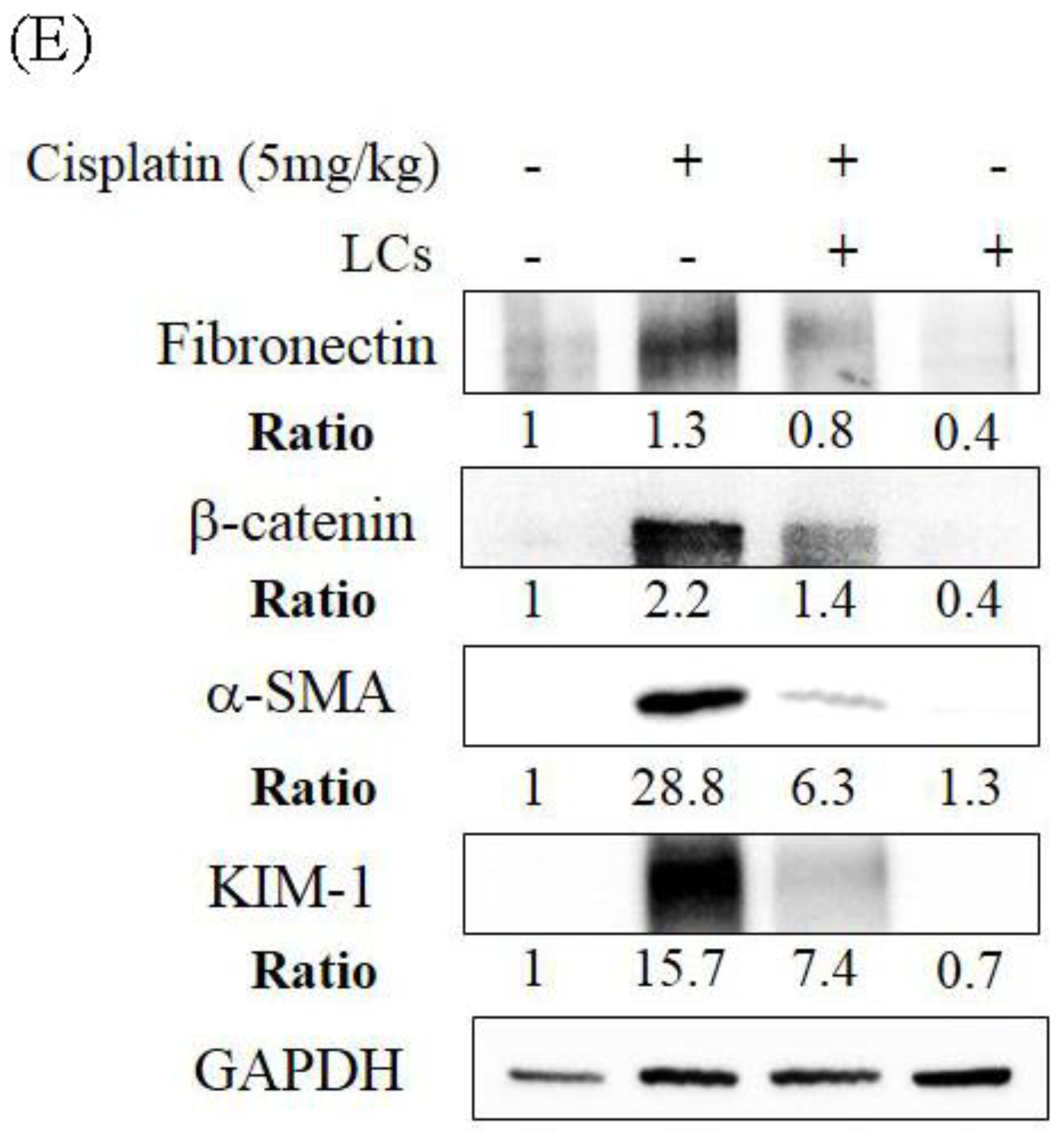
4.5. LCs Attenuates Kidney Inflammation, Apoptosis, and Fibrosis in Cisplatin-Induced Nephrotoxicity Models
4.6. LCs Promote the Output of Stool in Rats Treated with Cisplatin
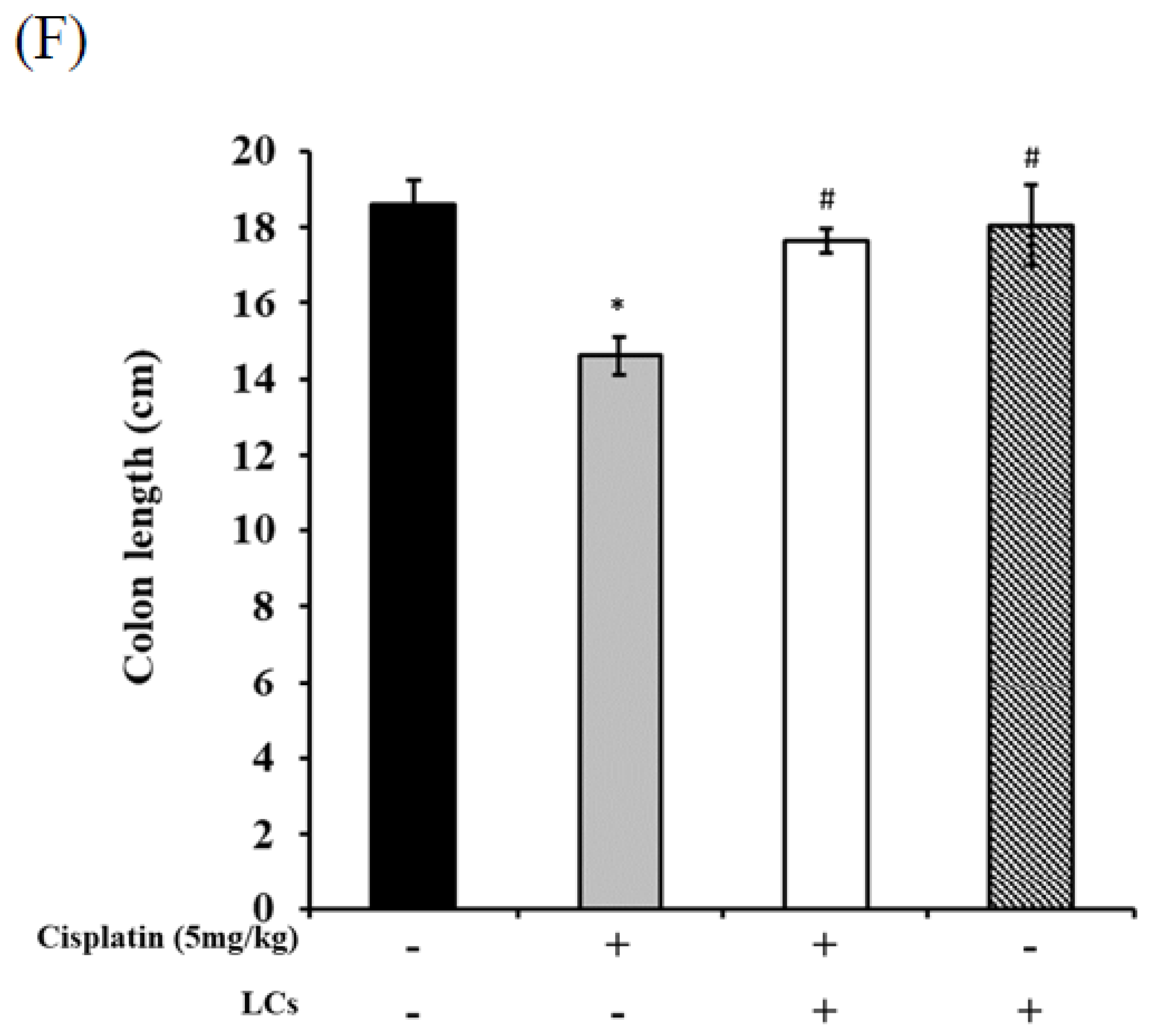
4.7. LCs Intervention Alleviates Intestinal Damage and Improves Shortened Colon Length in Cisplatin-Treated Rats
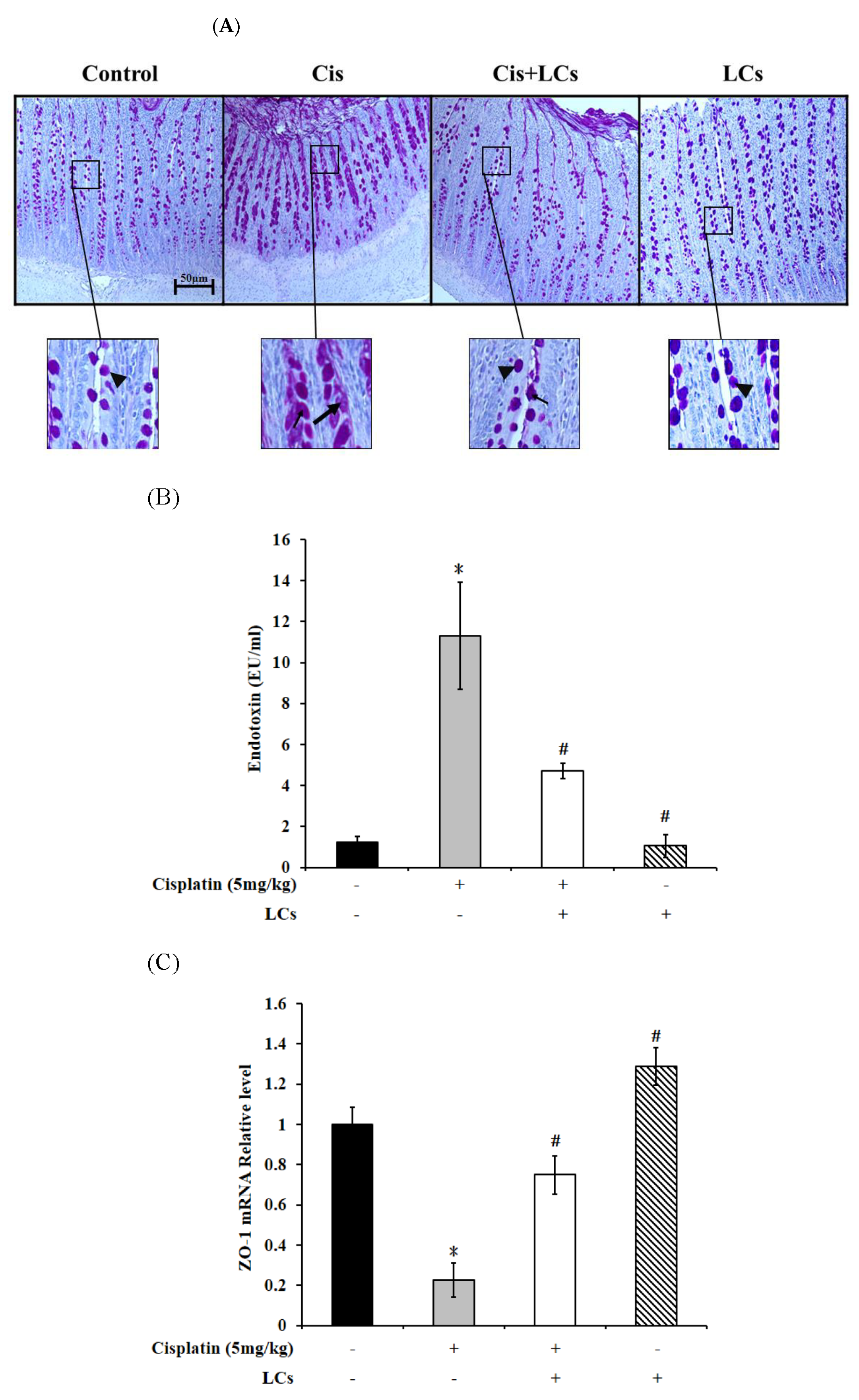
4.8. LCs Alleviates Goblet Cell Damage and Gut-Derived Endogenous Endotoxin Production and Improves Intestinal Permeability in Rats Treated with Cisplatin
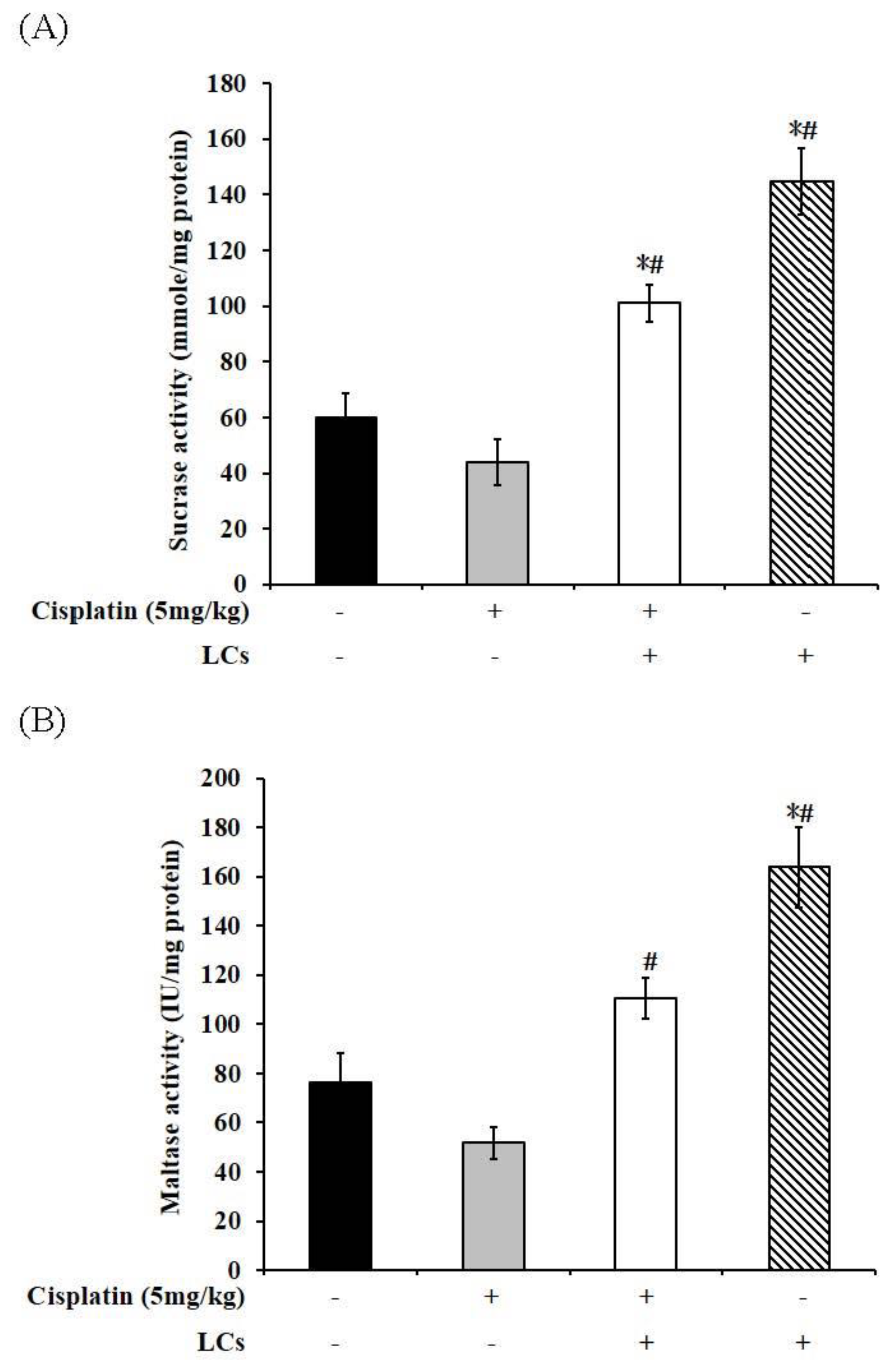
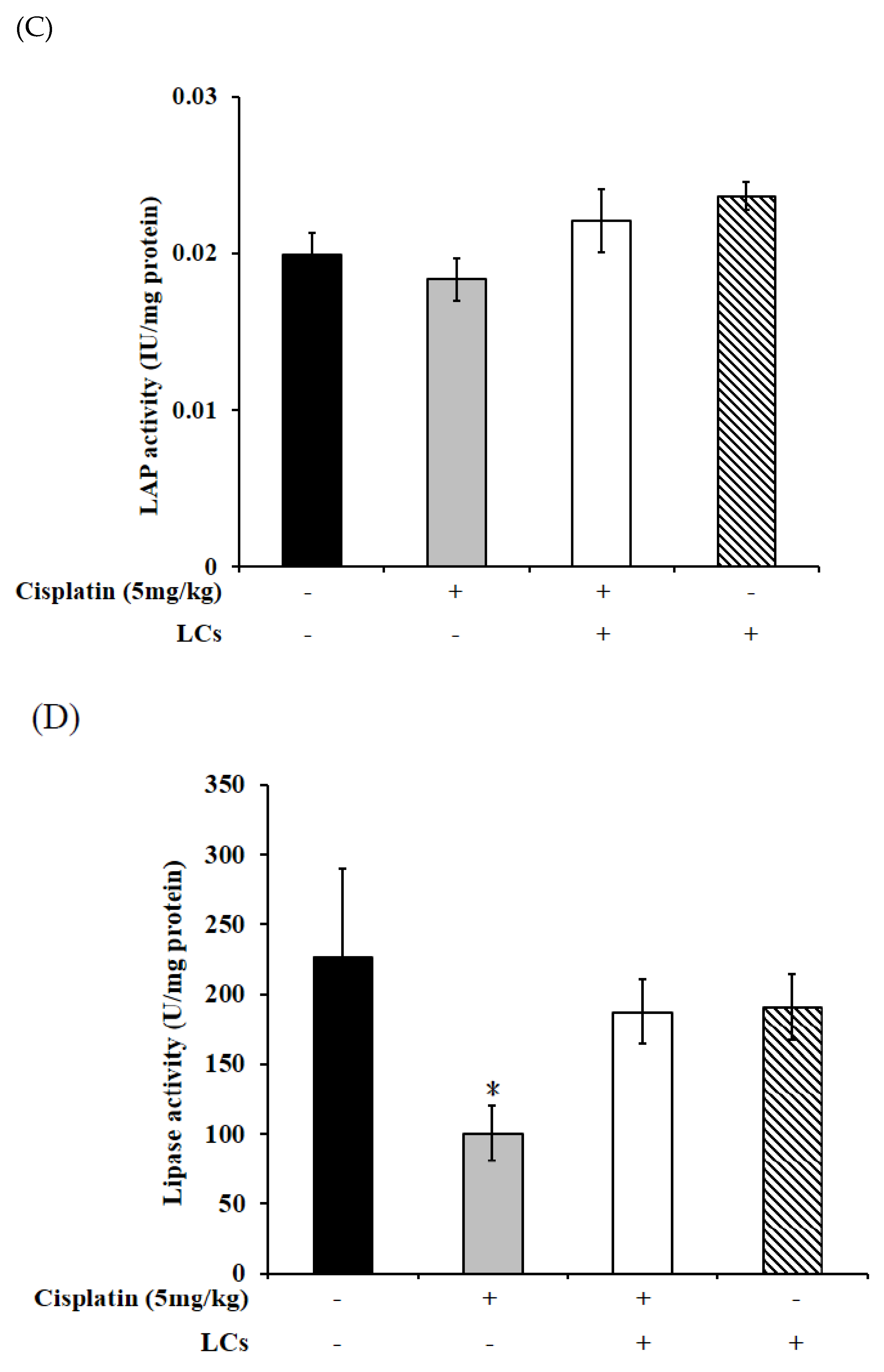
4.9. Effect of LCs on Intestinal Digestive Enzyme Activity in Cisplatin-Treated Rats
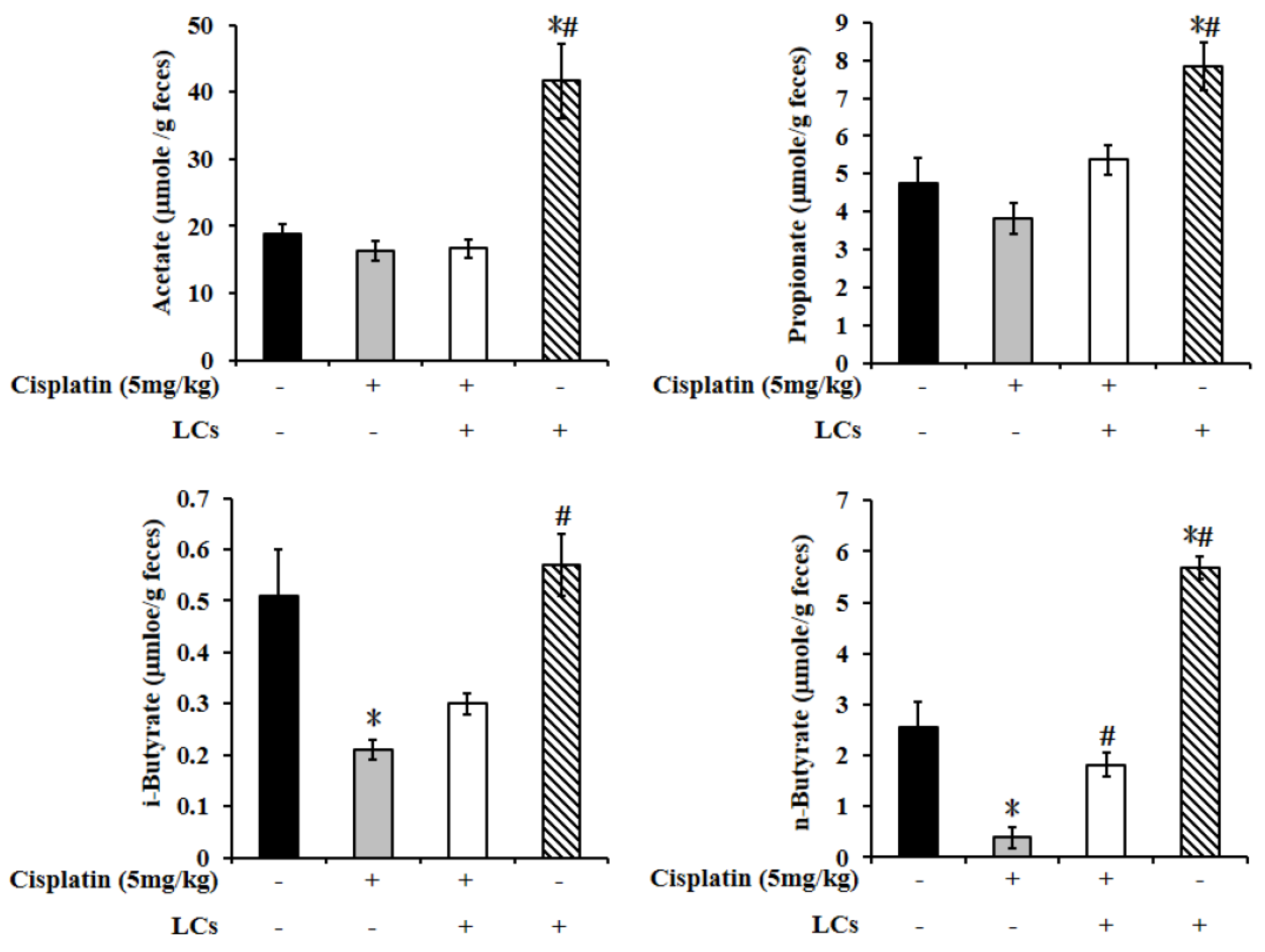
4.10. LCs Administration Promotes Butyrate Production after Cisplatin Treatment
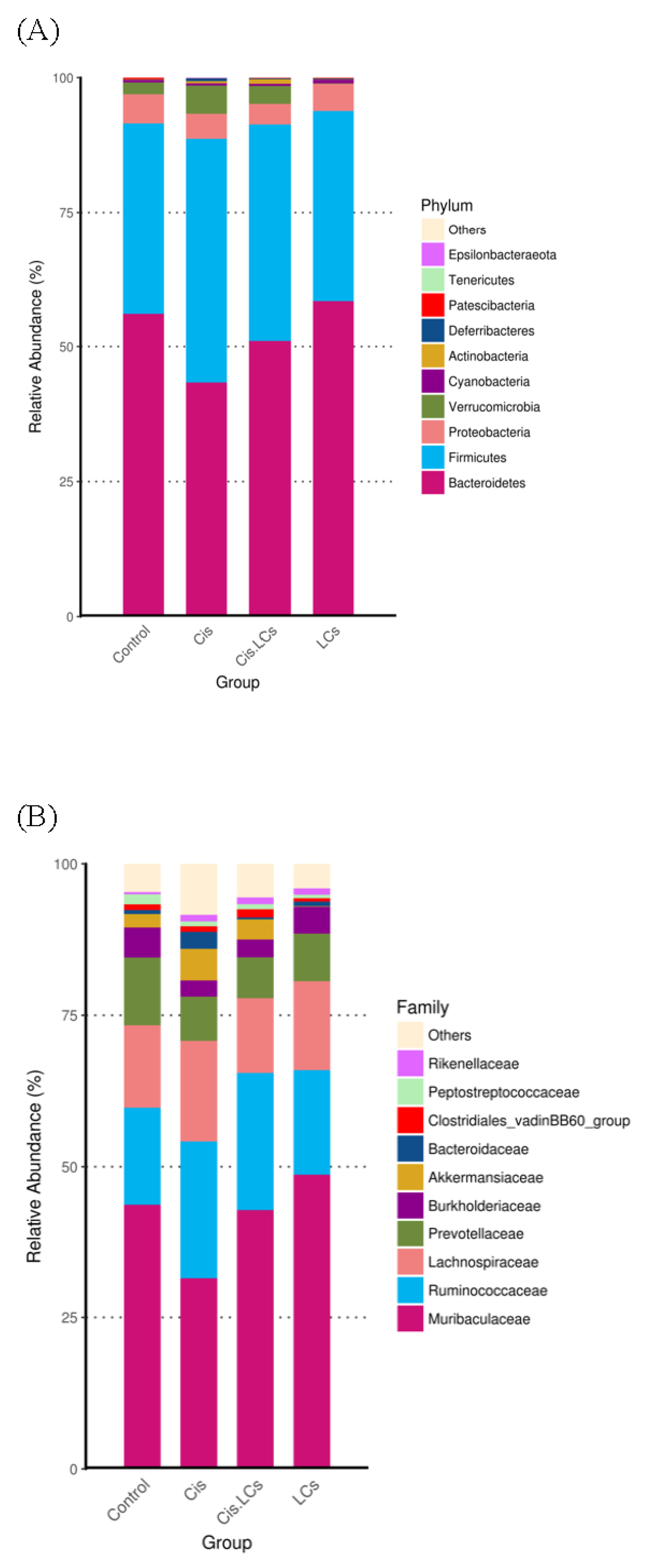
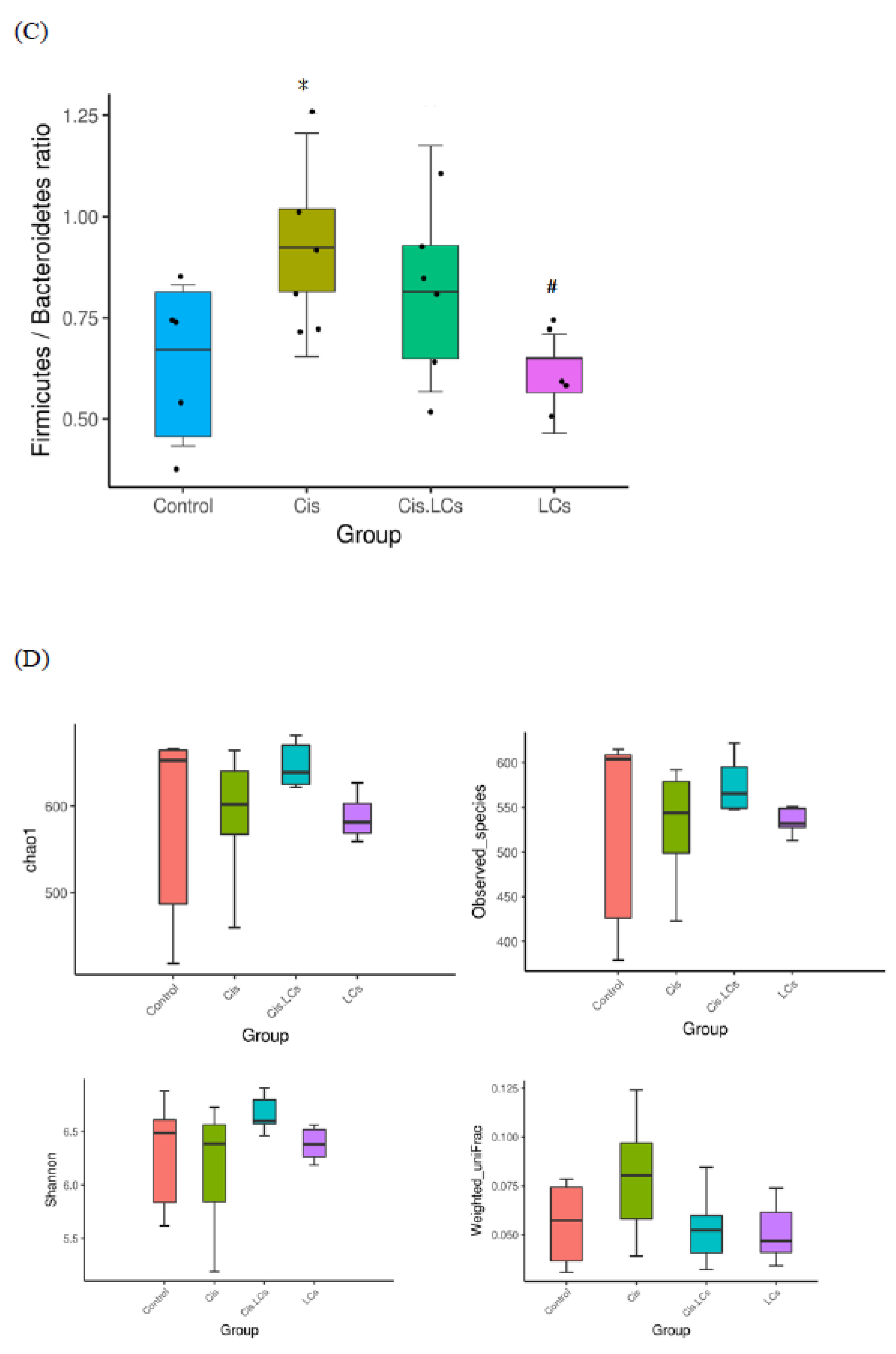
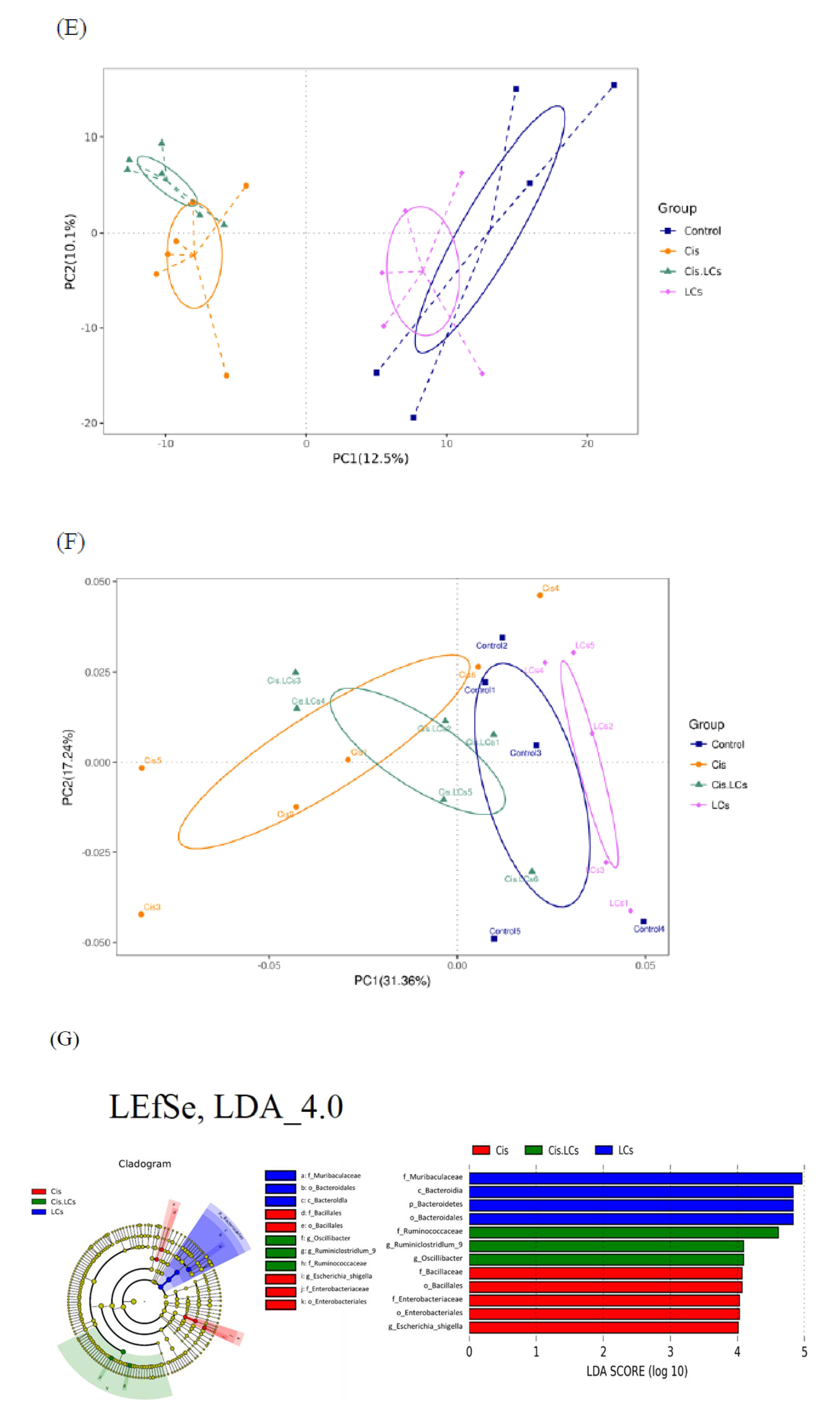
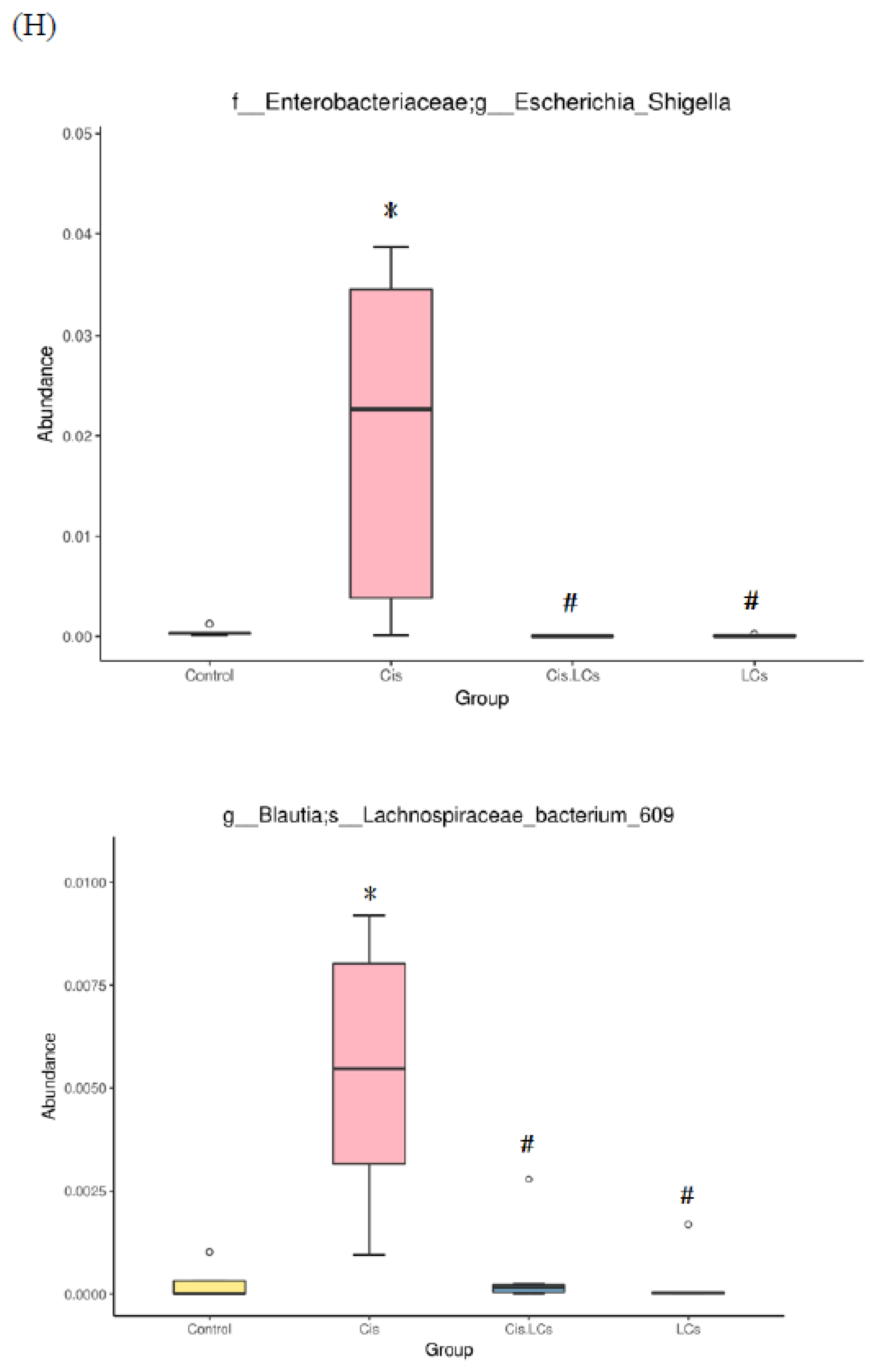
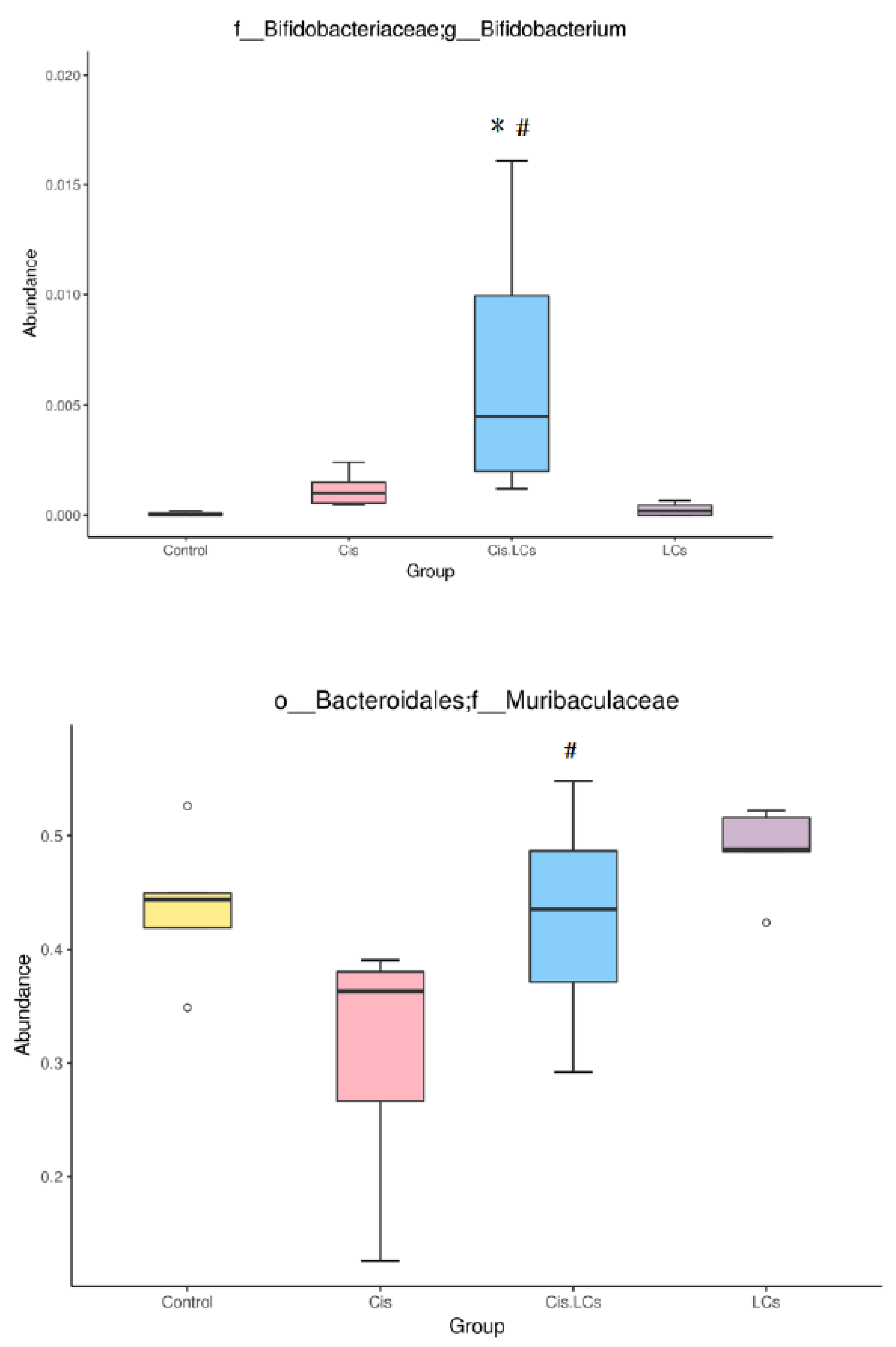
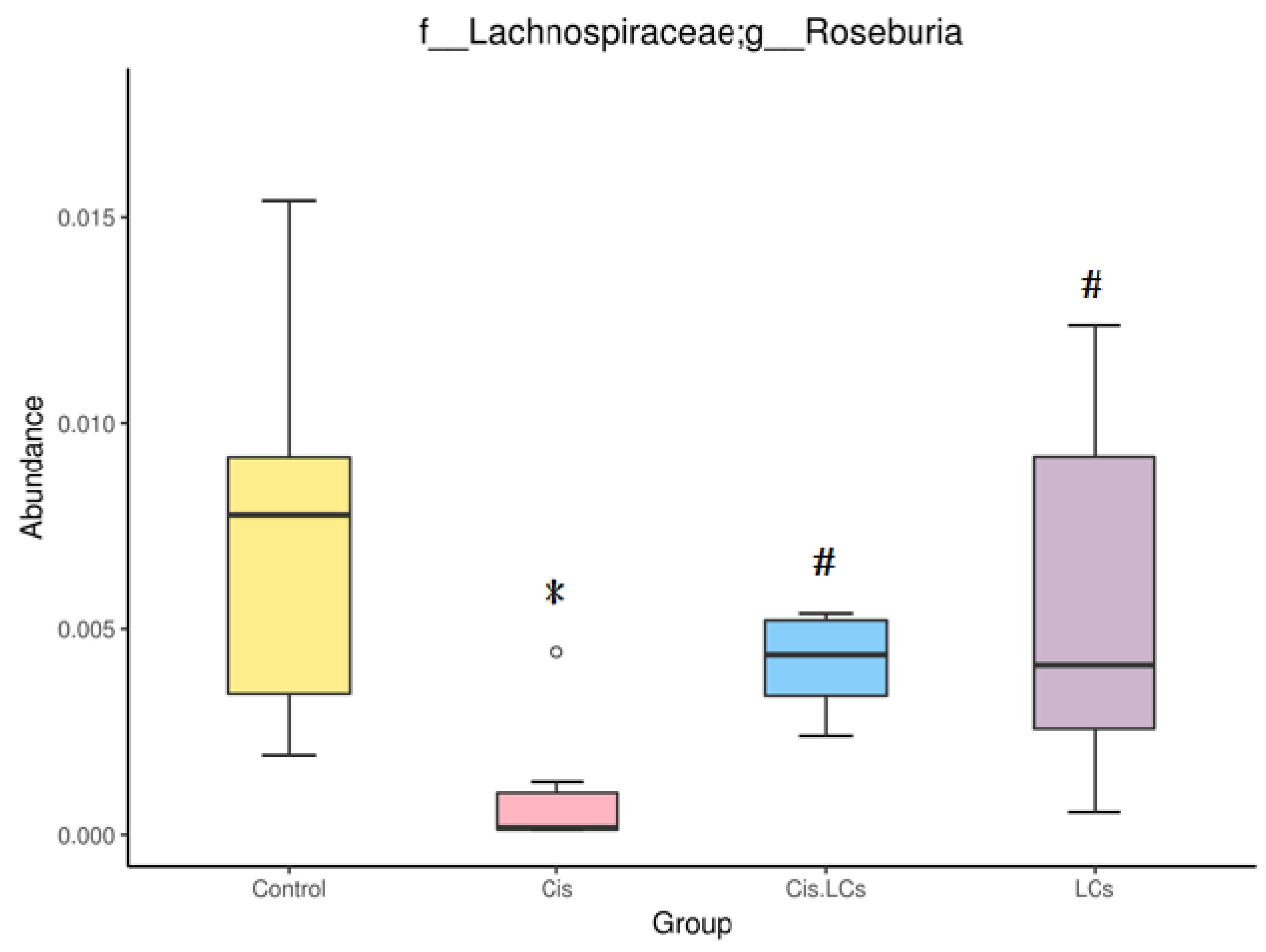
4.11. Restructuring of Gut Microbial Communities by LCs Intervention Prevents Cisplatin-Induced Dysbiosis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angelakis, E. Weight gain by gut microbiota manipulation in productive animals. Microb. Pathog. 2017, 106, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A. Sinapic acid modulates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Biomed. Pharmacother. 2017, 93, 646–653. [Google Scholar] [CrossRef]
- Arivarasu, N.A.; Priyamvada, S.; Mahmood, R. Oral administration of caffeic acid ameliorates the effect of cisplatin on brush border membrane enzymes and antioxidant system in rat intestine. Exp. Toxicol. Pathol. 2013, 65, 21–25. [Google Scholar] [CrossRef]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W.; et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef]
- Chen, X.; Wei, W.; Li, Y.; Huang, J.; Ci, X. Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chem. Biol. Interact. 2019, 308, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, J.; Su, Y.; Zhu, W. Effects of Intravenous Infusion with Sodium Butyrate on Colonic Microbiota, Intestinal Development- and Mucosal Immune-Related Gene Expression in Normal Growing Pigs. Front. Microbiol. 2018, 9, 1652. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, L.; Dou, X.; Wang, C.; Zhang, W.; Gao, K.; Liu, J.; Wang, H. Lactobacillus reuteri ZJ617 maintains intestinal integrity via regulating tight junction, autophagy and apoptosis in mice challenged with lipopolysaccharide. Oncotarget 2017, 8, 77489–77499. [Google Scholar] [CrossRef]
- Denk, S.; Weckbach, S.; Eisele, P.; Braun, C.K.; Wiegner, R.; Ohmann, J.J.; Wrba, L.; Hoenes, F.M.; Kellermann, P.; Radermacher, P.; et al. Role of Hemorrhagic Shock in Experimental Polytrauma. Shock 2018, 49, 154–163. [Google Scholar] [CrossRef]
- Dicksved, J.; Schreiber, O.; Willing, B.; Petersson, J.; Rang, S.; Phillipson, M.; Holm, L.; Roos, S. Lactobacillus reuteri maintains a functional mucosal barrier during DSS treatment despite mucus layer dysfunction. PLoS ONE 2012, 7, e46399–e46406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, H.; Niioka, M.; Kobayashi, N.; Tanaka, M.; Watanabe, T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: New insight into the probiotics for the gut-liver axis. PLoS ONE 2013, 8, e63388. [Google Scholar] [CrossRef] [Green Version]
- Espandiari, P.; Rosenzweig, B.; Zhang, J.; Zhou, Y.; Schnackenberg, L.; Vaidya, V.S.; Goering, P.L.; Brown, R.P.; Bonventre, J.V.; Mahjoob, K.; et al. Age-related differences in susceptibility to cisplatin-induced renal toxicity. J. Appl. Toxicol. 2010, 30, 172–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsgard, R.A.; Marrachelli, V.G.; Korpela, K.; Frias, R.; Collado, M.C.; Korpela, R.; Monleon, D.; Spillmann, T.; Osterlund, P. Chemotherapy-induced gastrointestinal toxicity is associated with changes in serum and urine metabolome and fecal microbiota in male Sprague-Dawley rats. Cancer Chemother. Pharmacol. 2017, 80, 317–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, A.; Krieg, R.; Massey, H.D.; Carl, D.; Ghosh, S.; Gehr, T.W.B.; Ghosh, S.S. Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression. Nephrol. Dial. Transplant 2019, 34, 783–794. [Google Scholar] [CrossRef]
- Grusovin, M.G.; Bossini, S.; Calza, S.; Cappa, V.; Garzetti, G.; Scotti, E.; Gherlone, E.F.; Mensi, M. Clinical efficacy of Lactobacillus reuteri-containing lozenges in the supportive therapy of generalized periodontitis stage III and IV, grade C: 1-year results of a double-blind randomized placebo-controlled pilot study. Clin. Oral. Investig. 2020, 24, 2015–2024. [Google Scholar] [CrossRef]
- Gryp, T.; Huys, G.R.B.; Joossens, M.; Van Biesen, W.; Glorieux, G.; Vaneechoutte, M. Isolation and Quantification of Uremic Toxin Precursor-Generating Gut Bacteria in Chronic Kidney Disease Patients. Int. J. Mol. Sci. 2020, 21, 1986. [Google Scholar] [CrossRef] [Green Version]
- Hagihara, M.; Kuroki, Y.; Ariyoshi, T.; Higashi, S.; Fukuda, K.; Yamashita, R.; Matsumoto, A.; Mori, T.; Mimura, K.; Yamaguchi, N.; et al. Clostridium butyricum Modulates the Microbiome to Protect Intestinal Barrier Function in Mice with Antibiotic-Induced Dysbiosis. iScience 2020, 23, 100772–100789. [Google Scholar] [CrossRef] [Green Version]
- Hagihara, M.; Yamashita, R.; Matsumoto, A.; Mori, T.; Kuroki, Y.; Kudo, H.; Oka, K.; Takahashi, M.; Nonogaki, T.; Yamagishi, Y.; et al. The impact of Clostridium butyricum MIYAIRI 588 on the murine gut microbiome and colonic tissue. Anaerobe 2018, 54, 8–18. [Google Scholar] [CrossRef]
- Hayashi, A.; Sato, T.; Kamada, N.; Mikami, Y.; Matsuoka, K.; Hisamatsu, T.; Hibi, T.; Roers, A.; Yagita, H.; Ohteki, T.; et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe 2013, 13, 711–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.; Yan, Y.; Chen, D.; Zhao, Y.; Dong, W.; Zeng, X.; Cao, Y. Ascorbic Acid Derivative 2-O-beta-d-Glucopyranosyl-l-Ascorbic Acid from the Fruit of Lycium barbarum Modulates Microbiota in the Small Intestine and Colon and Exerts an Immunomodulatory Effect on Cyclophosphamide-Treated BALB/c Mice. J. Agric. Food. Chem. 2020, 68, 11128–11143. [Google Scholar] [CrossRef]
- Hwang, S.; Park, J.; Kim, J.; Jang, H.R.; Kwon, G.Y.; Huh, W.; Kim, Y.G.; Kim, D.J.; Oh, H.Y.; Lee, J.E. Tissue expression of tubular injury markers is associated with renal function decline in diabetic nephropathy. J. Diabetes Complicat. 2017, 31, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Yang, W.; Jin, Y.; Huang, H.; Shi, C.; Jiang, Y.; Wang, J.; Kang, Y.; Wang, C.; Yang, G. Lactobacillus reuteri protects mice against Salmonella typhimurium challenge by activating macrophages to produce nitric oxide. Microb. Pathog. 2019, 137, 103754. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jo, J.; Kim, K.; An, H.J.; Gwon, M.G.; Gu, H.; Kim, H.J.; Yang, A.Y.; Kim, S.W.; Jeon, E.J.; et al. Pharmacological Activation of Sirt1 Ameliorates Cisplatin-Induced Acute Kidney Injury by Suppressing Apoptosis, Oxidative Stress, and Inflammation in Mice. Antioxidants 2019, 8, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koppe, L.; Mafra, D.; Fouque, D. Probiotics and chronic kidney disease. Kidney Int. 2015, 88, 958–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.H.; Lee, S.H.; Chen, K.M.; Lii, C.K.; Liu, C.T. Effect of garlic oil on neutrophil infiltration in the small intestine of endotoxin-injected rats and its association with levels of soluble and cellular adhesion molecules. J. Agric. Food Chem. 2011, 59, 7717–7725. [Google Scholar] [CrossRef]
- Lakritz, J.R.; Poutahidis, T.; Levkovich, T.; Varian, B.J.; Ibrahim, Y.M.; Chatzigiagkos, A.; Mirabal, S.; Alm, E.J.; Erdman, S.E. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int. J. Cancer 2014, 135, 529–540. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.H.; Park, D.; Kim, Y.J.; Lee, I.; Kim, S.; Oh, C.T.; Kim, J.Y.; Yang, J.; Jo, S.K. Lactobacillus salivarius BP121 prevents cisplatininduced acute kidney injury by inhibition of uremic toxins such as indoxyl sulfate and pcresol sulfate via alleviating dysbiosis. Int. J. Mol. Med. 2020, 45, 1130–1140. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Li, K.Y.; Wang, P.J.; Huang, H.W.; Chen, M.J. Alleviating chronic kidney disease progression through modulating the critical genus of gut microbiota in a cisplatin-induced Lanyu pig model. J. Food Drug Anal. 2020, 28, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Sung, C.Y.; Lee, N.; Ni, Y.; Pihlajamaki, J.; Panagiotou, G.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Qi, C.; Zhu, H.; Yu, R.; Xie, C.; Peng, Y.; Yin, S.W.; Fan, J.; Zhao, S.; Sun, J. Lactobacillus reuteri improves gut barrier function and affects diurnal variation of the gut microbiota in mice fed a high-fat diet. Food Funct. 2019, 10, 4705–4715. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Feng, L.X.; Zhu, X.J.; Liu, Q.; Wang, H.S.; Wu, X.; Yan, P.; Duan, X.J.; Xiao, Y.Q.; Cheng, W.; et al. Human umbilical cord blood mononuclear cells protect against renal tubulointerstitial fibrosis in cisplatin-treated rats. Biomed. Pharmacother. 2020, 121, 109310. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Rocha, C.S.; Dandekar, S.; Wan, Y.J. Functional analysis of the relationship between intestinal microbiota and the expression of hepatic genes and pathways during the course of liver regeneration. J. Hepatol. 2016, 64, 641–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.P.; Chang, C.Y.; Huang, W.H.; Fu, Y.S.; Chao, D.; Huang, H.T. Dimethylthiourea pretreatment inhibits endotoxin-induced compound exocytosis in goblet cells and plasma leakage of rat small intestine. J. Electron. Microsc. 2010, 59, 127–139. [Google Scholar] [CrossRef]
- Machado, R.A.; Constantino Lde, S.; Tomasi, C.D.; Rojas, H.A.; Vuolo, F.S.; Vitto, M.F.; Cesconetto, P.A.; de Souza, C.T.; Ritter, C.; Dal-Pizzol, F. Sodium butyrate decreases the activation of NF-kappaB reducing inflammation and oxidative damage in the kidney of rats subjected to contrast-induced nephropathy. Nephrol. Dial. Transplant 2012, 27, 3136–3140. [Google Scholar] [CrossRef] [Green Version]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Marietta, E.; Horwath, I.; Taneja, V. Microbiome, Immunomodulation, and the Neuronal System. Neurotherapeutics 2018, 15, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.A.; Bruley des Varannes, S.; Massart, S.; Moreau, P.; Potel, G.; de La Cochetiere, M.F.; Batard, E.; et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757–774. [Google Scholar] [CrossRef]
- Oka, K.; Osaki, T.; Hanawa, T.; Kurata, S.; Sugiyama, E.; Takahashi, M.; Tanaka, M.; Taguchi, H.; Kamiya, S. Establishment of an Endogenous Clostridium difficile Rat Infection Model and Evaluation of the Effects of Clostridium butyricum MIYAIRI 588 Probiotic Strain. Front. Microbiol. 2018, 9, 1264. [Google Scholar] [CrossRef]
- Pan, H.; Li, J.; Rankin, G.O.; Rojanasakul, Y.; Tu, Y.; Chen, Y.C. Synergistic effect of black tea polyphenol, theaflavin-3,3’-digallate with cisplatin against cisplatin resistant human ovarian cancer cells. J. Funct. Foods 2018, 46, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Perales-Puchalt, A.; Perez-Sanz, J.; Payne, K.K.; Svoronos, N.; Allegrezza, M.J.; Chaurio, R.A.; Anadon, C.; Calmette, J.; Biswas, S.; Mine, J.A.; et al. Frontline Science: Microbiota reconstitution restores intestinal integrity after cisplatin therapy. J. Leukoc. Biol. 2018, 103, 799–805. [Google Scholar] [CrossRef]
- Pianta, T.J.; Pickering, J.W.; Succar, L.; Chin, M.; Davidson, T.; Buckley, N.A.; Mohamed, F.; Endre, Z.H. Dexamethasone Modifies Cystatin C-Based Diagnosis of Acute Kidney Injury During Cisplatin-Based Chemotherapy. Kidney Blood Press. Res. 2017, 42, 62–75. [Google Scholar] [CrossRef] [Green Version]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Fang, Z.; Liu, X.; Hu, W.; Lu, W.; Lee, Y.K.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus reuteri attenuated allergic inflammation induced by HDM in the mouse and modulated gut microbes. PLoS ONE 2020, 15, e0231865. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Liu, L.; Liu, P.; Gamallat, Y.; Xin, Y.; Shang, D. Polysaccharide extracted from Enteromorpha ameliorates Cisplastininduced small intestine injury in mice. J. Funct. Foods 2018, 49, 371–378. [Google Scholar] [CrossRef]
- Rescigno, M. Intestinal microbiota and its effects on the immune system. Cell Microbiol. 2014, 16, 1004–1013. [Google Scholar] [CrossRef]
- Schnackenberg, L.K.; Sun, J.; Pence, L.M.; Bhattacharyya, S.; Gamboa da Costa, G.; Beger, R.D. Metabolomics evaluation of hydroxyproline as a potential marker of melamine and cyanuric acid nephrotoxicity in male and female Fischer F344 rats. Food Chem. Toxicol. 2012, 50, 3978–3983. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Birchenough, G.M.H.; Stahlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Backhed, F. Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe 2018, 23, 27–40.e27. [Google Scholar] [CrossRef] [Green Version]
- Seki, H.; Shiohara, M.; Matsumura, T.; Miyagawa, N.; Tanaka, M.; Komiyama, A.; Kurata, S. Prevention of antibiotic-associated diarrhea in children by Clostridium butyricum MIYAIRI. Pediatr. Int. 2003, 45, 86–90. [Google Scholar] [CrossRef]
- Seo, M.; Inoue, I.; Tanaka, M.; Matsuda, N.; Nakano, T.; Awata, T.; Katayama, S.; Alpers, D.H.; Komoda, T. Clostridium butyricum MIYAIRI 588 improves high-fat diet-induced non-alcoholic fatty liver disease in rats. Dig. Dis. Sci. 2013, 58, 3534–3544. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, C.; Long, G.; Wolf, J.; Okerberg, C.; Herbert, R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol. Pathol. 2002, 30, 93–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharp, C.N.; Doll, M.A.; Dupre, T.V.; Shah, P.P.; Subathra, M.; Siow, D.; Arteel, G.E.; Megyesi, J.; Beverly, L.J.; Siskind, L.J. Repeated administration of low-dose cisplatin in mice induces fibrosis. Am. J. Physiol. Renal. Physiol. 2016, 310, F560–F568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, F.; Zheng, R.D.; Sun, X.Q.; Ding, W.J.; Wang, X.Y.; Fan, J.G. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 375–381. [Google Scholar] [CrossRef]
- Shinnoh, M.; Horinaka, M.; Yasuda, T.; Yoshikawa, S.; Morita, M.; Yamada, T.; Miki, T.; Sakai, T. Clostridium butyricum MIYAIRI 588 shows antitumor effects by enhancing the release of TRAIL from neutrophils through MMP-8. Int. J. Oncol. 2013, 42, 903–911. [Google Scholar] [CrossRef] [Green Version]
- Takayama, F.; Taki, K.; Niwa, T. Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am. J. Kidney Dis. 2003, 41, S142–S145. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, Y.; Huang, Z.; Dong, W.; Deng, Y.; Wang, F.; Li, M.; Yuan, J. Administration of probiotic mixture DM#1 ameliorated 5-fluorouracil-induced intestinal mucositis and dysbiosis in rats. Nutrition 2017, 33, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Xu, B.; Qin, Y.; Fan, L.; Chen, J.; Zheng, P.; Gong, X.; Wang, H.; Bai, M.; Pu, J.; et al. Clostridium butyricum miyairi 588 has preventive effects on chronic social defeat stress-induced depressive-like behaviour and modulates microglial activation in mice. Biochem. Biophys. Res. Commun. 2019, 516, 430–436. [Google Scholar] [CrossRef]
- Wen, J.J.; Vyatkina, G.; Garg, N. Oxidative damage during chagasic cardiomyopathy development: Role of mitochondrial oxidant release and inefficient antioxidant defense. Free Radic. Biol. Med. 2004, 37, 1821–1833. [Google Scholar] [CrossRef]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Wong, Y.S.; Lin, M.Y.; Liu, P.F.; Ko, J.L.; Huang, G.T.; Tu, D.G.; Ou, C.C. D-methionine improves cisplatin-induced anorexia and dyspepsia syndrome by attenuating intestinal tryptophan hydroxylase 1 activity and increasing plasma leptin concentration. Neurogastroenterol. Motil. 2020, 32, e13803–e13813. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Ko, J.L.; Liao, J.M.; Huang, S.S.; Lin, M.Y.; Lee, L.H.; Chang, L.Y.; Ou, C.C. D-methionine alleviates cisplatin-induced mucositis by restoring the gut microbiota structure and improving intestinal inflammation. Ther. Adv. Med. Oncol. 2019, 11, 1758835918821021. [Google Scholar] [CrossRef] [Green Version]
- Wu, I.W.; Lin, C.Y.; Chang, L.C.; Lee, C.C.; Chiu, C.Y.; Hsu, H.J.; Sun, C.Y.; Chen, Y.C.; Kuo, Y.L.; Yang, C.W.; et al. Gut Microbiota as Diagnostic Tools for Mirroring Disease Progression and Circulating Nephrotoxin Levels in Chronic Kidney Disease: Discovery and Validation Study. Int. J. Biol. Sci. 2020, 16, 420–434. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.Y.; Xia, G.H.; Lu, J.Q.; Chen, M.X.; Zhen, X.; Wang, S.; You, C.; Nie, J.; Zhou, H.W.; Yin, J. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci. Rep. 2017, 7, 1445. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; El Nahas, A.M.; Thomas, G.L.; Haylor, J.L.; Watson, P.F.; Wagner, B.; Johnson, T.S. Caspase-3 and apoptosis in experimental chronic renal scarring. Kidney Int. 2001, 60, 1765–1776. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Lv, L.; Wu, W.; Li, Y.; Shi, D.; Fang, D.; Guo, F.; Jiang, H.; Yan, R.; Ye, W.; et al. Butyrate Protects Mice Against Methionine-Choline-Deficient Diet-Induced Non-alcoholic Steatohepatitis by Improving Gut Barrier Function, Attenuating Inflammation and Reducing Endotoxin Levels. Front. Microbiol. 2018, 9, 1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, C.H.; Tseng, Y.H.; Kuo, Y.W.; Lee, M.C.; Chen, H.L. Long-term supplementation of isomalto-oligosaccharides improved colonic microflora profile, bowel function, and blood cholesterol levels in constipated elderly people--a placebo-controlled, diet-controlled trial. Nutrition 2011, 27, 445–450. [Google Scholar] [CrossRef]
- Yoshifuji, A.; Wakino, S.; Irie, J.; Tajima, T.; Hasegawa, K.; Kanda, T.; Tokuyama, H.; Hayashi, K.; Itoh, H. Gut Lactobacillus protects against the progression of renal damage by modulating the gut environment in rats. Nephrol. Dial. Transplant 2016, 31, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Zhai, S.; Qin, S.; Li, L.; Zhu, L.; Zou, Z.; Wang, L. Dietary butyrate suppresses inflammation through modulating gut microbiota in high-fat diet-fed mice. FEMS Microbiol. Lett. 2019, 366, fnz153. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef]
- Zhou, P.; Li, Z.; Xu, D.; Wang, Y.; Bai, Q.; Feng, Y.; Su, G.; Chen, P.; Wang, Y.; Liu, H.; et al. Cepharanthine Hydrochloride Improves Cisplatin Chemotherapy and Enhances Immunity by Regulating Intestinal Microbes in Mice. Front. Cell Infect. Microbiol. 2019, 9, 225–240. [Google Scholar] [CrossRef] [PubMed]


| Group | Control | Cisplatin | Cis+LCs | LCs |
|---|---|---|---|---|
| AST (U/L) | 0.121 ± 6.38 | 109.14 ± 6.25 | 0101.89 ± 6.55 | 104.29 ± 6.73 |
| ALT (U/L) | 00.53 ± 2.50 | 0.49 ± 6.26 | 034.89 ± 2.45 *# | 038.14 ± 1.62 * |
| BUN (mg/dL) | 17.26 ± 1.18 | 119.61 ± 10.94 * | 0056.6 ± 8.30 *# | 015.73 ± 1.32 # |
| Creatinine (mg/dL) | 01.33 ± 0.06 | 02.87 ± 0.32 * | 001.99 ± 0.09 *# | 001.15 ± 0.06 # |
| Cystatin C (mg/L) | 01.27 ± 0.05 | 03.99 ± 0.44 * | .002.06 ± 0.19 # | 001.04 ± 0.02 # |
| UA (mg/dL) | 04.00 ± 0.39 | 01.37 ± 0.17 * | 02.66 ± 0.16 *# | 004.03 ± 0.26 # |
| IgA (mg/dL) | 00.90 ± 3.78 | 281.29 ± 27.25 * | 152.89 ± 13.12 *# | 076.43 ± 2.19 # |
| Indoxyl Sulfate (mg/mL) | 00.91 ± 0.02 | 02.76 ± 0.21 * | 01.61 ± 0.03 *# | 000.86 ± 0.01 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, Y.-P.; Chen, H.-L.; Tsai, J.-N.; Lin, M.-Y.; Liao, J.-W.; Wei, M.-S.; Ko, J.-L.; Ou, C.-C. Administration of Lactobacillus reuteri Combined with Clostridium butyricum Attenuates Cisplatin-Induced Renal Damage by Gut Microbiota Reconstitution, Increasing Butyric Acid Production, and Suppressing Renal Inflammation. Nutrients 2021, 13, 2792. https://doi.org/10.3390/nu13082792
Hsiao Y-P, Chen H-L, Tsai J-N, Lin M-Y, Liao J-W, Wei M-S, Ko J-L, Ou C-C. Administration of Lactobacillus reuteri Combined with Clostridium butyricum Attenuates Cisplatin-Induced Renal Damage by Gut Microbiota Reconstitution, Increasing Butyric Acid Production, and Suppressing Renal Inflammation. Nutrients. 2021; 13(8):2792. https://doi.org/10.3390/nu13082792
Chicago/Turabian StyleHsiao, Yu-Ping, Hsiao-Ling Chen, Jen-Ning Tsai, Meei-Yn Lin, Jiunn-Wang Liao, Meng-Syuan Wei, Jiunn-Liang Ko, and Chu-Chyn Ou. 2021. "Administration of Lactobacillus reuteri Combined with Clostridium butyricum Attenuates Cisplatin-Induced Renal Damage by Gut Microbiota Reconstitution, Increasing Butyric Acid Production, and Suppressing Renal Inflammation" Nutrients 13, no. 8: 2792. https://doi.org/10.3390/nu13082792
APA StyleHsiao, Y.-P., Chen, H.-L., Tsai, J.-N., Lin, M.-Y., Liao, J.-W., Wei, M.-S., Ko, J.-L., & Ou, C.-C. (2021). Administration of Lactobacillus reuteri Combined with Clostridium butyricum Attenuates Cisplatin-Induced Renal Damage by Gut Microbiota Reconstitution, Increasing Butyric Acid Production, and Suppressing Renal Inflammation. Nutrients, 13(8), 2792. https://doi.org/10.3390/nu13082792






