Vitamin D and Hashimoto’s Thyroiditis: Observations from CROHT Biobank
Abstract
:1. Introduction
2. Materials and Methods
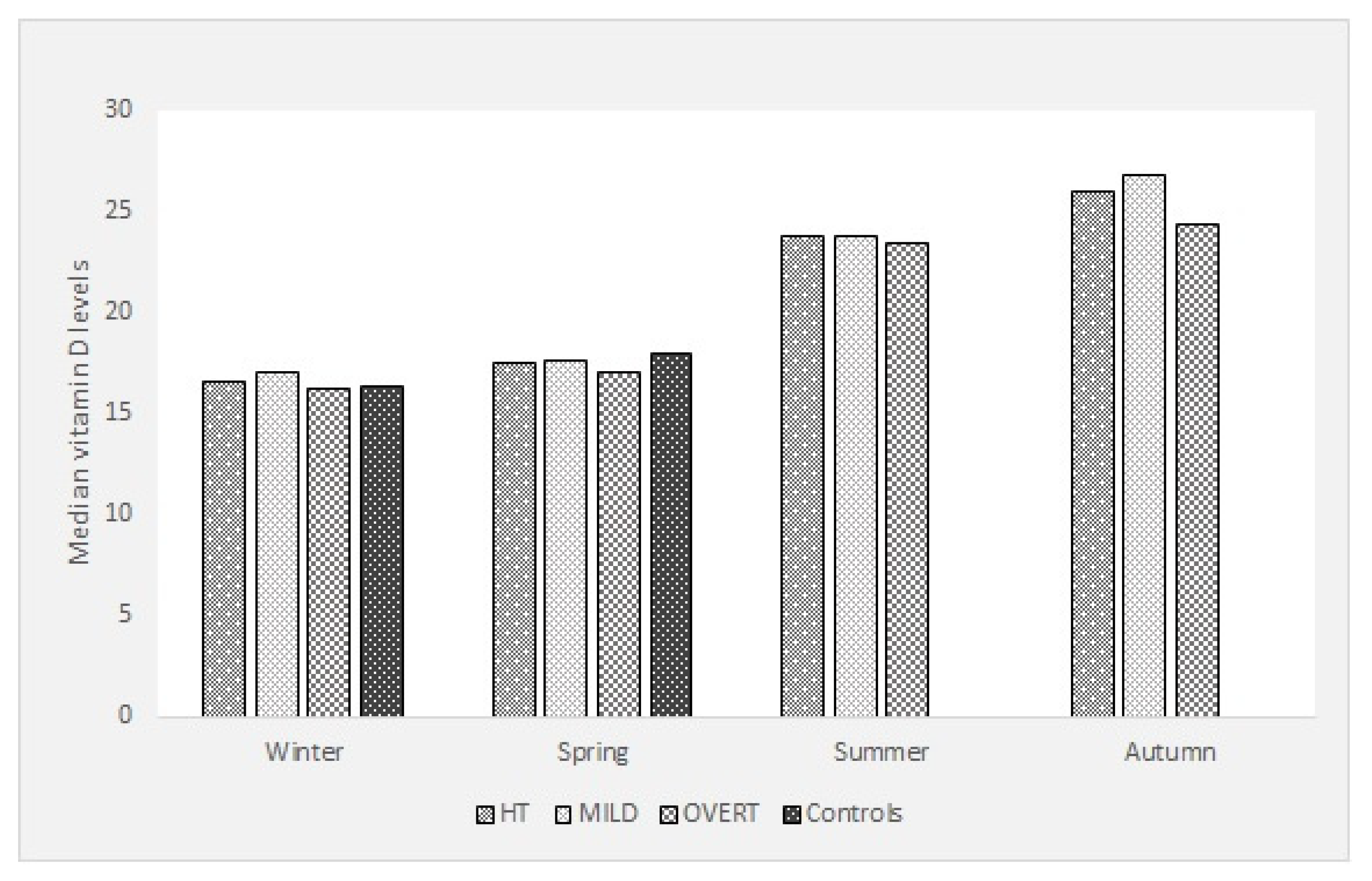
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nettore, I.C.; Albano, L.; Ungaro, P.; Colao, A.; Macchia, P.E. Sunshine vitamin and thyroid. Rev. Endocr. Metab. Disord. 2017, 18, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [Green Version]
- Muscogiuri, G.; Tirabassi, G.; Bizzaro, G.; Orio, F.; Paschou, S.A.; Vryonidou, A.; Balercia, G.; Shoenfeld, Y.; Colao, A. Vitamin D and thyroid disease: To D or not to D? Eur. J. Clin. Nutr. 2015, 69, 291–296. [Google Scholar] [CrossRef]
- Mele, C.; Caputo, M.; Bisceglia, A.; Samà, M.T.; Zavattaro, M.; Aimaretti, G.; Pagano, L.; Prodam, F.; Marzullo, P. Immunomodulatory Effects of Vitamin D in Thyroid Diseases. Nutrients 2020, 12, 1444. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhang, W.; Ma, C.; Zhao, Y.; Xiong, R.; Wang, H.; Chen, W.; Guo Zheng, S. Immunomodulatory Function of Vitamin D and Its Role in Auto-immune Thyroid Disease. Front. Immunol. 2021, 12, 352. [Google Scholar]
- Vondra, K.; Stárka, L.; Hampl, R. Vitamin D and Thyroid Diseases. Physiol. Res. 2015, 64, S95–S100. [Google Scholar] [CrossRef]
- Gallo, D.; Mortara, L.; Gariboldi, M.B.; Cattaneo, S.A.M.; Rosetti, S.; Gentile, L.; Noonan, D.M.; Premoli, P.; Cusini, C.; Tanda, M.L.; et al. Immunomodulatory effect of vitamin D and its potential role in the prevention and treatment of thyroid autoimmunity: A narrative review. J. Endocrinol. Investig. 2019, 43, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Kowalówka, M.; Główka, A.K.; Karaźniewicz-Łada, M.; Kosewski, G. Clinical Significance of Analysis of Vitamin D Status in Various Diseases. Nutrients 2020, 12, 2788. [Google Scholar] [CrossRef]
- Pilz, S.; Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; Keppel, M.H.; Grübler, M.R.; März, W.; Pandis, M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019, 8, R27–R43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.; Binkley, N.C.; Bischoff-Ferrari, H.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.S.; Hewison, M. Update in vitamin D. J. Clin. Endocrinol. Metab. 2010, 95, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.Y.; Kim, K.J.; Kim, D.; Hwang, S.; Lee, E.J. Low Serum Vitamin D Is Associated with Anti-Thyroid Peroxidase Antibody in Autoimmune Thyroiditis. Yonsei Med. J. 2014, 55, 476–481. [Google Scholar] [CrossRef] [Green Version]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caturegli, P.; De Remigis, A.; Rose, N. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.A.; Cooper, D.S. The incidence and prevalence of thyroid autoimmunity. Endocrine 2012, 42, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Merrill, S.J.; Mu, Y. Thyroid autoimmunity as a window to autoimmunity: An explanation for sex differences in the prevalence of thyroid autoimmunity. J. Theor. Biol. 2015, 375, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiromatsu, Y.; Satoh, H.; Amino, N. Hashimoto’s Thyroiditis: History and Future Outlook. Hormones 2013, 12, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Zaletel, K.; Gaberšček, S. Hashimoto’s Thyroiditis: From Genes to the Disease. Curr. Genom. 2011, 12, 576–588. [Google Scholar] [CrossRef] [Green Version]
- Brix, T.H.; Hegedüs, L. Twin studies as a model for exploring the aetiology of autoimmune thyroid disease. Clin. Endocrinol. 2012, 76, 457–464. [Google Scholar] [CrossRef]
- Kolypetri, P.; King, J.; Larijani, M.; Carayanniotis, G. Genes and Environment as Predisposing Factors in Autoimmunity: Acceleration of Spontaneous Thyroiditis by Dietary Iodide in NOD.H2(h4) Mice. Int. Rev. Immunol. 2015, 34, 542–556. [Google Scholar] [CrossRef]
- Wu, L.; Yu, J.-C.; Kang, W.-M.; Ma, Z.-Q. Iodine nutrition and thyroid diseases. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2013, 35, 1–8. [Google Scholar]
- Desailloud, R.; Hober, D. Viruses and thyroiditis: An update. Virol. J. 2009, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Burek, C.L.; Talor, M.V. Environmental triggers of autoimmune thyroiditis. J. Autoimmun. 2009, 33, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Liontiris, M.; Mazokopakis, E. A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients: Points that need more investigation. Hell. J. Nucl. Med. 2017, 20, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Tamer, G.; Arik, S.; Tamer, I.; Coksert, D. Relative vitamin D insufficiency in Hashimoto’s thyroiditis. Thyroid 2011, 21, 891–896. [Google Scholar] [CrossRef]
- Ventura, M.; Melo, M.; Carrilho, F. Selenium and Thyroid Disease: From Pathophysiology to Treatment. Int. J. Endocrinol. 2017, 2017, 1297658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goswami, R.; Marwaha, R.K.; Gupta, N.; Tandon, N.; Sreenivas, V.; Tomar, N.; Ray, D.; Kanwar, R.; Agarwal, R. Prevalence of vitamin D deficiency and its relationship with thyroid autoimmunity in Asian Indians: A community-based survey. Br. J. Nutr. 2009, 102, 382–386. [Google Scholar] [CrossRef] [Green Version]
- Chao, G.; Zhu, Y.; Fang, L. Correlation Between Hashimoto’s Thyroiditis-Related Thyroid Hormone Levels and 25-Hydroxyvitamin D. Front. Endocrinol. 2020, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Stefanić, M.; Tokić, S. Serum 25-hydoxyvitamin D concentrations in relation to Hashimoto’s thyroiditis: A systematic review, meta-analysis and meta-regression of observational studies. Eur. J. Nutr. 2020, 59, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Botelho, I.M.B.; Neto, A.M.; Silva, C.A.; Tambascia, M.A.; Alegre, S.M.; Zantut-Wittmann, D.E. Vitamin D in Hashimoto’s thyroiditis and its relationship with thyroid function and inflammatory status. Endocr. J. 2018, 65, 1029–1037. [Google Scholar] [CrossRef] [Green Version]
- Effraimidis, G.; Badenhoop, K.; Tijssen, J.G.P.; Wiersinga, W.M. Vitamin D deficiency is not associated with early stages of thyroid autoimmunity. Eur. J. Endocrinol. 2012, 167, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Musa, I.R.; Gasim, G.I.; Khan, S.; Ibrahim, I.A.; Abo-Alazm, H.; Adam, I. No Association between 25 (OH) Vitamin D Level and Hypothyroidism among Females. Open Access Maced. J. Med. Sci. 2017, 5, 126–130. [Google Scholar] [CrossRef] [Green Version]
- Yasmeh, J.; Farpour, F.; Rizzo, V.; Kheradnam, S.; Sachmechi, I. Hashimoto Thyroiditis not Associated with Vitamin D Deficiency. Endocr. Pract. 2016, 22, 809–813. [Google Scholar] [CrossRef]
- Brčić, L.; Barić, A.; Gračan, S.; Brekalo, M.; Kaličanin, D.; Gunjača, I.; Torlak Lovrić, V.; Tokić, S.; Radman, M.; Škrabić, V.; et al. Genome-wide association analysis suggests novel loci for Hashimoto’s thyroiditis. J. Endocrinol. Investig. 2018, 42, 567–576. [Google Scholar] [CrossRef]
- Brčić, L.; Barić, A.; Benzon, B.; Brekalo, M.; Gračan, S.; Kaličanin, D.; Škrabić, V.; Zemunik, T.; Barbalić, M.; Novak, I.; et al. AATF and SMARCA2 are associated with thyroid volume in Hashimoto’s thyroiditis patients. Sci. Rep. 2020, 10, 1754. [Google Scholar] [CrossRef]
- Ovesen, L.; Andersen, R.; Jakobsen, J. Geographical differences in vitamin D status, with particular reference to European coun-tries. Proc. Nutr. Soc. 2003, 62, 813–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.-W.; Liu, K.-Q.; Wang, P.-Y.; Liu, J.-Q.; Chen, J.-Y.; Xu, X.-J.; Xu, J.-J.; Qiu, M.-C.; Sun, Y.; Liu, C.; et al. Cohort profile: The Westlake BioBank for Chinese (WBBC) pilot project. BMJ Open 2021, 11, e045564. [Google Scholar] [CrossRef]
- Tirabassi, G.; Cutini, M.; Salvio, G.; Cerqueni, G.; Lenzi, A.; Balercia, G. Influence of vitamin D levels on the cardiovascular profile of hypogonadal men. J. Endocrinol. Investig. 2017, 40, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Lombardi, G.; Strollo, M.; Pontillo, M.; Motta, A.; Locatelli, M. Association between solar ultraviolet doses and vitamin D clinical routine data in European mid-latitude population between 2006 and 2018. Photochem. Photobiol. Sci. 2019, 18, 2696–2706. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, Q.; Xia, Z.; Zheng, S.; Zeng, L.; Han, L.; Yan, J.; Ke, P.; Zhuang, J.; Wu, X.; et al. Determination of serum 25-hydroxyvitamin D status among population in southern China by a high accuracy LC-MS/MS method traced to reference measurement procedure. Nutr. Metab. 2020, 17, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Won, J.W.; Jung, S.K.; Jung, I.A.; Lee, Y. Seasonal Changes in Vitamin D Levels of Healthy Children in Mid-Latitude, Asian Urban Area. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.; Brabant, G.; Duntas, L.; Monzani, F.; Peeters, R.P.; Razvi, S.; Wemeau, J.-L. 2013 ETA Guideline: Management of Subclinical Hypothyroidism. Eur. Thyroid J. 2013, 2, 215–228. [Google Scholar] [CrossRef] [Green Version]
- Brunn, J.; Block, U.; Ruf, G.; Bos, I.; Kunze, W.P.; Scriba, P.C. Volumetric analysis of thyroid lobes by real-time ultrasound. Dtsch. Med. Wochenschr. 1981, 106, 1338–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Aurizio, F.; Villalta, D.; Metus, P.; Doretto, P.; Tozzoli, R. Is vitamin D a player or not in the pathophysiology of autoimmune thyroid diseases? Autoimmun. Rev. 2015, 14, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Katrinaki, M.; Kampa, M.; Margioris, A.; Castanas, E.; Malliaraki, N. Vitamin D levels in a large Mediterranean cohort: Reconsidering normal cut-off values. Hormones 2016, 15, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Evliyaoğlu, O.; Acar, M.; Özcabı, B.; Erginöz, E.; Bucak, F.; Ercan, O.; Kucur, M. Vitamin D Deficiency and Hashimoto’s Thyroiditis in Children and Adolescents: A Critical Vitamin D Level for This Association? J. Clin. Res. Pediatr. Endocrinol. 2015, 7, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, D. Low vitamin D status is associated with hypothyroid Hashimoto’s thyroiditis. Hormones 2016, 15, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Kivity, S.; Agmon-Levin, N.; Zisappl, M.; Shapira, Y.; Nagy, E.V.; Dankó, K.; Szekanecz, Z.; Langevitz, P.; Shoenfeld, Y. Vitamin D and autoimmune thyroid diseases. Cell. Mol. Immunol. 2011, 8, 243–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chailurkit, L.-O.; Aekplakorn, W.; Ongphiphadhanakul, B. High Vitamin D Status in Younger Individuals Is Associated with Low Circulating Thyrotropin. Thyroid 2013, 23, 25–30. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Sun, M.; Cao, M.; Zhu, Z.; Fu, Q.; Gao, Y.; Mao, J.; Li, Y.; Shi, Y.; et al. Association of high vitamin d status with low circulating thyroid-stimulating hormone independent of thyroid hormone levels in middle-aged and elderly males. Int. J. Endocrinol. 2014, 631819, 16. [Google Scholar] [CrossRef]
- Barchetta, I.; Baroni, M.G.; Leonetti, F.; De Bernardinis, M.; Bertoccini, L.; Fontana, M.; Mazzei, E.; Fraioli, A.; Cavallo, M.G. TSH levels are associated with vitamin D status and seasonality in an adult population of euthyroid adults. Clin. Exp. Med. 2014, 15, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Taylor, P.N.; Javaid, H.; Tennant, B.P.; Geen, J.; Aldridge, A.; Okosieme, O. Seasonal Variation of Vitamin D and Serum Thyrotropin Levels and Its Relationship in a Euthyroid Caucasian Population. Endocr. Pract. 2018, 24, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Fonseca Valle, D.; Giannini, D.T. Correlation between vitamin D and blood pressure in adolescents. Int. J. Adolesc. Med. Health 2019, 32, 20170165. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, L.; Li, C.; Gai, Z.; Li, Y. Vitamin D and Vitamin D Receptor: New Insights in the Treatment of Hypertension. Curr. Protein Pept. Sci. 2019, 20, 984–995. [Google Scholar] [CrossRef] [PubMed]
| HT Patients | ||||
|---|---|---|---|---|
| Phenotype | ALL | MILD | OVERT | Controls |
| (N = 461) | (N = 240) | (N = 219) | (N = 176) | |
| Median (Q1–Q3) | Median (Q1–Q3) | Median (Q1–Q3) | Median (Q1–Q3) | |
| Age, years | 38.02 (27.76–48.49) | 35.81 (25.78–46.95) | 40.28 (31.04–50.37) | 35.17 (30.12–44.31) |
| BMI, kg/m2 | 23.52 (20.76–26.85) | 23.15 (20.72–26.59) | 24.01 (21.02–26.99) | 22.66 (20.96–25.24) |
| TSH, mIU/L | 3.33 (1.74–5.68) | 3.23 (1.82–4.74) | 3.52 (1.67–12.30) | 1.48 (1.14–1.95) |
| T3, nmol/L | 1.60 (1.30–1.80) | 1.70 (1.50–1.90) | 1.50 (1.20–1.80) | 1.50 (1.40–1.70) |
| T4, nmol/L | 105 (89–118) | 106 (91–117.25) | 103 (84.85–121) | 101 (89.37–116) |
| fT4, pmol/L | 12.10 (10.20–13.20) | 12.10 (10.90–13.10) | 11.90 (9.90–13.70) | 12.70 (11.90–13.70) |
| TgAb, IU/ml | 135 (36.40–422.40) | 121.50 (26.40–321.30) | 192 (49.30–596.05) | 10.75 (9.10–18.00) |
| TPOAb, IU/ml | 212 (27.60–652.90) | 161.50 (17.40–529.75) | 273 (66.40–945.50) | 3.40 (1.20–8.70) |
| Thyroid volume, cm3 | 9.85 (7.30–13.91) | 9.89 (7.72–13.26) | 9.59 (6.82–14.90) | 8.69 (6.63–10.60) |
| No. of symptoms | 4 (1–7) | 3 (1–6) | 5 (2–8) | / |
| Systolic bp, mmHg | 120 (110–130) | 115 (110–130) | 120 (110–130) | 110 (100–120) |
| Diastolic bp, mmHg | 70 (65–80) | 70 (65–78.75) | 70 (65–80) | 65 (60–80) |
| Tested Group | N 1 | N 2 | Median 1 (Q1–Q3) | Median 2 (Q1–Q3) | OR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| HT vs. controls a | 461 | 175 c | 19.7 (14.4–25.2) | 17.3 (13.2–22.7) | 0.987 (0.958–1.017) | 0.401 |
| HT vs. controls b | 303 | 175 | 17.1 (13.2–22.2) | 17.3 (13.2–22.7) | 0.983 (0.954–1.014) | 0.277 |
| MILD vs. controls a | 240 | 175 | 20.7 (14.9–25.8) | 17.3 (13.2–22.7) | 1.005 (0.970–1.041) | 0.788 |
| MILD vs. controls b | 155 | 175 | 17.7 (14.0–22.6) | 17.3 (13.2–22.7) | 1.002 (0.966–1.038) | 0.927 |
| OVERT vs. controls a | 219 | 175 | 19 (13.9–24.5) | 17.3 (13.2–22.7) | 0.971 (0.937–1.006) | 0.105 |
| OVERT vs. controls b | 147 | 175 | 16.7 (12.1–21.7) | 17.3 (13.2–22.7) | 0.966 (0.930–1.002) | 0.065 |
| MILD vs. OVERT a | 240 | 219 | 20.7 (14.9–25.8) | 19 (13.9–24.5) | 1.038 (1.005–1.071) | 0.023 |
| Tested Group | N 1 | N 2 | Proportion 1 (%) | Proportion 2 (%) | p-Value (χ2-Test) |
|---|---|---|---|---|---|
| HT vs. controls a | 196 | 107 | 64.69 | 60.79 | 0.394 |
| MILD vs. controls a | 94 | 107 | 60.64 | 60.79 | 0.977 |
| OVERT vs. controls a | 101 | 107 | 68.7 | 60.79 | 0.139 |
| MILD vs. OVERT b | 115 | 123 | 47.92 | 56.16 | 0.077 |
| Phenotype | ALL (N = 461) | MILD (N = 240) | OVERT (N = 219) | |
|---|---|---|---|---|
| TSH | r | −0.113 | −0.092 | −0.015 |
| p | 0.029 | 0.2 | 0.837 | |
| T3 | r | −0.008 | −0.013 | −0.135 |
| p | 0.882 | 0.857 | 0.07 | |
| T4 | r | 0.026 | −0.025 | 0.053 |
| p | 0.622 | 0.73 | 0.478 | |
| fT4 | r | 0.011 | −0.009 | −0.006 |
| p | 0.832 | 0.906 | 0.941 | |
| TgAb | r | −0.048 | −0.002 | −0.026 |
| p | 0.357 | 0.979 | 0.724 | |
| TPOAb | r | −0.039 | −0.058 | 0.047 |
| p | 0.45 | 0.419 | 0.53 | |
| Thyroid volume | r | −0.004 | 0.009 | 0.013 |
| p | 0.947 | 0.899 | 0.867 | |
| No. of hypothyroid sym. | r | 0.018 | 0.11 | −0.032 |
| p | 0.755 | 0.178 | 0.693 | |
| Systolic blood pressure | r | 0.073 | −0.03 | 0.205 |
| p | 0.249 | 0.731 | 0.025 | |
| Diastolic blood pressure | r | −0.001 | −0.04 | 0.112 |
| p | 0.987 | 0.644 | 0.227 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cvek, M.; Kaličanin, D.; Barić, A.; Vuletić, M.; Gunjača, I.; Torlak Lovrić, V.; Škrabić, V.; Punda, A.; Boraska Perica, V. Vitamin D and Hashimoto’s Thyroiditis: Observations from CROHT Biobank. Nutrients 2021, 13, 2793. https://doi.org/10.3390/nu13082793
Cvek M, Kaličanin D, Barić A, Vuletić M, Gunjača I, Torlak Lovrić V, Škrabić V, Punda A, Boraska Perica V. Vitamin D and Hashimoto’s Thyroiditis: Observations from CROHT Biobank. Nutrients. 2021; 13(8):2793. https://doi.org/10.3390/nu13082793
Chicago/Turabian StyleCvek, Maja, Dean Kaličanin, Ana Barić, Marko Vuletić, Ivana Gunjača, Vesela Torlak Lovrić, Veselin Škrabić, Ante Punda, and Vesna Boraska Perica. 2021. "Vitamin D and Hashimoto’s Thyroiditis: Observations from CROHT Biobank" Nutrients 13, no. 8: 2793. https://doi.org/10.3390/nu13082793
APA StyleCvek, M., Kaličanin, D., Barić, A., Vuletić, M., Gunjača, I., Torlak Lovrić, V., Škrabić, V., Punda, A., & Boraska Perica, V. (2021). Vitamin D and Hashimoto’s Thyroiditis: Observations from CROHT Biobank. Nutrients, 13(8), 2793. https://doi.org/10.3390/nu13082793






