Physiological Functions of Threonine in Animals: Beyond Nutrition Metabolism
Abstract
1. Introduction
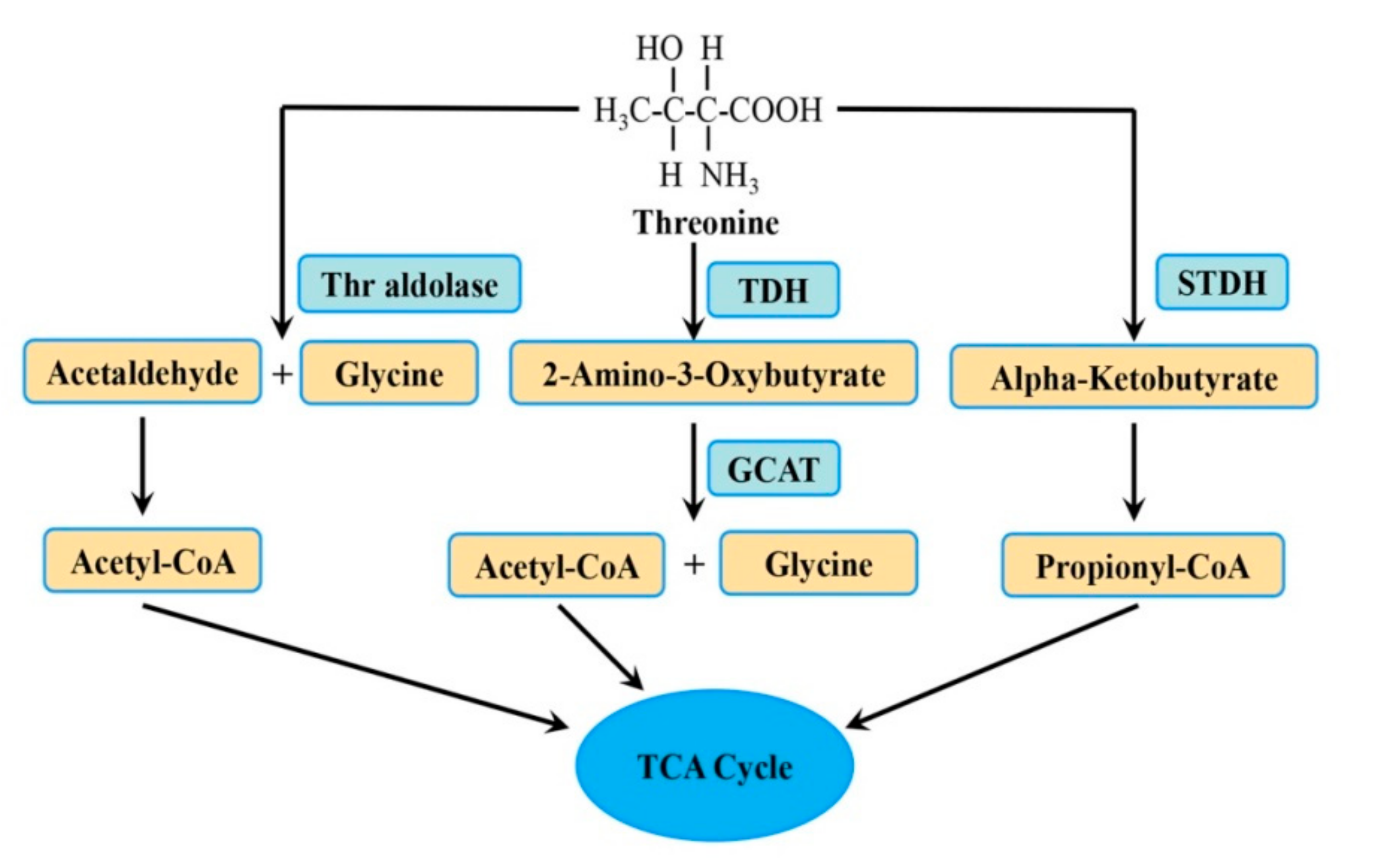
2. The Metabolic Pathway of Thr
3. Role of Thr in Nutrition Metabolism
3.1. Lipid Metabolism
3.2. Protein Synthesis
4. The Effects of Thr Metabolism on ESC Function
4.1. Proliferation and Differentiation
4.2. Epigenetic Regulation
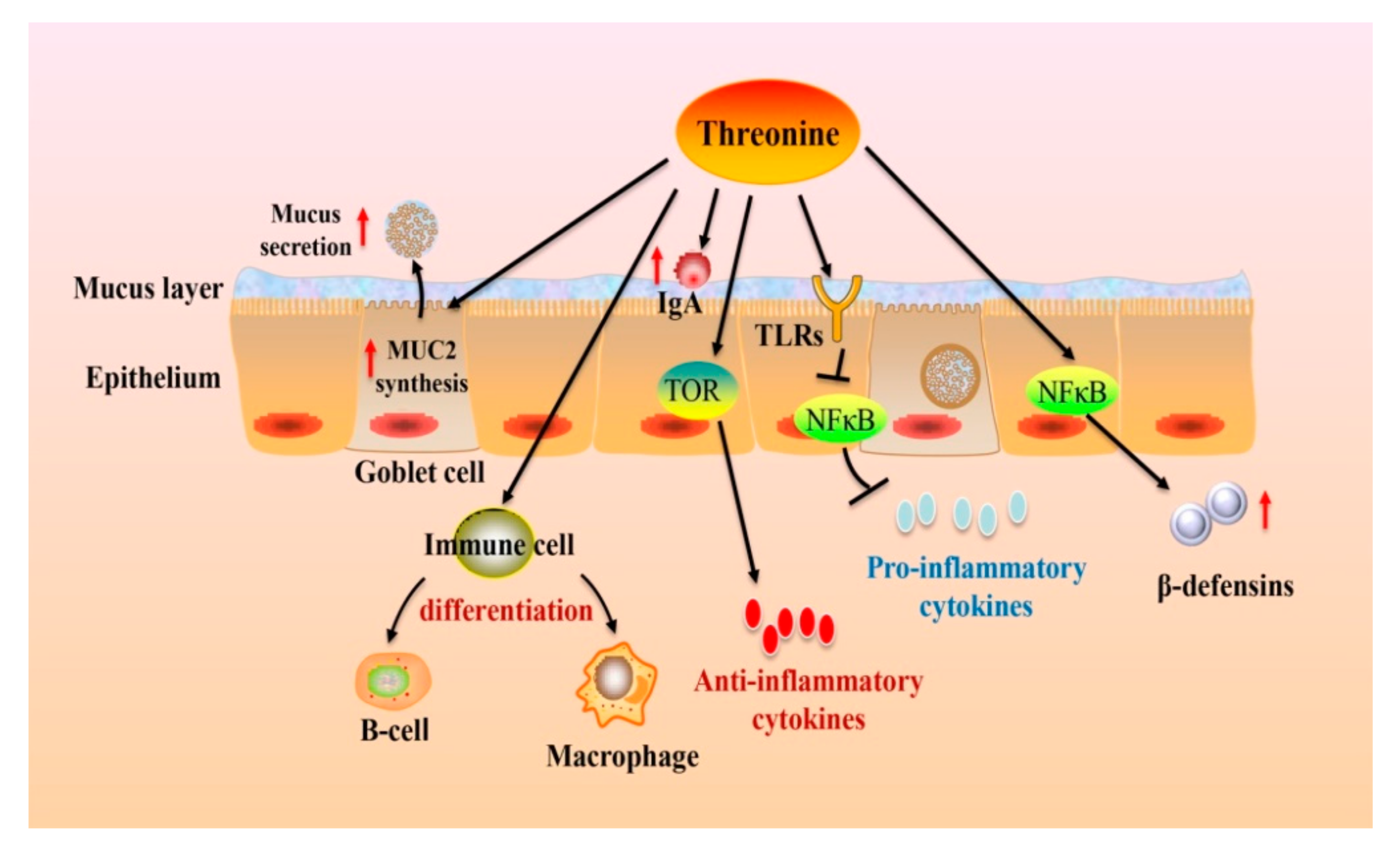
5. The Roles of Thr on Intestinal Health and Functions
5.1. Intestinal Thr Uptake and Utilization
5.2. The Effects of Thr on Intestinal Health and Function
5.2.1. Nutrient Digestibility
5.2.2. Gut Microbiota
5.2.3. Barrier Function
5.2.4. Immune Function
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kidd, M.T.; Kerr, B.J. L-Threonine for Poultry: A Review. J. Appl. Poult. Res. 1996, 5, 358–367. [Google Scholar] [CrossRef]
- Chen, Y.P.; Cheng, Y.F.; Li, X.H.; Yang, W.L.; Wen, C.; Zhuang, S.; Zhou, Y.M. Effects of threonine supplementation on the growth performance, immunity, oxidative status, intestinal integrity, and barrier function of broilers at the early age. Poult. Sci. 2017, 96, 405–413. [Google Scholar] [CrossRef]
- Karau, A.; Grayson, I. Amino acids in human and animal nutrition. Adv. Biochem. Eng. Biotechnol. 2014, 143, 189–228. [Google Scholar] [CrossRef]
- Estalkhzir, F.M.; Khojasteh, S.; Jafari, M. The Effect of Different Levels of Threonine on Performance and Carcass Characteristics of Broiler Chickens. J. Nov. Appl. Sci. 2013, 9, 382–386. [Google Scholar]
- Doepel, L.; Hewage, I.I.; Lapierre, H. Milk protein yield and mammary metabolism are affected by phenylalanine deficiency but not by threonine or tryptophan deficiency. J. Dairy Sci. 2016, 99, 3144–3156. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, X.; Mao, X.; Wu, G.; Qiao, S. Optimal dietary true ileal digestible threonine for supporting the mucosal barrier in small intestine of weanling pigs. J. Nutr. 2010, 140, 981–986. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Cheng, Y.; Li, Y.; Wen, C.; Zhou, Y. Dietary l-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Br. J. Nutr. 2018, 119, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Ross-Inta, C.M.; Zhang, Y.F.; Almendares, A.; Giulivi, C. Threonine-deficient diets induced changes in hepatic bioenergetics. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1130–G1139. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhou, X.; Sun, Y.; Hu, L.; Zhu, J.; Shao, C.; Meng, Q.; Shan, A. Threonine, but Not Lysine and Methionine, Reduces Fat Accumulation by Regulating Lipid Metabolism in Obese Mice. J. Agric. Food Chem. 2020, 68, 4876–4883. [Google Scholar] [CrossRef] [PubMed]
- Ochocki, J.D.; Simon, M.C. Nutrient-sensing pathways and metabolic regulation in stem cells. J. Cell Biol. 2013, 203, 23–33. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, J.H.; Wang, P.; Go, C.D.; Hesketh, G.G.; Gingras, A.C.; Jafarnejad, S.M.; Sonenberg, N. Mitochondrial Threonyl-tRNA Synthetase TARS2 Is Required for Threonine-Sensitive mTORC1 Activation. Mol. Cell. 2021, 81, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Daley, G.Q. Stem cells and the evolving notion of cellular identity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140376. [Google Scholar] [CrossRef]
- Boyd, A.; Newsome, P.; Lu, W.Y. The role of stem cells in liver injury and repair. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 623–631. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J. Threonine metabolism and embryonic stem cell self-renewal. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Schaart, M.W.; Schierbeek, H.; van der Schoor, S.R.; Stoll, B.; Burrin, D.G.; Reeds, P.J.; van Goudoever, J.B. Threonine utilization is high in the intestine of piglets. J. Nutr. 2005, 135, 765–770. [Google Scholar] [CrossRef]
- Habte-Tsion, H.M.; Ge, X.; Liu, B.; Xie, J.; Ren, M.; Zhou, Q.; Miao, L.; Pan, L.; Chen, R. A deficiency or an excess of dietary threonine level affects weight gain, enzyme activity, immune response and immune-related gene expression in juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2015, 42, 439–446. [Google Scholar] [CrossRef]
- Gaifem, J.; Gonçalves, L.G.; Dinis-Oliveira, R.J.; Cunha, C.; Carvalho, A.; Torrado, E.; Rodrigues, F.; Saraiva, M.; Castro, A.G.; Silvestre, R. L-Threonine Supplementation during Colitis Onset Delays Disease Recovery. Front. Physiol. 2018, 9, 1247. [Google Scholar] [CrossRef]
- House, J.D.; Hall, B.N.; Brosnan, J.T. Threonine metabolism in isolated rat hepatocytes. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1300–E1307. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Sève, B. Catabolism through the threonine dehydrogenase pathway does not account for the high first-pass extraction rate of dietary threonine by the portal drained viscera in pigs. Br. J. Nutr. 2005, 93, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Edgar, A.J. Mice have a transcribed L-threonine aldolase/GLY1 gene, but the human GLY1 gene is a non-processed pseudogene. BMC Genom. 2005, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Parimi, P.S.; Gruca, L.L.; Kalhan, S.C. Metabolism of threonine in newborn infants. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E981–E985. [Google Scholar] [CrossRef]
- Darling, P.B.; Grunow, J.; Rafii, M.; Brookes, S.; Ball, R.O.; Pencharz, P.B. Threonine dehydrogenase is a minor degradative pathway of threonine catabolism in adult humans. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E877–E884. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xie, M.; Fan, W.; Xue, J.; Zhou, Z.; Tang, J.; Chen, G.; Hou, S. Transcriptome Analysis Reveals Differential Expression of Genes Regulating Hepatic Triglyceride Metabolism in Pekin Ducks During Dietary Threonine Deficiency. Front. Genet. 2019, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, N.; Song, P.; He, T.; Levesque, C.; Bai, Y.; Zhang, A.; Ma, X. Grape Seed Proanthocyanidin Affects Lipid Metabolism via Changing Gut Microflora and Enhancing Propionate Production in Weaned Pigs. J. Nutr. 2019, 149, 1523–1532. [Google Scholar] [CrossRef]
- Lasar, D.; Rosenwald, M.; Kiehlmann, E.; Balaz, M.; Tall, B.; Opitz, L.; Lidell, M.E.; Zamboni, N.; Krznar, P.; Sun, W.; et al. Peroxisome Proliferator Activated Receptor Gamma Controls Mature Brown Adipocyte Inducibility through Glycerol Kinase. Cell Rep. 2018, 22, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef]
- Wang, F.H.; Liu, J.; Deng, Q.J.; Qi, Y.; Wang, M.; Wang, Y.; Zhang, X.G.; Zhao, D. Association between plasma essential amino acids and atherogenic lipid profile in a Chinese population: A cross-sectional study. Atherosclerosis 2019, 286, 7–13. [Google Scholar] [CrossRef]
- Piper, M.D.W.; Soultoukis, G.A.; Blanc, E.; Mesaros, A.; Herbert, S.L.; Juricic, P.; He, X.; Atanassov, I.; Salmonowicz, H.; Yang, M.; et al. Matching Dietary Amino Acid Balance to the In Silico-Translated Exome Optimizes Growth and Reproduction without Cost to Lifespan. Cell Metab. 2017, 25, 1206. [Google Scholar] [CrossRef]
- Sjøberg, K.A.; Schmoll, D.; Piper, M.D.W.; Kiens, B.; Rose, A.J. Effects of Short-Term Dietary Protein Restriction on Blood Amino Acid Levels in Young Men. Nutrients 2020, 12, 2195. [Google Scholar] [CrossRef] [PubMed]
- Maida, A.; Zota, A.; Sjøberg, K.A.; Schumacher, J.; Sijmonsma, T.P.; Pfenninger, A.; Christensen, M.M.; Gantert, T.; Fuhrmeister, J.; Rothermel, U.; et al. A liver stress-endocrine nexus promotes metabolic integrity during dietary protein dilution. J. Clin. Investig. 2016, 126, 3263–3278. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Cummings, N.E.; Arriola Apelo, S.I.; Neuman, J.C.; Kasza, I.; Schmidt, B.A.; Cava, E.; Spelta, F.; Tosti, V.; Syed, F.A.; et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep. 2016, 16, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.W.; Rusu, P.M.; Chan, A.Y.; Fam, B.C.; Jungmann, A.; Solon-Biet, S.M.; Barlow, C.K.; Creek, D.J.; Huang, C.; Schittenhelm, R.B.; et al. Restriction of essential amino acids dictates the systemic metabolic response to dietary protein dilution. Nat. Commun. 2020, 11, 2894. [Google Scholar] [CrossRef]
- Green, C.L.; Lamming, D.W. Regulation of metabolic health by essential dietary amino acids. Mech. Ageing Dev. 2019, 177, 186–200. [Google Scholar] [CrossRef]
- Faure, M.; Moënnoz, D.; Montigon, F.; Mettraux, C.; Breuillé, D.; Ballèvre, O. Dietary threonine restriction specifically reduces intestinal mucin synthesis in rats. J. Nutr. 2005, 135, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiao, S.; Yin, Y.; Yue, L.; Wang, Z.; Wu, G. A deficiency or excess of dietary threonine reduces protein synthesis in jejunum and skeletal muscle of young pigs. J. Nutr. 2007, 137, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Jiang, W.; Kuang, S.; Hu, K.; Tang, L.; Liu, Y.; Jiang, J.; Zhang, Y.; Zhou, X.; Feng, L. Growth, digestive and absorptive capacity and antioxidant status in intestine and hepatopancreas of sub-adult grass carp Ctenopharyngodonidella fed graded levels of dietary threonine. J. Anim. Sci. Biotechnol. 2015, 6, 34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rabinovsky, E.D.; Gelir, E.; Gelir, S.; Lui, H.; Kattash, M.; DeMayo, F.J.; Shenaq, S.M.; Schwartz, R.J. Targeted expression of IGF-1 transgene to skeletal muscle accelerates muscle and motor neuron regeneration. FASEB J. 2003, 17, 53–55. [Google Scholar] [CrossRef]
- Yoon, M.S. mTOR as a Key Regulator in Maintaining Skeletal Muscle Mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Q.; Zhou, X.Q.; Xu, S.X.; Feng, L.; Liu, Y.; Jiang, W.D.; Wu, P.; Zhao, J.; Jiang, J. Effect of dietary threonine on growth performance and muscle growth, protein synthesis and antioxidant-related signalling pathways of hybrid catfish Pelteobagrus vachelli × Leiocassis longirostris. Br. J. Nutr. 2020, 123, 121–134. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Lin, G.; Hu, S.; Wang, B.; Dai, Z.; Wu, G. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J. Nutr. 2014, 144, 1540–1548. [Google Scholar] [CrossRef]
- Wang, J.; Alexander, P.; Wu, L.; Hammer, R.; Cleaver, O.; McKnight, S.L. Dependence of mouse embryonic stem cells on threonine catabolism. Science 2009, 325, 435–439. [Google Scholar] [CrossRef]
- Liu, K.; Cao, J.; Shi, X.; Wang, L.; Zhao, T. Cellular metabolism and homeostasis in pluripotency regulation. Protein Cell 2020, 11, 630–640. [Google Scholar] [CrossRef]
- Washington, J.M.; Rathjen, J.; Felquer, F.; Lonic, A.; Bettess, M.D.; Hamra, N.; Semendric, L.; Tan, B.S.; Lake, J.A.; Keough, R.A.; et al. L-Proline induces differentiation of ES cells: A novel role for an amino acid in the regulation of pluripotent cells in culture. Am. J. Physiol. Cell Physiol. 2010, 298, C982–C992. [Google Scholar] [CrossRef]
- Kilberg, M.S.; Terada, N.; Shan, J. Influence of Amino Acid Metabolism on Embryonic Stem Cell Function and Differentiation. Adv. Nutr. 2016, 7, 780s–789s. [Google Scholar] [CrossRef] [PubMed]
- Alexander, P.B.; Wang, J.; McKnight, S.L. Targeted killing of a mammalian cell based upon its specialized metabolic state. Proc. Natl. Acad. Sci. USA 2011, 108, 15828–15833. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Gu, H.; Wang, J.; Lu, W.; Mei, Y.; Wu, M. Regulation of L-threonine dehydrogenase in somatic cell reprogramming. Stem Cells 2013, 31, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Shyh-Chang, N.; Locasale, J.W.; Lyssiotis, C.A.; Zheng, Y.; Teo, R.Y.; Ratanasirintrawoot, S.; Zhang, J.; Onder, T.; Unternaehrer, J.J.; Zhu, H.; et al. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science 2013, 339, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.M.; Han, H.J. L-threonine regulates G1/S phase transition of mouse embryonic stem cells via PI3K/Akt, MAPKs, and mTORC pathways. J. Biol. Chem. 2011, 286, 23667–23678. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J. A regulatory circuitry locking pluripotent stemness to embryonic stem cell: Interaction between threonine catabolism and histone methylation. Semin. Cancer Biol. 2019, 57, 72–78. [Google Scholar] [CrossRef]
- Bertolo, R.F.; Chen, C.Z.; Law, G.; Pencharz, P.B.; Ball, R.O. Threonine requirement of neonatal piglets receiving total parenteral nutrition is considerably lower than that of piglets receiving an identical diet intragastrically. J. Nutr. 1998, 128, 1752–1759. [Google Scholar] [CrossRef]
- Law, G.K.; Bertolo, R.F.; Adjiri-Awere, A.; Pencharz, P.B.; Ball, R.O. Adequate oral threonine is critical for mucin production and gut function in neonatal piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1293–G1301. [Google Scholar] [CrossRef]
- Paris, N.E.; Wong, E.A. Expression of digestive enzymes and nutrient transporters in the intestine of Eimeria maxima-infected chickens. Poult. Sci. 2013, 92, 1331–1335. [Google Scholar] [CrossRef]
- Ma, N.; Ma, X. Dietary Amino Acids and the Gut-Microbiome-Immune Axis: Physiological Metabolism and Therapeutic Prospects. Compr. Rev. Food Sci. Food Saf. 2019, 18, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Moreira Filho, A.L.B.; Ferket, P.R.; Malheiros, R.D.; Oliveira, C.J.B.; Aristimunha, P.C.; Wilsmann, D.E.; Givisiez, P.E.N. Enrichment of the amnion with threonine in chicken embryos affects the small intestine development, ileal gene expression and performance of broilers between 1 and 21 days of age. Poult. Sci. 2019, 98, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, K.; Zhu, W. Amino acid sensing in the gut and its mediation in gut-brain signal transduction. Anim. Nutr. 2016, 2, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.; Lee, J.; Nyachoti, C.M. Diet complexity and l-threonine supplementation: Effects on nutrient digestibility, nitrogen and energy balance, and body composition in nursery pigs. J. Anim. Sci. 2020, 98, skaa124. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, X.; Eicher, S.D.; Ajuwon, K.M.; Applegate, T.J. Effect of threonine deficiency on intestinal integrity and immune response to feed withdrawal combined with coccidial vaccine challenge in broiler chicks. Br. J. Nutr. 2016, 116, 2030–2043. [Google Scholar] [CrossRef]
- Ma, N.; He, T.; Johnston, L.J.; Ma, X. Host-microbiome interactions: The aryl hydrocarbon receptor as a critical node in tryptophan metabolites to brain signaling. Gut Microbes 2020, 11, 1203–1219. [Google Scholar] [CrossRef]
- Munasinghe, L.L.; Robinson, J.L.; Harding, S.V.; Brunton, J.A.; Bertolo, R.F. Protein Synthesis in Mucin-Producing Tissues Is Conserved When Dietary Threonine Is Limiting in Piglets. J. Nutr. 2017, 147, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Neis, E.P.; Dejong, C.H.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, J.; Xie, F.; He, H.; Johnston, L.J.; Dai, X.; Wu, C.; Ma, X. Dietary fiber-derived short-chain fatty acids: A potential therapeutic target to alleviate obesity-related nonalcoholic fatty liver disease. Obes. Rev. 2021. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, N.; Johnston, L.J.; Ma, X. Dietary Nutrients Mediate Intestinal Host Defense Peptide Expression. Adv. Nutr. 2020, 11, 92–102. [Google Scholar] [CrossRef]
- Zaefarian, F.; Zaghari, M.; Shivazad, M. The Threonine Requirements and its Effects on Growth Performance and Gut Morphology of Broiler Chicken Fed Different Levels of Protein. Int. J. Poult. Sci. 2008, 12, 1207–1215. [Google Scholar] [CrossRef]
- Min, Y.N.; Liu, S.G.; Qu, Z.X.; Meng, G.H.; Gao, Y.P. Effects of dietary threonine levels on growth performance, serum biochemical indexes, antioxidant capacities, and gut morphology in broiler chickens. Poult. Sci. 2017, 96, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Hamard, A.; Mazurais, D.; Boudry, G.; Le Huërou-Luron, I.; Sève, B.; Le Floc’h, N. A moderate threonine deficiency affects gene expression profile, paracellular permeability and glucose absorption capacity in the ileum of piglets. J. Nutr. Biochem. 2010, 21, 914–921. [Google Scholar] [CrossRef]
- Dozier, W.A., III; Moran, E.T., Jr.; Kidd, M.T. Male and female broiler responses to low and adequate dietary threonine on nitrogen and energy balance. Poult. Sci. 2001, 80, 926–930. [Google Scholar] [CrossRef]
- Dong, X.Y.; Azzam, M.M.M.; Zou, X.T. Effects of dietary threonine supplementation on intestinal barrier function and gut microbiota of laying hens. Poult. Sci. 2017, 96, 3654–3663. [Google Scholar] [CrossRef]
- Faure, M.; Mettraux, C.; Moennoz, D.; Godin, J.P.; Vuichoud, J.; Rochat, F.; Breuillé, D.; Obled, C.; Corthésy-Theulaz, I. Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium-treated rats. J. Nutr. 2006, 136, 1558–1564. [Google Scholar] [CrossRef]
- Liao, S.F. Invited Review: Maintain or Improve Piglet Gut Health around Weanling: The Fundamental Effects of Dietary Amino Acids. Animals 2021, 11, 1110. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.; Choi, J.; Yang, C.; Nyachoti, C.M. Diet complexity and l-threonine supplementation: Effects on growth performance, immune response, intestinal barrier function, and microbial metabolites in nursery pigs. J. Anim. Sci. 2020, 98. [Google Scholar] [CrossRef]
- Ma, N.; Guo, P.; Zhang, J.; He, T.; Kim, S.W.; Zhang, G.; Ma, X. Nutrients Mediate Intestinal Bacteria-Mucosal Immune Crosstalk. Front. Immunol. 2018, 9, 5. [Google Scholar] [CrossRef]
- McAuley, J.L.; Linden, S.K.; Png, C.W.; King, R.M.; Pennington, H.L.; Gendler, S.J.; Florin, T.H.; Hill, G.R.; Korolik, V.; McGuckin, M.A. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Investig. 2007, 117, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- Montagne, L.; Piel, C.; Lallès, J.P. Effect of diet on mucin kinetics and composition: Nutrition and health implications. Nutr. Rev. 2004, 62, 105–114. [Google Scholar] [CrossRef]
- Wu, G. Intestinal mucosal amino acid catabolism. J. Nutr. 1998, 128, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Azzam, M.M.; Dong, X.Y.; Xie, P.; Zou, X.T. Influence of L-threonine supplementation on goblet cell numbers, histological structure and antioxidant enzyme activities of laying hens reared in a hot and humid climate. Br. Poult. Sci. 2012, 53, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Y.; Li, Y.; Zhang, T.; Ying, Z.; Su, W.; Zhang, L.; Wang, T. l-Threonine improves intestinal mucin synthesis and immune function of intrauterine growth-retarded weanling piglets. Nutrition 2019, 59, 182–187. [Google Scholar] [CrossRef]
- Stanger, B.Z.; Datar, R.; Murtaugh, L.C.; Melton, D.A. Direct regulation of intestinal fate by Notch. Proc. Natl. Acad. Sci. USA 2005, 102, 12443–12448. [Google Scholar] [CrossRef] [PubMed]
- Van der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Park, E.T.; Oh, H.K.; Gum, J.R., Jr.; Crawley, S.C.; Kakar, S.; Engel, J.; Leow, C.C.; Gao, W.Q.; Kim, Y.S. HATH1 expression in mucinous cancers of the colorectum and related lesions. Clin. Cancer Res. 2006, 12, 5403–5410. [Google Scholar] [CrossRef]
- Yang, Q.; Bermingham, N.A.; Finegold, M.J.; Zoghbi, H.Y. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 2001, 294, 2155–2158. [Google Scholar] [CrossRef] [PubMed]
- Wellington, M.O.; Hamonic, K.; Krone, J.E.C.; Htoo, J.K.; Van Kessel, A.G.; Columbus, D.A. Effect of dietary fiber and threonine content on intestinal barrier function in pigs challenged with either systemic E. coli lipopolysaccharide or enteric Salmonella Typhimurium. J. Anim. Sci. Biotechnol. 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Saadatmand, N.; Toghyani, M.; Gheisari, A. Effects of dietary fiber and threonine on performance, intestinal morphology and immune responses in broiler chickens. Anim. Nutr. 2019, 5, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Wils-Plotz, E.L.; Dilger, R.N. Combined dietary effects of supplemental threonine and purified fiber on growth performance and intestinal health of young chicks. Poult. Sci. 2013, 92, 726–734. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Chen, X.; Nie, C.; Zhao, J.; Guan, W.; Lei, L.; He, T.; Chen, Y.; Johnston, L.J.; et al. Combination of Clostridium butyricum and Corn Bran Optimized Intestinal Microbial Fermentation Using a Weaned Pig Model. Front. Microbiol. 2018, 9, 3091. [Google Scholar] [CrossRef]
- Blank, B.; Schlecht, E.; Susenbeth, A. Effect of dietary fibre on nitrogen retention and fibre associated threonine losses in growing pigs. Arch. Anim. Nutr. 2012, 66, 86–101. [Google Scholar] [CrossRef]
- Wellington, M.O.; Thiessen, R.B.; Van Kessel, A.G.; Columbus, D.A. Intestinal Health and Threonine Requirement of Growing Pigs Fed Diets Containing High Dietary Fibre and Fermentable Protein. Animals 2020, 10, 2055. [Google Scholar] [CrossRef] [PubMed]
- Rémond, D.; Buffière, C.; Godin, J.P.; Mirand, P.P.; Obled, C.; Papet, I.; Dardevet, D.; Williamson, G.; Breuillé, D.; Faure, M. Intestinal inflammation increases gastrointestinal threonine uptake and mucin synthesis in enterally fed minipigs. J. Nutr. 2009, 139, 720–726. [Google Scholar] [CrossRef]
- Faure, M.; Choné, F.; Mettraux, C.; Godin, J.P.; Béchereau, F.; Vuichoud, J.; Papet, I.; Breuillé, D.; Obled, C. Threonine utilization for synthesis of acute phase proteins, intestinal proteins, and mucins is increased during sepsis in rats. J. Nutr. 2007, 137, 1802–1807. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Eicher, S.D.; Ajuwon, K.M.; Applegate, T.J. Effect of threonine on secretory immune system using a chicken intestinal ex vivo model with lipopolysaccharide challenge. Poult. Sci. 2017, 96, 3043–3051. [Google Scholar] [CrossRef]
- Zhang, Q.; Zeng, Q.F.; Cotter, P.; Applegate, T.J. Dietary threonine response of Pekin ducks from hatch to 14 d of age based on performance, serology, and intestinal mucin secretion. Poult. Sci. 2016, 95, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Kaňková, Z.; Zeman, M.; Schwarz, S.; Kaspers, B. Preliminary results on intersexual differences in gene expression of chemokine K203 in mononuclear cells of chicken. Acta Vet. Hung. 2016, 64, 56–64. [Google Scholar] [CrossRef][Green Version]
- Papadakis, K.A.; Prehn, J.; Nelson, V.; Cheng, L.; Binder, S.W.; Ponath, P.D.; Andrew, D.P.; Targan, S.R. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J. Immunol. 2000, 165, 5069–5076. [Google Scholar] [CrossRef] [PubMed]
- Ebert, L.M.; Schaerli, P.; Moser, B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol. Immunol. 2005, 42, 799–809. [Google Scholar] [CrossRef]
- Kaetzel, C.S. The polymeric immunoglobulin receptor: Bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 2005, 206, 83–99. [Google Scholar] [CrossRef]
- Wils-Plotz, E.L.; Jenkins, M.C.; Dilger, R.N. Modulation of the intestinal environment, innate immune response, and barrier function by dietary threonine and purified fiber during a coccidiosis challenge in broiler chicks. Poult. Sci. 2013, 92, 735–745. [Google Scholar] [CrossRef] [PubMed]
- An, J.M.; Kang, E.A.; Han, Y.M.; Kim, Y.S.; Hong, Y.G.; Hah, B.S.; Hong, S.P.; Hahm, K.B. Dietary threonine prevented stress-related mucosal diseases in rats. J. Physiol. Pharmacol. 2019, 70, 467–478. [Google Scholar] [CrossRef]
- Dong, Y.W.; Jiang, W.D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q.; et al. Threonine deficiency decreased intestinal immunity and aggravated inflammation associated with NF-κB and target of rapamycin signalling pathways in juvenile grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Br. J. Nutr. 2017, 118, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Fu, H.Y.; Ma, X. Design, optimization, and nanotechnology of antimicrobial peptides: From exploration to applications. Nano Today 2021, 39. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Gao, N.; Lan, J.; Dou, X.; Li, J.; Shan, A. L-Threonine upregulates the expression of β-defensins by activating the NF-κB signaling pathway and suppressing SIRT1 expression in porcine intestinal epithelial cells. Food Funct. 2021, 12, 5821–5836. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Study Design | Main Findings | Reference |
|---|---|---|---|
| 0.57%, 1.07%, and 2.07% dietary Thr | male Sprague-Dawley rats, 10 months, n = 8/treatment, 20 days, intestinal inflammation | Increased the number of goblet cells and enhanced mucin synthesis and the mucosal mass | [36] |
| 140 mg/kg/d Thr, intragastric administration 4 µmol/kg/d L-[15N]Thr, intravenous administration 4 µmol/kg/d L-[U-13C]Thr | Pitmann-Moore minipigs, 10 months, n = 4/treatment, 7 days, ileitis | Promoted intestinal mucin synthesis and PDV utilization of Thr | [91] |
| intravenous administration 500 µmol/100 g weight L-[ U-13C]Thr | male Sprague-Dawley rats, 300 g body weight, n = 12/treatment or 14/treatment, 6 days, sepsis | Promoted the synthesis of mucin and mucosal protein | [92] |
| the basal diet supplemented with 3 · 0 g/kg L-Thr | male Arbor Acres Plus broilers, 1 day, n = 48/treatment, 21 days, lipopolysaccharide -challenged | Enhanced intestinal goblet cell density and MUC2 mRNA expression | [7] |
| the basal diet supplemented with 2.0 g/kg L-Thr | piglets, newborn, n = 18/treatment, 21 days, piglets with intrauterine growth retardation | Increased the production of MUC2 and the density of goblet cells | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Tan, P.; Ma, N.; Ma, X. Physiological Functions of Threonine in Animals: Beyond Nutrition Metabolism. Nutrients 2021, 13, 2592. https://doi.org/10.3390/nu13082592
Tang Q, Tan P, Ma N, Ma X. Physiological Functions of Threonine in Animals: Beyond Nutrition Metabolism. Nutrients. 2021; 13(8):2592. https://doi.org/10.3390/nu13082592
Chicago/Turabian StyleTang, Qi, Peng Tan, Ning Ma, and Xi Ma. 2021. "Physiological Functions of Threonine in Animals: Beyond Nutrition Metabolism" Nutrients 13, no. 8: 2592. https://doi.org/10.3390/nu13082592
APA StyleTang, Q., Tan, P., Ma, N., & Ma, X. (2021). Physiological Functions of Threonine in Animals: Beyond Nutrition Metabolism. Nutrients, 13(8), 2592. https://doi.org/10.3390/nu13082592






